Effect of Astaxanthin on the Expression and Activity of Aquaporin-3 in Skin in an In-Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PHK16-0b Cell Culture
2.3. HaCaT Cell Culture
2.4. EpiSkin 3D Human Epidermis Model
2.5. Real-Time RT-PCR
2.6. Preparation of Fractions from HaCaT Cells for Immunoblotting
2.7. Electrophoresis and Western Blotting
2.8. Glycerol Permeability Assay
2.9. Statistical Analysis
3. Results
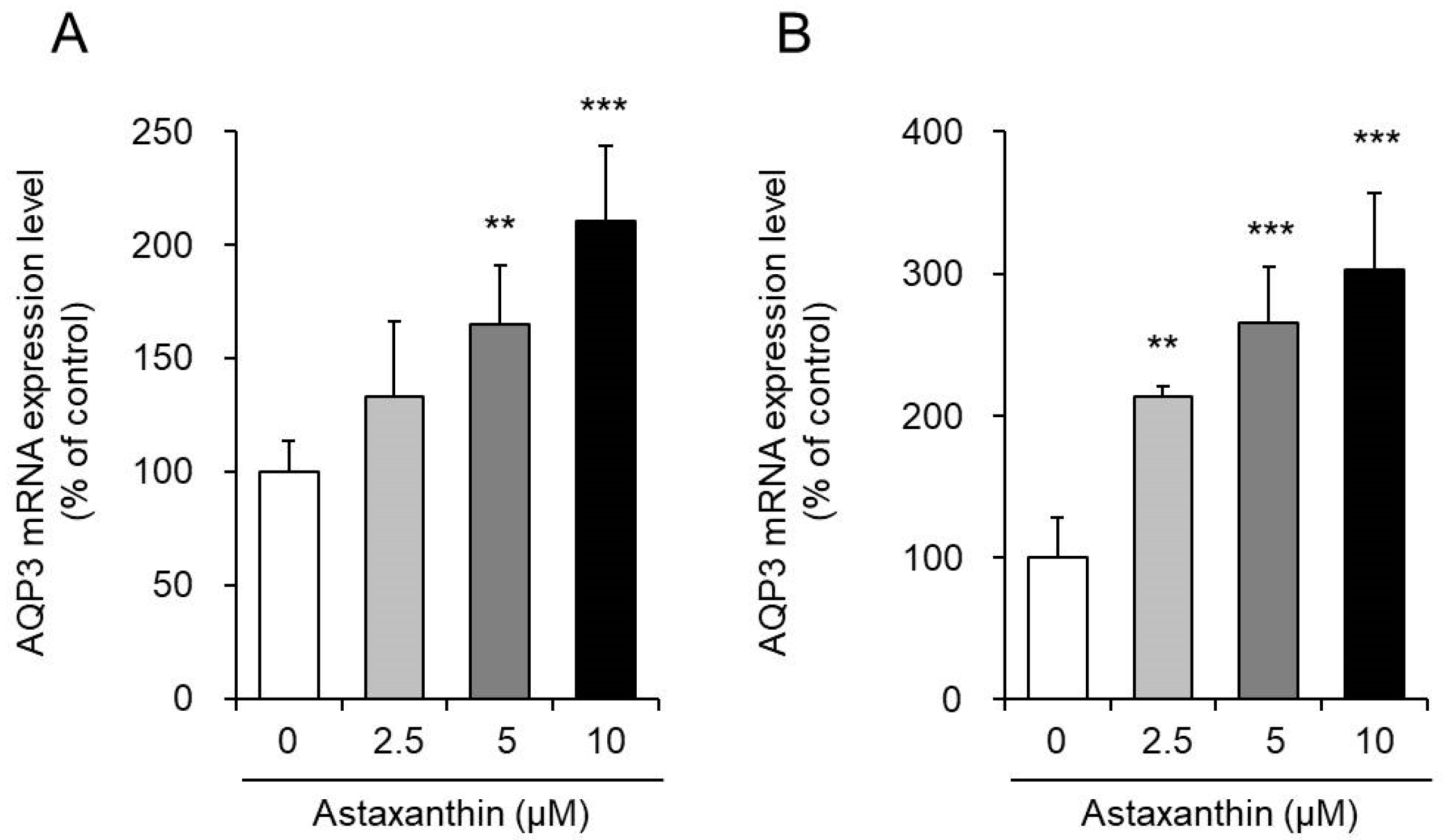
3.1. Effect of Astaxanthin on the mRNA Expression Levels of AQP3 in PHK16-0b and HaCaT Cells
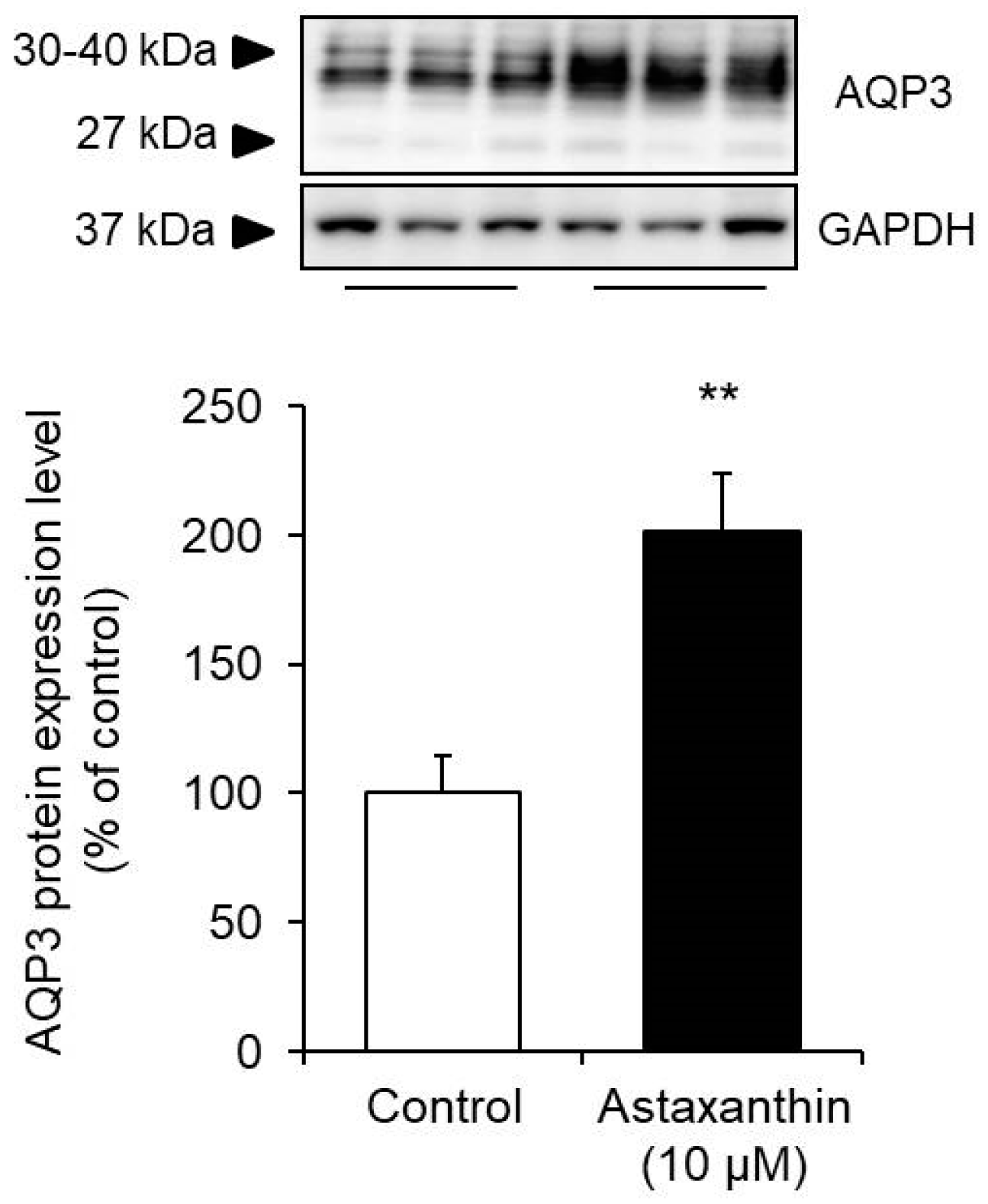
3.2. Effect of Astaxanthin on the Protein Expression Level of AQP3 in HaCaT Cells
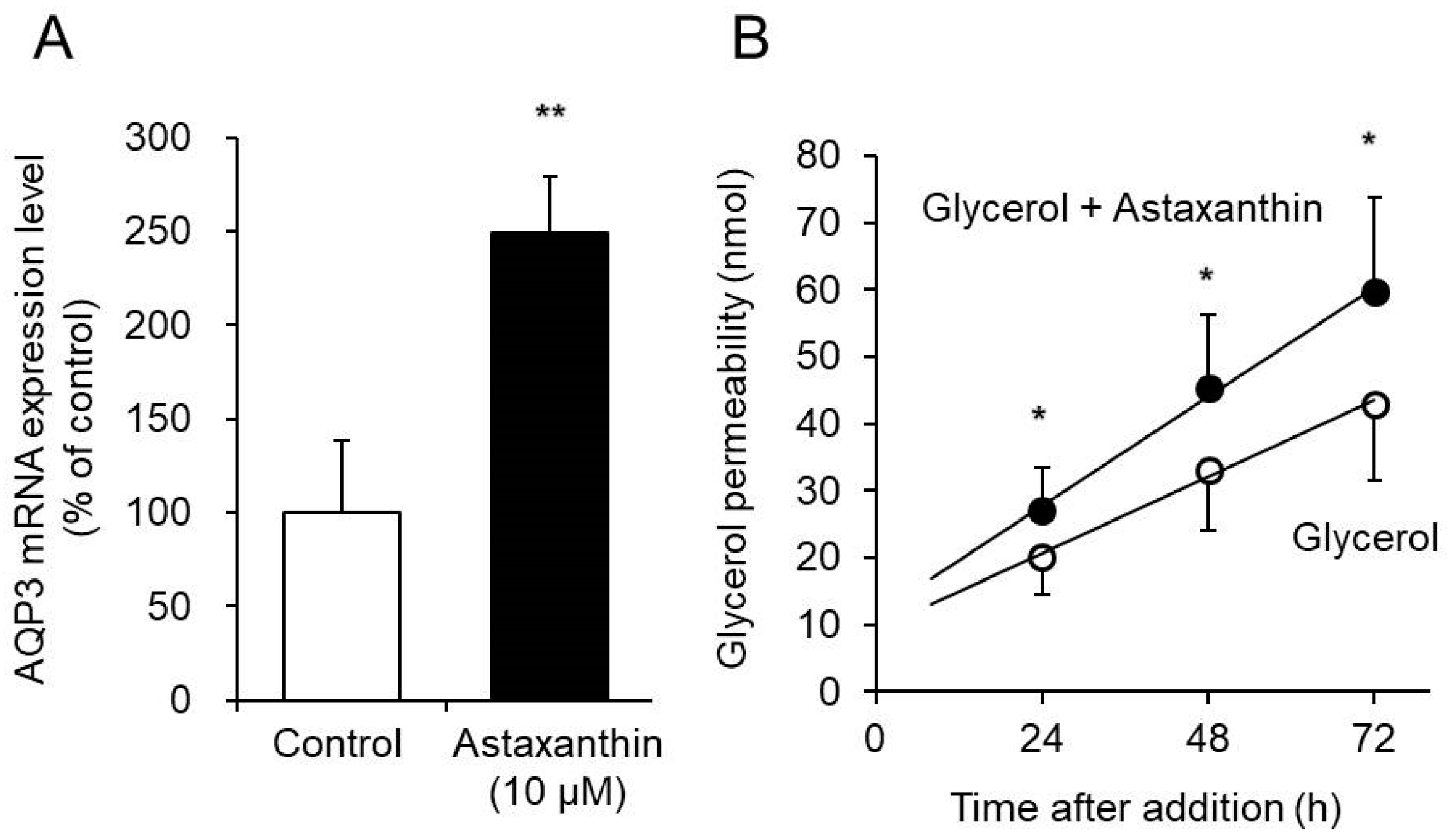
3.3. Effect of Astaxanthin on Glycerol Permeability via AQP3 in the EpiSkin Epidermal System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naguib, Y.M.A. Antioxidant Activities of Astaxanthin and Related Carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of Astaxanthin on Lipopolysaccharide-Induced Inflammation in vitro and in vivo. Investig. Opthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, G.; Nakagawa, T.; Goto, H.; Shimada, Y.; Matsumoto, K.; Sankawa, U.; Watanabe, H. Astaxanthin Ameliorates Features of Metabolic Syndrome in SHR/NDmcr-cp. Life Sci. 2007, 80, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Nagashimada, M.; Zhuge, F.; Zhan, L.; Nagata, N.; Tsutsui, A.; Nakanuma, Y.; Kaneko, S.; Ota, T. Astaxanthin Prevents and Reverses Diet-Induced Insulin Resistance and Steatohepatitis in Mice: A Comparison with Vitamin E. Sci. Rep. 2015, 5, 17192. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of Astaxanthin in Obese Mice Fed a High-Fat Diet. Biosci. Biotechnol. Biochem. 2007, 71, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, S.; Wang, H.; Xiao, S.; Li, C.; Li, Y.; Liu, B. Xanthophyllomyces Dendrorhous-Derived Astaxanthin Regulates Lipid Metabolism and Gut Microbiota in Obese Mice Induced by A High-Fat Diet. Mar. Drugs 2019, 17, 337. [Google Scholar] [CrossRef] [Green Version]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous Astaxanthin Intake Reduces Oxidative Stress and Reverses Age-Related Morphological Changes of Residual Skin Surface Components in Middle-Aged Volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus Astaxanthin: Applications for Human Health and Nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Hama, S.; Takahashi, K.; Inai, Y.; Shiota, K.; Sakamoto, R.; Yamada, A.; Tsuchiya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective Effects of Topical Application of a Poorly Soluble Antioxidant Astaxanthin Liposomal Formulation on Ultraviolet-Induced Skin Damage. J. Pharm. Sci. 2012, 101, 2909–2916. [Google Scholar] [CrossRef]
- Ng, Q.X.; De Deyn, M.L.Z.Q.; Loke, W.; Foo, N.X.; Chan, H.W.; Yeo, W.S. Effects of Astaxanthin Supplementation on Skin Health: A Systematic Review of Clinical Studies. J. Diet. Suppl. 2020, 1–14. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic Benefits of Astaxanthin on Humans Subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Edwin, D.L. Equol’s Effecacy is Greater than Astaxanthin for Antioxidants, Extracellular Matrix Integrity & Breakdown, Growth Factors and Inflammatory Biomarkers via Human Skin Gene Expression Analysis. J. Funct. Foods 2019, 59, 380–393. [Google Scholar]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive Effect of Dietary Astaxanthin on UVA-Induced Skin Photoaging in Hairless Mice. PLoS ONE 2017, 12, e0171178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Fujiyoshia, Y.; Mitsuoka, K.; De Groot, B.L.; Philippsen, A.; Grubmüller, H.; Agre, P.; Engel, A. Structure and Function of Water Channels. Curr. Opin. Struct. Biol. 2002, 12, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Kitchen, P.; Day, R.E.; Salman, M.M.; Conner, M.T.; Bill, R.M.; Conner, A.C. Beyond Water Homeostasis: Diverse Functional Roles of Mammalian Aquaporins. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 2410–2421. [Google Scholar] [CrossRef] [Green Version]
- Kitchen, P.; Salman, M.M.; Pickel, S.U.; Jennings, J.; Törnroth-Horsefield, S.; Conner, M.T.; Bill, R.M.; Conner, A.C. Water Channel Pore Size Determines Exclusion Properties but not Solute Selectivity. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hara-Chikuma, M.; Verkman, A.S. Physiological Roles of Glycerol-Transporting Aquaporins: The Aquaglyceroporins. Cell. Mol. Life Sci. 2006, 63, 1386–1392. [Google Scholar] [CrossRef]
- Hara, M.; Ma, T.; Verkman, A.S. Selectively Reduced Glycerol in Skin of Aquaporin-3-Deficient Mice May Account for Impaired Skin Hydration, Elasticity, and Barrier Recovery. J. Biol. Chem. 2002, 277, 46616–46621. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Hara, M.; Sougrat, R.; Verbavatz, J.-M.; Verkman, A.S. Impaired Stratum Corneum Hydration in Mice Lacking Epidermal Water Channel Aquaporin-3. J. Biol. Chem. 2002, 277, 17147–17153. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Zheng, X.; Zhong, X.; Shetty, A.K.; Elias, P.M.; Bollag, W.B. Aquaporin-3 in Keratinocytes and Skin: Its Role and Interaction with Phospholipase D2. Arch. Biochem. Biophys. 2011, 508, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Je, Y.-J.; Lee, S.-S.; Li, Z.J.; Choi, D.-K.; Kwon, Y.-B.; Sohn, K.-C.; Im, M.; Seo, Y.J.; Lee, J.H. Changes in Transepidermal Water Loss and Skin Hydration According to Expression of Aquaporin-3 in Psoriasis. Ann. Dermatol. 2012, 24, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-H.; Lee, A.-Y. Reduced Aquaporin3 Expression and Survival of Keratinocytes in the Depigmented Epidermis of Vitiligo. J. Investig. Dermatol. 2010, 130, 2231–2239. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.-Y. Role of Keratinocytes in the Development of Vitiligo. Ann. Dermatol. 2012, 24, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Ikarashi, N.; Mizukami, N.; Kon, R.; Kaneko, M.; Uchino, R.; Fujisawa, I.; Fukuda, N.; Sakai, H.; Kamei, J. Study of the Mechanism Underlying the Onset of Diabetic Xeroderma Focusing on an Aquaporin-3 in a Streptozotocin-Induced Diabetic Mouse Model. Int. J. Mol. Sci. 2019, 20, 3782. [Google Scholar] [CrossRef] [Green Version]
- Ikarashi, N.; Kon, R.; Kaneko, M.; Mizukami, N.; Kusunoki, Y.; Sugiyama, K. Relationship between Aging-Related Skin Dryness and Aquaporins. Int. J. Mol. Sci. 2017, 18, 1559. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kaneko, M.; Watanabe, T.; Kon, R.; Yoshino, M.; Yokoyama, T.; Tanaka, R.; Takayama, N.; Sakai, H.; Kamei, J. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Erlotinib Induces Dry Skin via Decreased in Aquaporin-3 Expression. Biomolecules 2020, 10, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomczynski, P.; Sacchi, N. Single-step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Silberstein, C.; Kierbel, A.; Amodeo, G.; Zotta, E.; Bigi, F.; Berkowski, D.; Ibarra, C. Functional Characterization and Localization of AQP3 in the Human Colon. Braz. J. Med. Biol. Res. 1999, 32, 1303–1313. [Google Scholar] [CrossRef] [Green Version]
- Spector, D.A.; Wade, J.B.; Dillow, R.; Steplock, D.A.; Weinman, E.J. Expression, Localization, and Regulation of Aquaporin-1 to -3 in Rat Urothelia. Am. J. Physiol. Physiol. 2002, 282, F1034–F1042. [Google Scholar] [CrossRef]
- Baumgarten, R.; Van De Pol, M.H.; Wetzels, J.F.; Van Os, C.H.; Deen, P.M. Glycosylation is not Essential for Vasopressin-Dependent Routing of Aquaporin-2 in Transfected Madin-Darby Canine Kidney Cells. J. Am. Soc. Nephrol. 1998, 9, 1553–1559. [Google Scholar] [PubMed]
- Hendriks, G.; Wang, F.; Thirumurugan, K.; Stafford, W.F.; Hammer, J.A.; Knight, P.J.; Sellers, J.R.; Koudijs, M.; Van Balkom, B.W.M.; Oorschot, V.; et al. Glycosylation Is Important for Cell Surface Expression of the Water Channel Aquaporin-2 but Is Not Essential for Tetramerization in the Endoplasmic Reticulum. J. Biol. Chem. 2003, 279, 2975–2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umenishi, F.; Narikiyo, T.; Schrier, R.W. Effect on Stability, Degradation, Expression, and Targeting of Aquaporin-2 Water Channel by Hyperosmolality in Renal Epithelial Cells. Biochem. Biophys. Res. Commun. 2005, 338, 1593–1599. [Google Scholar] [CrossRef]
- Ikarashi, N.; Nagoya, C.; Kon, R.; Kitaoka, S.; Kajiwara, S.; Saito, M.; Kawabata, A.; Ochiai, W.; Sugiyama, K. Changes in the Expression of Aquaporin-3 in the Gastrointestinal Tract Affect Drug Absorption. Int. J. Mol. Sci. 2019, 20, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luu-The, V.; Duche, D.; Ferraris, C.; Meunier, J.-R.; LeClaire, J.; Labrie, F. Expression Profiles of Phases 1 and 2 Metabolizing Enzymes in Human Skin and the Reconstructed Skin Models Episkin™ and Full Thickness Model from Episkin™. J. Steroid Biochem. Mol. Biol. 2009, 116, 178–186. [Google Scholar] [CrossRef]
- Roguet, R.; Cohen, C.; Dossou, K.; Rougier, A. Episkin, a Reconstituted Human Epidermis for Assessing in vitro the Irritancy of Topically Applied Compounds. Toxicol. Vitro 1994, 8, 283–291. [Google Scholar] [CrossRef]
- Abir-Awan, M.; Kitchen, P.; Salman, M.M.; Conner, M.; Conner, A.C.; Bill, R.M. Inhibitors of Mammalian Aquaporin Water Channels. Int. J. Mol. Sci. 2019, 20, 1589. [Google Scholar] [CrossRef] [Green Version]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but Elusive Drug Targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [Green Version]
- Hashida, T.; Yasumoto, S. Induction of Chromosome Abnormalities in Mouse and Human Epidermal Keratinocytes by the Human Papillomavirus Type 16 E7 Oncogene. J. Gen. Virol. 1991, 72, 1569–1577. [Google Scholar] [CrossRef]
- Ikarashi, N.; Ogiue, N.; Toyoda, E.; Nakamura, M.; Kon, R.; Kusunoki, Y.; Aburada, T.; Ishii, M.; Tanaka, Y.; Machida, Y.; et al. Elucidating the Mechanism by Which Gypsum fibrosum, a Traditional Chinese Medicine, Maintains Cutaneous Water Content. Biol. Pharm. Bull. 2013, 36, 1615–1621. [Google Scholar] [CrossRef] [Green Version]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.; Rafiq, M.; Paramesh, R. In vitro Anti-Inflammatory and Skin Protective Properties of Virgin Coconut Oil. J. Tradit. Complement. Med. 2019, 9, 5–14. [Google Scholar] [CrossRef]
- Xing, F.; Liao, W.; Jiang, P.; Xu, W.; Jin, X. Effect of Retinoic Acid on Aquaporin 3 Expression in Keratinocytes. Genet. Mol. Res. 2016, 15, 15016951. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-I. Astaxanthin as a Peroxisome Proliferator-Activated Receptor (PPAR) Modulator: Its Therapeutic Implications. Mar. Drugs 2019, 17, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, M.; Tanabe, H.; Matsumoto, A.; Takagi, M.; Umegaki, K.; Amagaya, S.; Takahashi, J. Astaxanthin Functions Differently as a Selective Peroxisome Proliferator-Activated Receptor Gamma Modulator in Adipocytes and Macrophages. Biochem. Pharmacol. 2012, 84, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Kim, P.; Lu, Y.F.; Feingold, K.R. PPARgamma Activators Stimulate Aquaporin 3 Expression in Keratinocytes/Epidermis. Exp. Dermatol. 2011, 20, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Schmuth, M.; Moosbrugger-Martinz, V.; Blunder, S.; Dubrac, S. Role of PPAR, LXR, and PXR in Epidermal Homeostasis and Inflammation. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2014, 1841, 463–473. [Google Scholar] [CrossRef]
- Yang, R.; Chowdhury, S.; Choudhary, V.; Chen, X.; Bollag, W. Keratinocyte Aquaporin-3 Expression Induced by Histone Deacetylase Inhibitors is Mediated in Part by Peroxisome Proliferator-Activated Receptors (PPARs). Exp. Dermatol. 2020, 29, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Ikarashi, N.; Kon, R.; Sugiyama, K. Aquaporins in the Colon as a New Therapeutic Target in Diarrhea and Constipation. Int. J. Mol. Sci. 2016, 17, 1172. [Google Scholar] [CrossRef]
| Target | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| AQP3 | AGACAGCCCCTTCAGGATTT | TCCCTTGCCCTGAATATCTG |
| GAPDH | ATGGGGAAGGTGAAGGTCG | GGGGTCATTGATGGCAACAATA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikarashi, N.; Kon, R.; Nagoya, C.; Ishikura, A.; Sugiyama, Y.; Takahashi, J.; Sugiyama, K. Effect of Astaxanthin on the Expression and Activity of Aquaporin-3 in Skin in an In-Vitro Study. Life 2020, 10, 193. https://doi.org/10.3390/life10090193
Ikarashi N, Kon R, Nagoya C, Ishikura A, Sugiyama Y, Takahashi J, Sugiyama K. Effect of Astaxanthin on the Expression and Activity of Aquaporin-3 in Skin in an In-Vitro Study. Life. 2020; 10(9):193. https://doi.org/10.3390/life10090193
Chicago/Turabian StyleIkarashi, Nobutomo, Risako Kon, Chika Nagoya, Airi Ishikura, Yuri Sugiyama, Jiro Takahashi, and Kiyoshi Sugiyama. 2020. "Effect of Astaxanthin on the Expression and Activity of Aquaporin-3 in Skin in an In-Vitro Study" Life 10, no. 9: 193. https://doi.org/10.3390/life10090193
APA StyleIkarashi, N., Kon, R., Nagoya, C., Ishikura, A., Sugiyama, Y., Takahashi, J., & Sugiyama, K. (2020). Effect of Astaxanthin on the Expression and Activity of Aquaporin-3 in Skin in an In-Vitro Study. Life, 10(9), 193. https://doi.org/10.3390/life10090193





