Zebrafish Vascular Mural Cell Biology: Recent Advances, Development, and Functions
Abstract
:1. Introduction
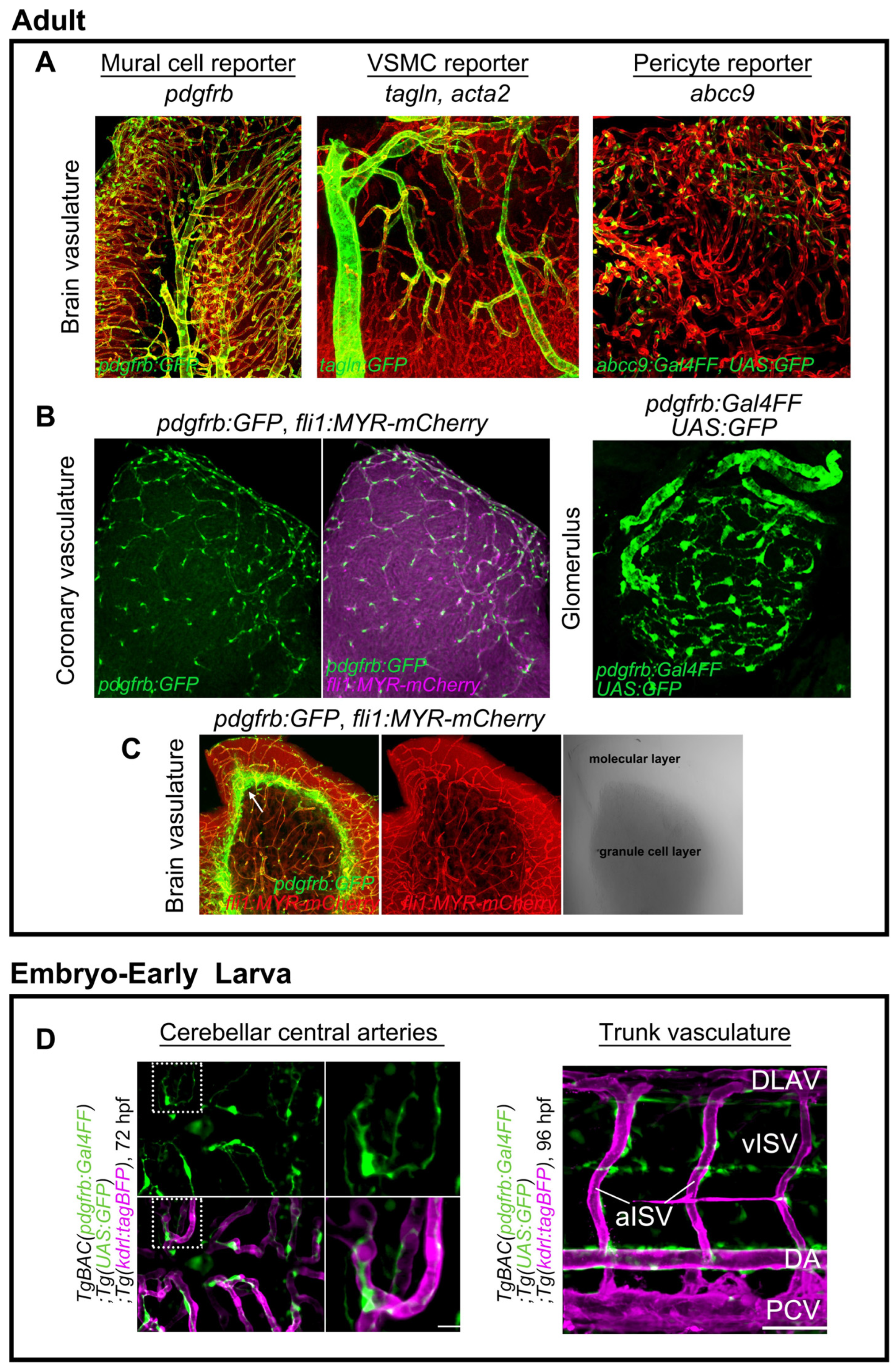
2. Genetic Tools for Mural Cell Biology in Zebrafish
3. Mural Cell Development in Zebrafish
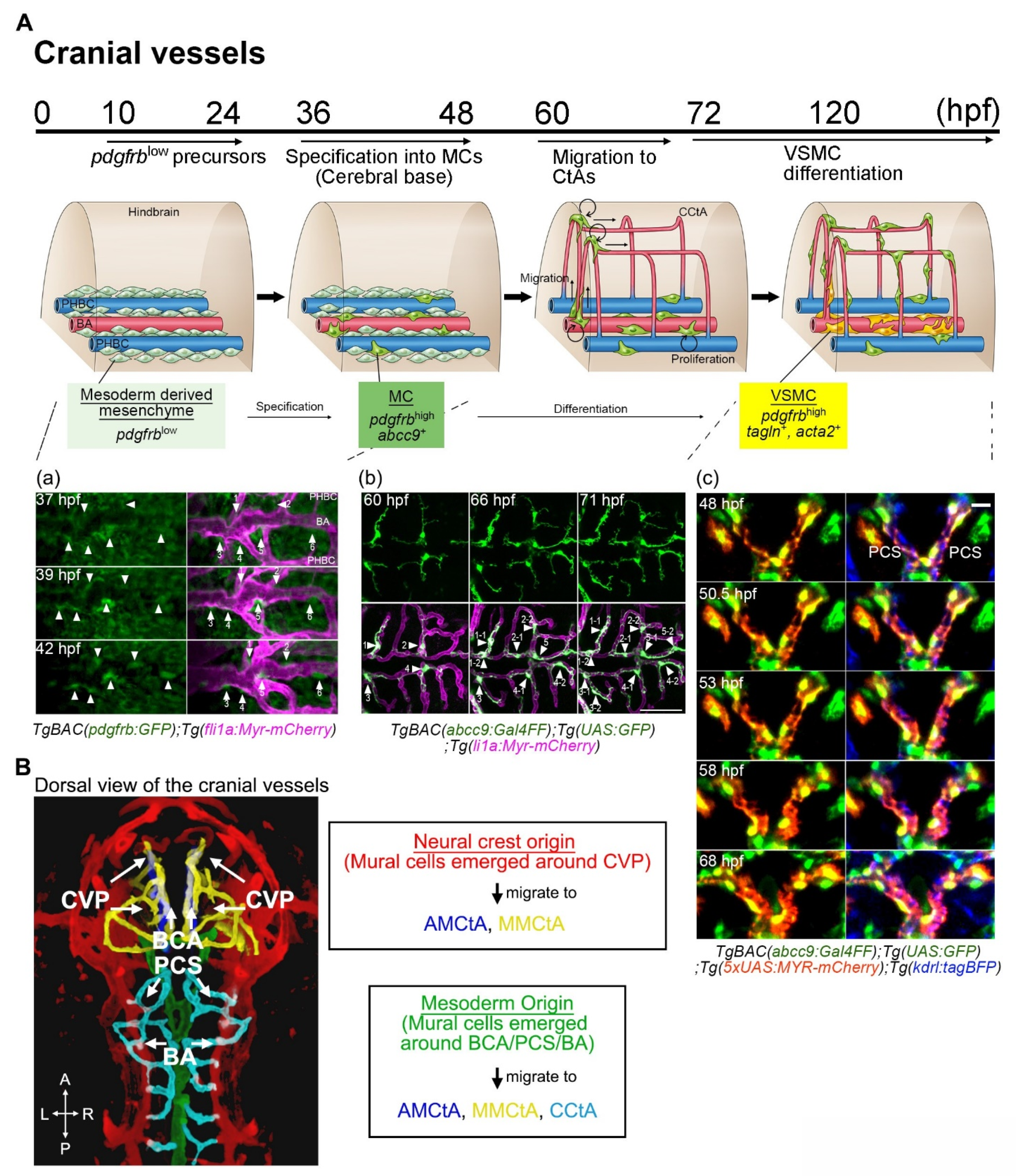
3.1. Overview of Mural Cell Development during the Early Developmental Stages in Zebrafish
3.2. Mural Cell Development in Axial Vessels in the Trunk
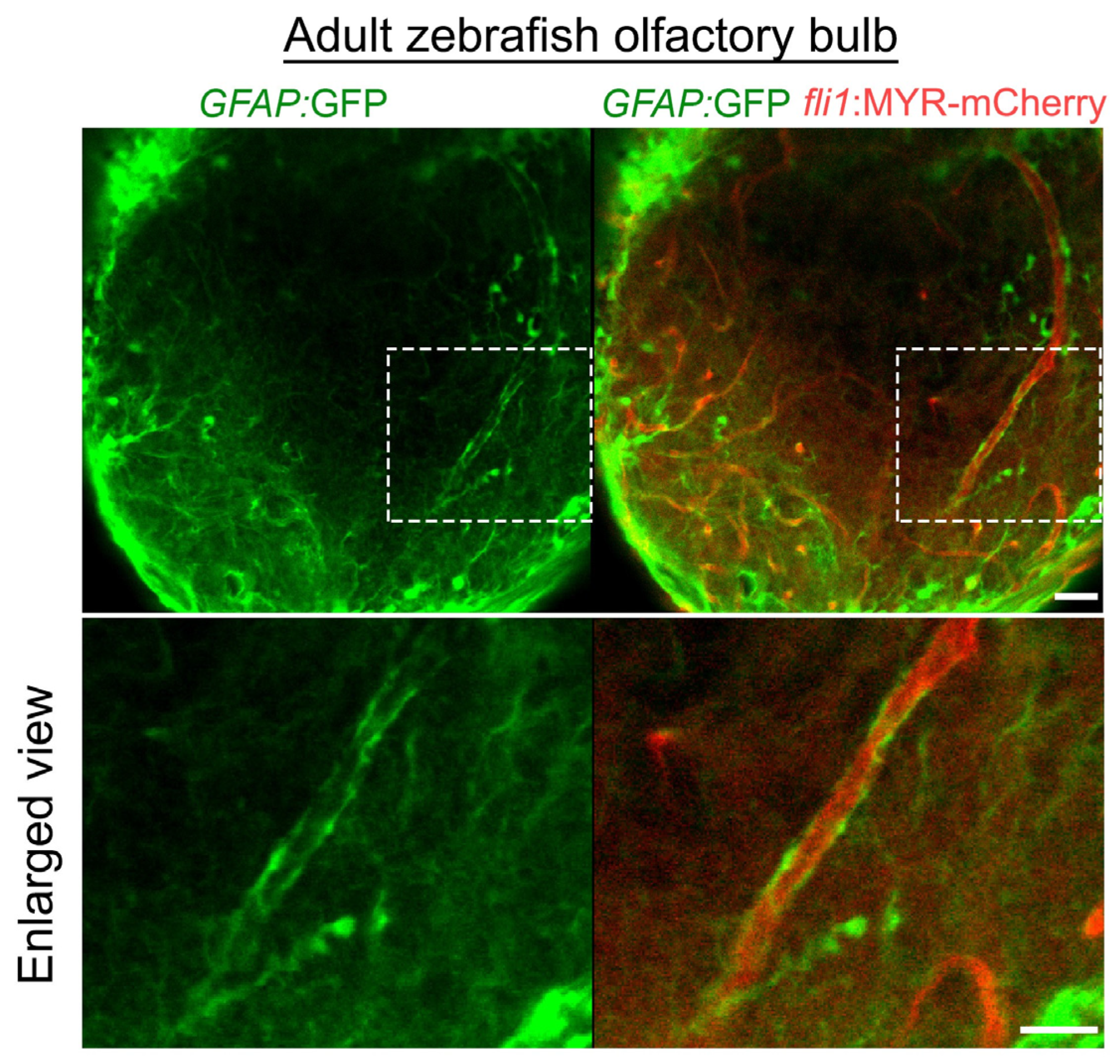
3.3. Mural Cell Development in the Brain
3.4. Mural Cell Development in the Ventral Head
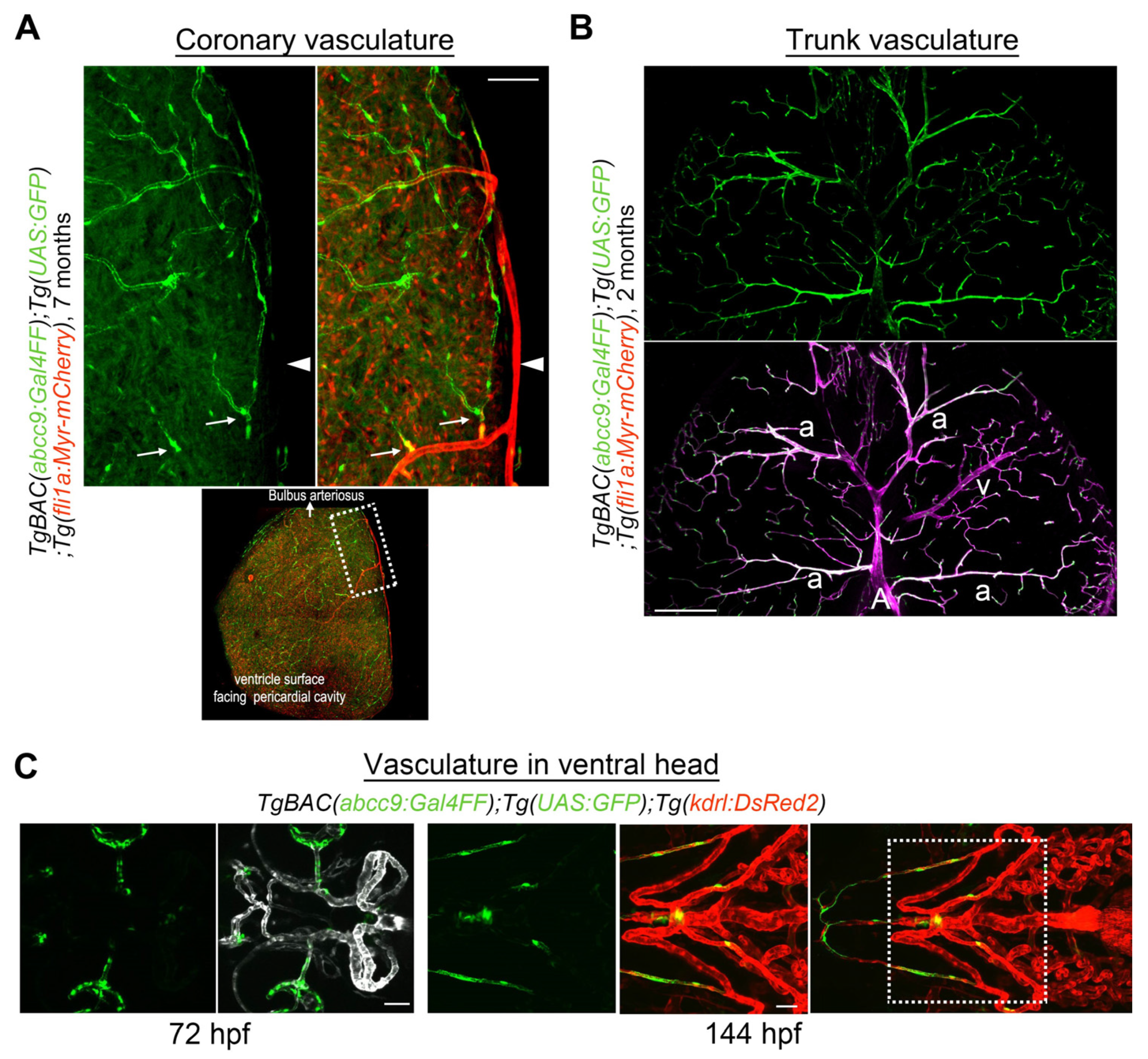
3.5. Mural Cell Development in the Kidney
3.6. Mural Cell Development during Regeneration
4. Molecular Mechanisms Underlying Mural Cell Development
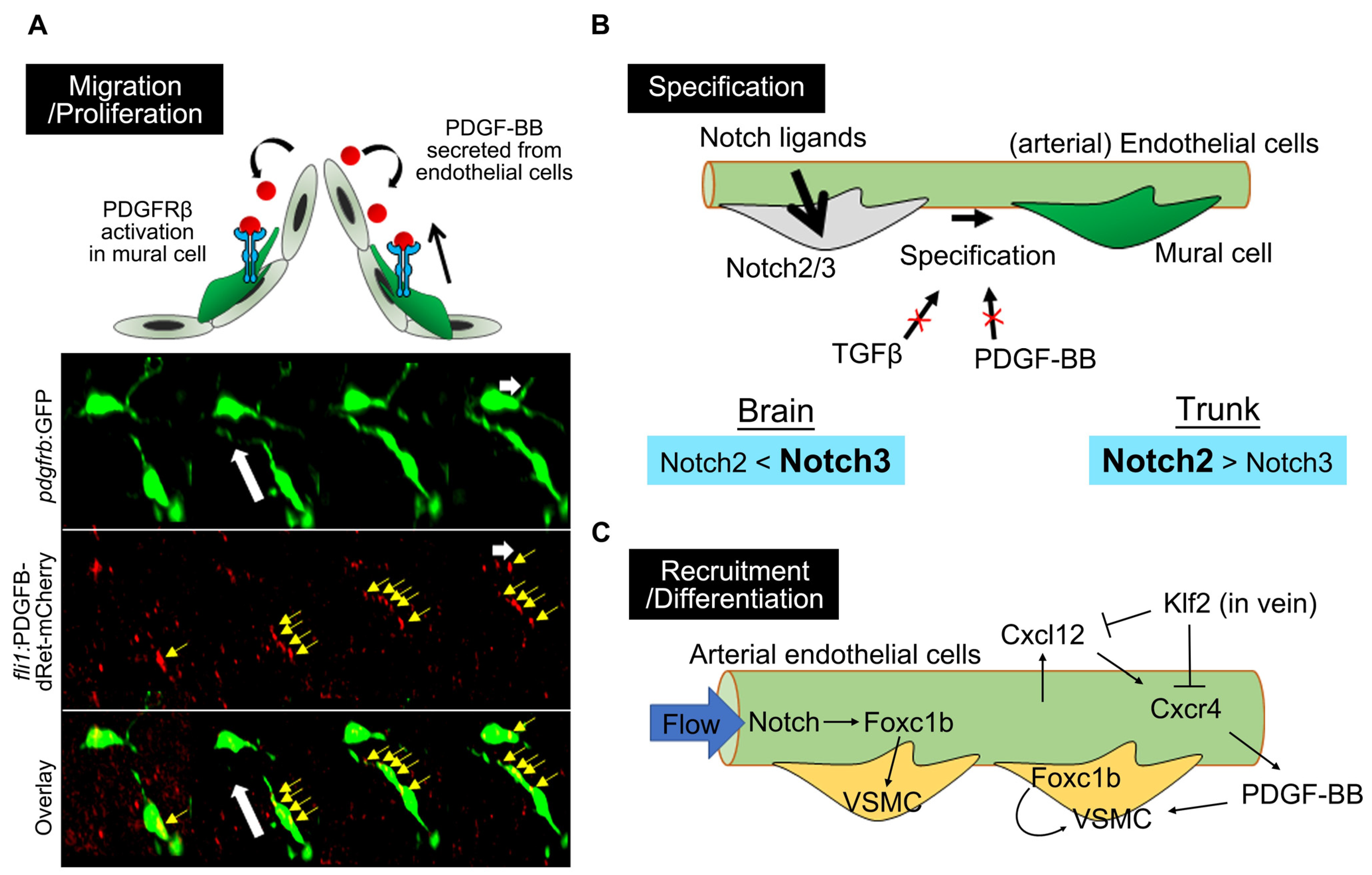
4.1. PDGFRβ-Mediated Signaling in Mural Cell Development
4.2. Notch Signaling in Mural Cell Development
4.3. Forkhead Box Transcription Factors
4.4. TGFβ Signaling
5. Function of Mural Cells in Zebrafish
5.1. Observations Made in the Phenotyping Portion of pdgfrb Mutant Zebrafish Studies
5.2. BBB
5.3. Regulation of Vascular Tone
6. Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Develop. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475. [Google Scholar] [CrossRef] [Green Version]
- Uemura, M.T.; Maki, T.; Ihara, M.; Lee, V.M.Y.; Trojanowski, J.Q. Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia. Front. Aging Neurosci. 2020, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Muhl, L.; Genové, G.; Leptidis, S.; Liu, J.; He, L.; Mocci, G.; Sun, Y.; Gustafsson, S.; Buyandelger, B.; Chivukula, I.V.; et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nature Commun. 2020, 11, 3953. [Google Scholar] [CrossRef]
- Fukuhara, S.; Fukui, H.; Wakayama, Y.; Ando, K.; Nakajima, H.; Mochizuki, N. Looking back and moving forward: Recent advances in understanding of cardiovascular development by imaging of zebrafish. Develop. Growth Differ. 2015, 57, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Gebala, V.; Collins, R.; Geudens, I.; Phng, L.-K.; Gerhardt, H. Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo. Nat. Cell Biol. 2016, 18, 443–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwada, T.; Fukuhara, S.; Terai, K.; Tanaka, T.; Wakayama, Y.; Ando, K.; Nakajima, H.; Fukui, H.; Yuge, S.; Saito, Y. β-catenin-dependent transcription is central to Bmp-mediated formation of venous vessels. Development 2015, 142, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Develop. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, K.S.; Hogan, B.M. Endothelial Cell Dynamics in Vascular Development: Insights From Live-Imaging in Zebrafish. Front. Physiol. 2020, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Nakajima, H.; Wakayama, Y.; Muto, A.; Kawakami, K.; Fukuhara, S.; Mochizuki, N. Endothelial Ca2+ oscillations reflect VEGFR signaling-regulated angiogenic capacity in vivo. eLife 2015, 4, e08817. [Google Scholar] [CrossRef]
- Vanhollebeke, B.; Stone, O.A.; Bostaille, N.; Cho, C.; Zhou, Y.; Maquet, E.; Gauquier, A.; Cabochette, P.; Fukuhara, S.; Mochizuki, N.; et al. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/beta-catenin pathway during brain angiogenesis. eLife 2015, 4, e06489. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Fukuhara, S.; Izumi, N.; Nakajima, H.; Fukui, H.; Kelsh, R.N.; Mochizuki, N. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development 2016, 143, 1328–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bower, N.I.; Koltowska, K.; Pichol-Thievend, C.; Virshup, I.; Paterson, S.; Lagendijk, A.K.; Wang, W.; Lindsey, B.W.; Bent, S.J.; Baek, S.; et al. Mural lymphatic endothelial cells regulate meningeal angiogenesis in the zebrafish. Nat. Neurosci. 2017, 20, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Rider, S.A.; Christian, H.C.; Mullins, L.J.; Howarth, A.R.; MacRae, C.A.; Mullins, J.J. Zebrafish mesonephric renin cells are functionally conserved and comprise two distinct morphological populations. Am. J. Physiol. Renal Physiol. 2017, 312, F778–F790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, E.V.; Figueroa, R.J.; Bussmann, J.; Lawson, N.D.; Amigo, J.D.; Siekmann, A.F. Regenerating vascular mural cells in zebrafish fin blood vessels are not derived from pre-existing ones and differentially require pdgfrb signaling for their development. bioRxiv 2021. [Google Scholar] [CrossRef]
- Tsata, V.; Möllmert, S.; Schweitzer, C.; Kolb, J.; Möckel, C.; Böhm, B.; Rosso, G.; Lange, C.; Lesche, M.; Hammer, J.; et al. A switch in pdgfrb+ cell-derived ECM composition prevents inhibitory scarring and promotes axon regeneration in the zebrafish spinal cord. Develop. Cell 2021, 56, 509–524. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Y.; Du, X.-F.; Li, J.; Zi, H.-X.; Bu, J.-W.; Yan, Y.; Han, H.; Du, J.-L. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017, 27, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C.; Abrams, J.; Pack, M. Characterization of zebrafish intestinal smooth muscle development using a novel sm22α-b promoter. Develop. Dyn. 2010, 239, 2806–2812. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Gays, D.; Milia, C.; Santoro, M.M. Cilia Control Vascular Mural Cell Recruitment in Vertebrates. Cell Rep. 2017, 18, 1033–1047. [Google Scholar] [CrossRef] [Green Version]
- Stratman, A.N.; Pezoa, S.A.; Farrelly, O.M.; Castranova, D.; Dye, L.E.; Butler, M.G.; Sidik, H.; Talbot, W.S.; Weinstein, B.M. Interactions between mural cells and endothelial cells stabilize the developing zebrafish dorsal aorta. Development 2017, 144, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Elworthy, S.; Savage, A.M.; Wilkinson, R.N.; Malicki, J.J.; Chico, T.J.A. The role of endothelial cilia in postembryonic vascular development. Develop. Dyn. 2019, 248, 410–425. [Google Scholar] [CrossRef]
- Chhabria, K.; Vouros, A.; Gray, C.; MacDonald, R.B.; Jiang, Z.; Wilkinson, R.N.; Plant, K.; Vasilaki, E.; Howarth, C.; Chico, T.J.A. Sodium nitroprusside prevents the detrimental effects of glucose on the neurovascular unit and behaviour in zebrafish. Dis. Models Mech. 2019, 12, dmm039867. [Google Scholar] [CrossRef] [Green Version]
- Whitesell, T.R.; Kennedy, R.M.; Carter, A.D.; Rollins, E.L.; Georgijevic, S.; Santoro, M.M.; Childs, S.J. An α-smooth muscle actin (acta2/αsma) zebrafish transgenic line marking vascular mural cells and visceral smooth muscle cells. PLoS ONE 2014, 9, e90590. [Google Scholar] [CrossRef] [Green Version]
- Whitesell, T.R.; Chrystal, P.W.; Ryu, J.-R.; Munsie, N.; Grosse, A.; French, C.R.; Workentine, M.L.; Li, R.; Zhu, L.J.; Waskiewicz, A.; et al. foxc1 is required for embryonic head vascular smooth muscle differentiation in zebrafish. Dev. Biol. 2019, 453, 34–47. [Google Scholar] [CrossRef] [PubMed]
- French, C.R.; Seshadri, S.; Destefano, A.L.; Fornage, M.; Arnold, C.R.; Gage, P.J.; Skarie, J.M.; Dobyns, W.B.; Millen, K.J.; Liu, T. Mutation of FOXC1 and PITX2 induces cerebral small-vessel disease. J. Clin. Investig. 2014, 124, 4877–4881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Fan, X.; Wang, Y.; Jin, H.; Song, Y.; Han, Y.; Huang, S.; Meng, Y.; Tang, F.; Meng, A. Embryonic hematopoiesis in vertebrate somites gives rise to definitive hematopoietic stem cells. J. Mol. Cell Biol. 2016, 8, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Miesfeld, J.B.; Gestri, G.; Clark, B.S.; Flinn, M.A.; Poole, R.J.; Bader, J.R.; Besharse, J.C.; Wilson, S.W.; Link, B.A. Yap and Taz regulate retinal pigment epithelial cell fate. Development 2015, 142, 3021–3032. [Google Scholar] [PubMed] [Green Version]
- Ando, K.; Wang, W.; Peng, D.; Chiba, A.; Lagendijk, A.K.; Barske, L.; Crump, J.G.; Stainier, D.Y.R.; Lendahl, U.; Koltowska, K.; et al. Peri-arterial specification of vascular mural cells from naïve mesenchyme requires Notch signaling. Development 2019, 146, dev165589. [Google Scholar] [CrossRef] [Green Version]
- Berthiaume, A.-A.; Hartmann, D.A.; Majesky, M.W.; Bhat, N.R.; Shih, A.Y. Pericyte Structural Remodeling in Cerebrovascular Health and Homeostasis. Front. Aging Neurosci. 2018, 10, 210. [Google Scholar] [CrossRef]
- Holm, A.; Heumann, T.; Augustin, H.G. Microvascular Mural Cell Organotypic Heterogeneity and Functional Plasticity. Trends Cell Biol. 2018, 28, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Lendahl, U.; Nilsson, P.; Betsholtz, C. Emerging links between cerebrovascular and neurodegenerative diseases-a special role for pericytes. EMBO Rep. 2019, 20, e48070. [Google Scholar] [CrossRef]
- Yamazaki, T.; Mukouyama, Y.-S. Tissue Specific Origin, Development, and Pathological Perspectives of Pericytes. Front. Cardiovasc. Med. 2018, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.M.; Pesce, G.; Stainier, D.Y. Characterization of vascular mural cells during zebrafish development. Mech. Dev. 2009, 126, 638–649. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, L.; Moens, C.B.; Appel, B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development 2014, 141, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Kaslin, J.; Ganz, J.; Geffarth, M.; Grandel, H.; Hans, S.; Brand, M. Stem cells in the adult zebrafish cerebellum: Initiation and maintenance of a novel stem cell niche. J. Neurosci. 2009, 29, 6142–6153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, A.C.; Maldonado-Soto, A.R.; Silva-Vargas, V.; Mizrak, D.; von Känel, T.; Tan, K.R.; Paul, A.; Madar, A.; Cuervo, H.; Kitajewski, J.; et al. Release of stem cells from quiescence reveals gliogenic domains in the adult mouse brain. Science 2021, 372, 1205–1209. [Google Scholar] [CrossRef]
- Wang, S.; Mo, M.; Wang, J.; Sadia, S.; Shi, B.; Fu, X.; Yu, L.; Tredget, E.E.; Wu, Y. Platelet-derived growth factor receptor beta identifies mesenchymal stem cells with enhanced engraftment to tissue injury and pro-angiogenic property. Cell. Mol. Life Sci. 2018, 75, 547–561. [Google Scholar] [CrossRef]
- Rajan, A.M.; Ma, R.C.; Kocha, K.M.; Zhang, D.J.; Huang, P. Dual function of perivascular fibroblasts in vascular stabilization in zebrafish. PLOS Genet. 2020, 16, e1008800. [Google Scholar] [CrossRef] [PubMed]
- Wasteson, P.; Johansson, B.R.; Jukkola, T.; Breuer, S.; Akyürek, L.M.; Partanen, J.; Lindahl, P. Developmental origin of smooth muscle cells in the descending aorta in mice. Development 2008, 135, 1823–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, Y. Dorsal aorta formation: Separate origins, lateral-to-medial migration, and remodeling. Dev. Growth Differ. 2013, 55, 113–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardanaud, L.; Luton, D.; Prigent, M.; Bourcheix, L.M.; Catala, M.; Dieterlen-Lievre, F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development 1996, 122, 1363–1371. [Google Scholar] [CrossRef]
- Pouget, C.; Gautier, R.; Teillet, M.A.; Jaffredo, T. Somite-derived cells replace ventral aortic hemangioblasts and provide aortic smooth muscle cells of the trunk. Development 2006, 133, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Perlin, J.R.; Robertson, A.L.; Zon, L.I. Efforts to enhance blood stem cell engraftment: Recent insights from zebrafish hematopoiesis. J. Exp. Med. 2017, 214, 2817–2827. [Google Scholar] [CrossRef] [Green Version]
- Rho, S.S.; Kobayashi, I.; Oguri-Nakamura, E.; Ando, K.; Fujiwara, M.; Kamimura, N.; Hirata, H.; Iida, A.; Iwai, Y.; Mochizuki, N.; et al. Rap1b Promotes Notch-Signal-Mediated Hematopoietic Stem Cell Development by Enhancing Integrin-Mediated Cell Adhesion. Dev. Cell 2019, 49, 681–696. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Hollway, G.E.; Sonntag, C.; Miles, L.B.; Hall, T.E.; Berger, S.; Fernandez, K.J.; Gurevich, D.B.; Cole, N.J.; Alaei, S.; et al. Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature 2014, 512, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Pouget, C.; Peterkin, T.; Simões, F.C.; Lee, Y.; Traver, D.; Patient, R. FGF signalling restricts haematopoietic stem cell specification via modulation of the BMP pathway. Nat. Commun. 2014, 5, 5588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, R.N.; Pouget, C.; Gering, M.; Russell, A.J.; Davies, S.G.; Kimelman, D.; Patient, R. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev. Cell 2009, 16, 909–916. [Google Scholar] [CrossRef] [Green Version]
- Souilhol, C.; Gonneau, C.; Lendinez, J.G.; Batsivari, A.; Rybtsov, S.; Wilson, H.; Morgado-Palacin, L.; Hills, D.; Taoudi, S.; Antonchuk, J.; et al. Inductive interactions mediated by interplay of asymmetric signalling underlie development of adult haematopoietic stem cells. Nat. Commun. 2016, 7, 10784. [Google Scholar] [CrossRef] [Green Version]
- Damm, E.W.; Clements, W.K. Pdgf signalling guides neural crest contribution to the haematopoietic stem cell specification niche. Nat. Cell Biol. 2017, 19, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.E.; Esain, V.; Kwan, W.; Theodore, L.N.; Cortes, M.; Frost, I.M.; Liu, S.Y.; North, T.E. HIF1α-induced PDGFRβ signaling promotes developmental HSC production via IL-6 activation. Exp. Hematol. 2017, 46, 83–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noishiki, C.; Yuge, S.; Ando, K.; Wakayama, Y.; Mochizuki, N.; Ogawa, R.; Fukuhara, S. Live imaging of angiogenesis during cutaneous wound healing in adult zebrafish. Angiogenesis 2019, 22, 341–354. [Google Scholar] [CrossRef]
- Bahrami, N.; Childs, S.J. Development of vascular regulation in the zebrafish embryo. Development 2020, 147, dev183061. [Google Scholar] [CrossRef]
- Ando, K.; Shih, Y.H.; Ebarasi, L.; Grosse, A.; Portman, D.; Chiba, A.; Mattonet, K.; Gerri, C.; Stainier, D.Y.R.; Mochizuki, N.; et al. Conserved and context-dependent roles for pdgfrb signaling during zebrafish vascular mural cell development. Dev. Biol 2021. [Google Scholar] [CrossRef] [PubMed]
- Wanjare, M.; Kusuma, S.; Gerecht, S. Defining differences among perivascular cells derived from human pluripotent stem cells. Stem Cell Rep. 2014, 2, 561–575. [Google Scholar] [CrossRef] [Green Version]
- Ichimura, K.; Bubenshchikova, E.; Powell, R.; Fukuyo, Y.; Nakamura, T.; Tran, U.; Oda, S.; Tanaka, M.; Wessely, O.; Kurihara, H.; et al. A Comparative Analysis of Glomerulus Development in the Pronephros of Medaka and Zebrafish. PLoS ONE 2012, 7, e45286. [Google Scholar] [CrossRef] [Green Version]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Hellstrom, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Stratman, A.N.; Burns, M.C.; Farrelly, O.M.; Davis, A.E.; Li, W.; Pham, V.N.; Castranova, D.; Yano, J.J.; Goddard, L.M.; Nguyen, O.; et al. Chemokine mediated signalling within arteries promotes vascular smooth muscle cell recruitment. Commun. Biol. 2020, 3, 734. [Google Scholar] [CrossRef]
- Wiens, K.M.; Lee, H.L.; Shimada, H.; Metcalf, A.E.; Chao, M.Y.; Lien, C.L. Platelet-derived growth factor receptor beta is critical for zebrafish intersegmental vessel formation. PLoS ONE 2010, 5, e11324. [Google Scholar] [CrossRef] [Green Version]
- El-Brolosy, M.A.; Kontarakis, Z.; Rossi, A.; Kuenne, C.; Gunther, S.; Fukuda, N.; Kikhi, K.; Boezio, G.L.M.; Takacs, C.M.; Lai, S.L.; et al. Genetic compensation triggered by mutant mRNA degradation. Nature 2019, 568, 193–197. [Google Scholar] [CrossRef] [PubMed]
- El-Brolosy, M.A.; Stainier, D.Y. Genetic compensation: A phenomenon in search of mechanisms. PLoS Genet. 2017, 13, 7. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Kennard, S.; Lilly, B. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ. Res. 2009, 104, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Villa, N.; Walker, L.; Lindsell, C.E.; Gasson, J.; Iruela-Arispe, M.L.; Weinmaster, G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001, 108, 161–164. [Google Scholar] [CrossRef]
- Quillien, A.; Moore, J.C.; Shin, M.; Siekmann, A.F.; Smith, T.; Pan, L.; Moens, C.B.; Parsons, M.J.; Lawson, N.D. Distinct Notch signaling outputs pattern the developing arterial system. Development 2014, 141, 1544–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegenthaler, J.A.; Choe, Y.; Patterson, K.P.; Hsieh, I.; Li, D.; Jaminet, S.-C.; Daneman, R.; Kume, T.; Huang, E.J.; Pleasure, S.J. Foxc1 is required by pericytes during fetal brain angiogenesis. Biol. Open 2013, 2, 647–659. [Google Scholar] [CrossRef] [Green Version]
- Reyahi, A.; Nik, A.M.; Ghiami, M.; Gritli-Linde, A.; Pontén, F.; Johansson, B.R.; Carlsson, P. Foxf2 Is Required for Brain Pericyte Differentiation and Development and Maintenance of the Blood-Brain Barrier. Dev. Cell 2015, 34, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, G.; Arnold, C.R.; Chu, A.Y.; Fornage, M.; Reyahi, A.; Bis, J.C.; Havulinna, A.S.; Sargurupremraj, M.; Smith, A.V.; Adams, H.H.H.; et al. Identification of additional risk loci for stroke and small vessel disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2016, 15, 695–707. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Guo, Y.; Walker, E.J.; Shen, F.; Jun, K.; Oh, S.P.; Degos, V.; Lawton, M.T.; Tihan, T.; Davalos, D.; et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Garg, N.; Khunger, M.; Gupta, A.; Kumar, N. Optimal management of hereditary hemorrhagic telangiectasia. J. Blood Med. 2014, 5, 191–206. [Google Scholar]
- Tang, Y.; Urs, S.; Boucher, J.; Bernaiche, T.; Venkatesh, D.; Spicer, D.B.; Vary, C.P.H.; Liaw, L. Notch and Transforming Growth Factor-β (TGFβ) Signaling Pathways Cooperatively Regulate Vascular Smooth Muscle Cell Differentiation. J. Biol. Chem. 2010, 285, 17556–17563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, T.; Nalbandian, A.; Uchida, Y.; Li, W.; Arnold, T.D.; Kubota, Y.; Yamamoto, S.; Ema, M.; Mukouyama, Y.S. Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-β Signaling in Developing Skin Vasculature. Cell Rep. 2017, 18, 2991–3004. [Google Scholar] [CrossRef]
- Boezio, G.L.M.; Bensimon-Brito, A.; Piesker, J.; Guenther, S.; Helker, C.S.M.; Stainier, D.Y.R. Endothelial TGF-β signaling instructs smooth muscle cell development in the cardiac outflow tract. eLife 2020, 9, e57603. [Google Scholar] [CrossRef]
- O’Brown, N.M.; Megason, S.G.; Gu, C. Suppression of transcytosis regulates zebrafish blood-brain barrier function. eLife 2019, 8, e47326. [Google Scholar] [CrossRef]
- O’Brown, N.M.; Pfau, S.J.; Gu, C. Bridging barriers: A comparative look at the blood-brain barrier across organisms. Genes Dev. 2018, 32, 466–478. [Google Scholar] [CrossRef] [Green Version]
- Quiñonez-Silvero, C.; Hübner, K.; Herzog, W. Development of the brain vasculature and the blood-brain barrier in zebrafish. Dev. Biol. 2020, 457, 181–190. [Google Scholar] [CrossRef]
- Umans, R.A.; Henson, H.E.; Mu, F.; Parupalli, C.; Ju, B.; Peters, J.L.; Lanham, K.A.; Plavicki, J.S.; Taylor, M.R. CNS angiogenesis and barriergenesis occur simultaneously. Dev. Biol. 2017, 425, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Farage, E.; Sugimoto, M.; Anand-Apte, B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Develop. Biol. 2010, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guemez-Gamboa, A.; Nguyen, L.N.; Yang, H.; Zaki, M.S.; Kara, M.; Ben-Omran, T.; Akizu, N.; Rosti, R.O.; Rosti, B.; Scott, E.; et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat. Genet. 2015, 47, 809–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594. [Google Scholar] [CrossRef] [Green Version]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [Green Version]
- Nagai, J.; Yu, X.; Papouin, T.; Cheong, E.; Freeman, M.R.; Monk, K.R.; Hastings, M.H.; Haydon, P.G.; Rowitch, D.; Shaham, S.; et al. Behaviorally consequential astrocytic regulation of neural circuits. Neuron 2021, 109, 576–596. [Google Scholar] [CrossRef]
- Scheib, J.; Byrd-Jacobs, C. Zebrafish Astroglial Morphology in the Olfactory Bulb Is Altered With Repetitive Peripheral Damage. Front. Neuroanat. 2020, 14, 4. [Google Scholar] [CrossRef] [PubMed]



| Regulatory Regions/Promoter | Types of Labeled Mural Cells | Expressed Gene | Name/Construct | Original Reference | ZFIN ID | Note |
|---|---|---|---|---|---|---|
| pdgfrb | All mural cells (Pericyte, VSMC) | mCitrin | TgBAC(pdgfrb:Citrine)s1010 | Vanhollebeke et al., 2015 [11] | ZDB-TGCONSTRCT-150911-2 | BAC clone; CH73-289D6. Inserted position of Citrin is different from that of GFP in TgBAC(pdgfrb:EGFP)ncv22Tg |
| GFP | TgBAC(pdgfrb:EGFP)ncv22Tg | Ando et al., 2016 [12] | ZDB-TGCONSTRCT-160609-1 | BAC clone; CH1073-606I16 | ||
| GFP | TgBAC(pdgfrb:EGFP)uq15bhTg | Bower et al., 2017 [13] | ZDB-ALT-180306-11 | BAC clone; CH1073-606I16 | ||
| GFP | Tg(pdgfrb:EGFP)ue302Tg | Rider et al., 2017 [14] | ZDB-TGCONSTRCT-170830-1 | Use 7.16-kbp pdgfrb promoter region | ||
| mCherry | TgBAC(pdgfrb:mCherry)ncv23Tg | Ando et al., 2016 [12] | ZDB-ALT-160609-2 | BAC clone; CH1073-606I16 | ||
| H2B-dendra | Tg(pdgfrb:H2B-dendra)mu158 | Leonard et al., 2021 [15] | Not assigned yet | BAC clone; CH1073-606I16 | ||
| TetA-2A-AmCyan | Tg(pdgfrb:TETA-2A-AmCyan)mps7Tg | Tsata et al., 2021 [16] | ZDB-ALT-200915-4 | CRISPR-Cas9-mediated targeted knock-in of CreERT2 at pdgfrb coding locus | ||
| Gal4FF | TgBAC(pdgfrb:GAL4FF)ncv24Tg | Ando et al., 2016 [12] | ZDB-ALT-160609-3 | BAC clone; CH1073-606I16 | ||
| Gal4-VP16 | Ki(pdgfrβ:Gal4) | Xu et al., 2017 [17] | ZDB-ALT-171120-1 | CRISPR/Cas9-mediated targeted knock-in of Gal4-VP16 at pdgfrb coding locus | ||
| CreERT2 | Tg(pdgfrb:CreERT2)mps6Tg | Tsata et al., 2021 [16] | ZDB-ALT-200911-6 | CRISPR-Cas9-mediated targeted knock-in of CreERT2 at pdgfrb coding locus | ||
| tagln | VSMC | GFP | Tg(tagln:GFP)p151 | Seiler et al., 2010 [18] | ZDB-ALT-101123-2 | Use tagln promoter region containing ECR5 |
| GFP | TgBAC(tagln:EGFP)ncv25Tg | Ando et al., 2016 [12] | ZDB-ALT-160609-4 | BAC clone; CH1073-307D13 | ||
| Caax-EGFP | Tg(tagln:CAAX-EGFP)uto37Tg | Chen et al., 2017 [19] | ZDB-ALT-170323-2 | Use 2 kbp tagln promoter region | ||
| NLS-EGFP-2A-CFP-FTASE | Tg(tagln:NLS-EGFP-2A-CFP-FTASE)y450Tg | Stratman et al., 2017 [20] | ZDB-TGCONSTRCT-170227-2 | NA | ||
| mCherry | Tg(tagln:mCherry)sh441Tg | Elworthy et al., 2019 [21] | ZDB-TGCONSTRCT-191111-1 | Use tagln promoter region containing ECR5 | ||
| NLS-mCherry | Tg(tagln:NLS-mCherry)sh480Tg | Chhabria et al., 2019 [22] | ZDB-TGCONSTRCT-201222-1 | NA | ||
| ECR-GAL4 | Tg(tagln:ECR-GAL4)y449Tg | Stratman et al., 2017 [20] | ZDB-TGCONSTRCT-170227-1 | NA | ||
| acta2 | VSMC | GFP | Tg(acta2:GFP)ca7Tg | Whitesell et al., 2014 [23] | ZDB-TGCONSTRCT-120508-1 | Use 2.4-kbp acta2 promoter region |
| GFP | TgBAC(acta2:EGFP)uq17bh | Bower et al., 2017 [13] | ZDB-TGCONSTRCT-180306-9 | BAC clone; DKEY-256C3 | ||
| mCherry | Tg(acta2:mCherry)uto5Tg | Chen et al., 2017 [19] | ZDB-TGCONSTRCT-170323-1 | Use acta2 promoter region | ||
| mCherry | Tg(acta2:mCherry)ca8Tg | Whitesell et al., 2014 [23] | ZDB-TGCONSTRCT-120508-2 | Use 2.4 kbp acta2 promoter region | ||
| Gal4FF | Tg(acta2:GAL4FF,myl7:EGFP)ca62Tg | Whitesell et al., 2019 [24] | ZDB-TGCONSTRCT-200102-2 | Use 2.4 kbp acta2 promoter region | ||
| foxc1b | VSMC | GFP | Tg(foxc1b:EGFP)mw44Tg | French et al., 2014 [25] | ZDB-TGCONSTRCT-150312-6 | Use 5 kbp foxc1b promoter region |
| EOS | Tg(foxc1b:Eos)tsu2013Tg | Qiu et al., 2016 [26] | ZDB-TGCONSTRCT-161212-5 | Use 3.6 kbp foxc1b promoter region | ||
| Gal4-VP16 | Tg(-5foxc1b:GAL4-VP16)mw72Tg | Miesfeld et al., 2015 [27] | ZDB-TGCONSTRCT-151218-7 | NA | ||
| Gal4FF | Tg(foxc1b:GAL4FF,myl7:EGFP) | Whitesell et al., 2019 [24] | ZDB-TGCONSTRCT-200102-3 | Use 5 kbp foxc1b promoter region | ||
| myh11a | VSMC | YFP | Tg(myh11a:YFP)mu125 | Leonard et al., 2021 [15] | Not assigned yet | BAC clone; CH73-223E22 |
| abcc9 | Pericyte, (VSMC in the trunk) | Gal4FF | TgBAC(abcc9:GAL4FF)ncv34Tg | Ando et al., 2019 [28] | ZDB-TGCONSTRCT-210412-1 | BAC clone; CH211-58C15. IRES-Gal4FF fragment is inserted at the c-tail of abcc9 (ENSDART00000079987), which resembles SUR2B isoform. Construction method is described in Vanlandewijck et al., 2018 [2]. abcc9 reporter become selective for pericyte in brain or coronary vessels, but arteriolar VSMCs are also labeled in the trunk. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, K.; Ishii, T.; Fukuhara, S. Zebrafish Vascular Mural Cell Biology: Recent Advances, Development, and Functions. Life 2021, 11, 1041. https://doi.org/10.3390/life11101041
Ando K, Ishii T, Fukuhara S. Zebrafish Vascular Mural Cell Biology: Recent Advances, Development, and Functions. Life. 2021; 11(10):1041. https://doi.org/10.3390/life11101041
Chicago/Turabian StyleAndo, Koji, Tomohiro Ishii, and Shigetomo Fukuhara. 2021. "Zebrafish Vascular Mural Cell Biology: Recent Advances, Development, and Functions" Life 11, no. 10: 1041. https://doi.org/10.3390/life11101041
APA StyleAndo, K., Ishii, T., & Fukuhara, S. (2021). Zebrafish Vascular Mural Cell Biology: Recent Advances, Development, and Functions. Life, 11(10), 1041. https://doi.org/10.3390/life11101041






