The Effect of Fat Distribution on the Inflammatory Response of Multiple Trauma Patients—A Retrospective Study
Abstract
:1. Introduction
2. Methods
2.1. Patient Population
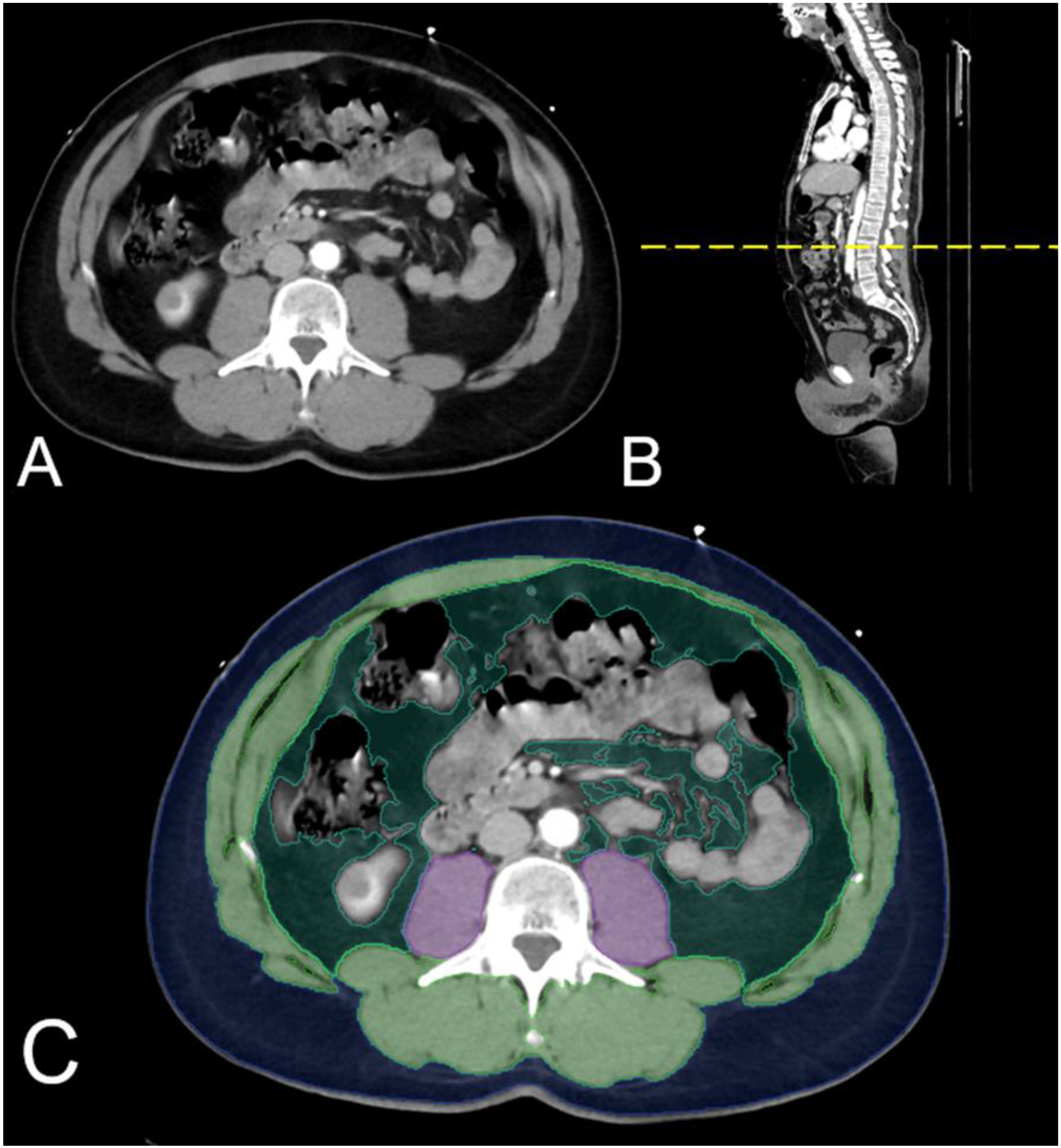
2.2. Image Post-Processing—Measurement of the Adipose Tissue Distribution
2.3. Body Composition
2.4. Evaluation of Inflammatory Response
2.5. Evaluation of Physiological Parameters
2.6. Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. Baseline Characteristics
3.3. Association between Ratio of Visceral Adipose Tissue to Subcutaneous Adipose Tissue (VSr) and Body Mass Index (BMI)
3.4. Injury Pattern and Physiological Situation upon Admission
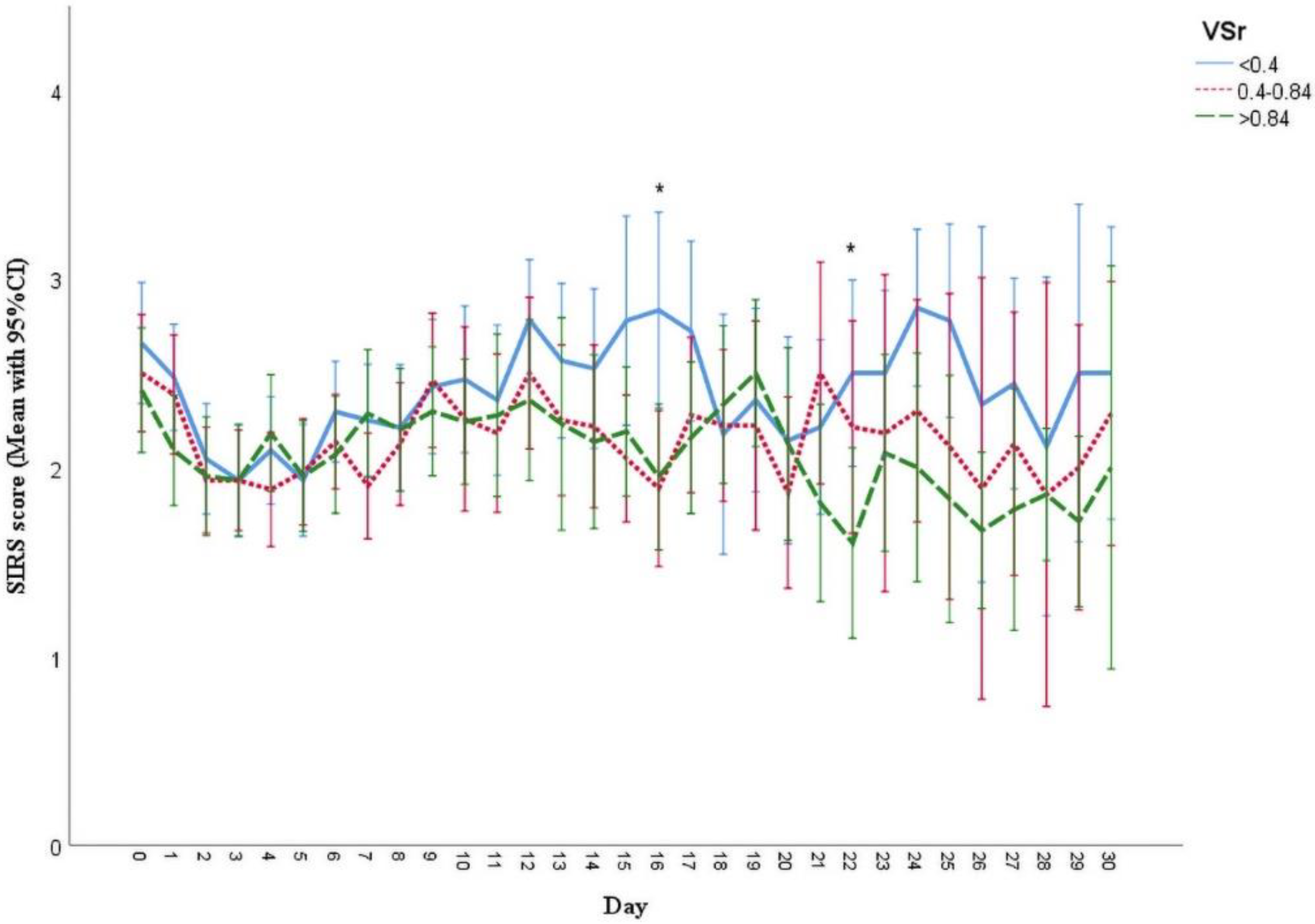
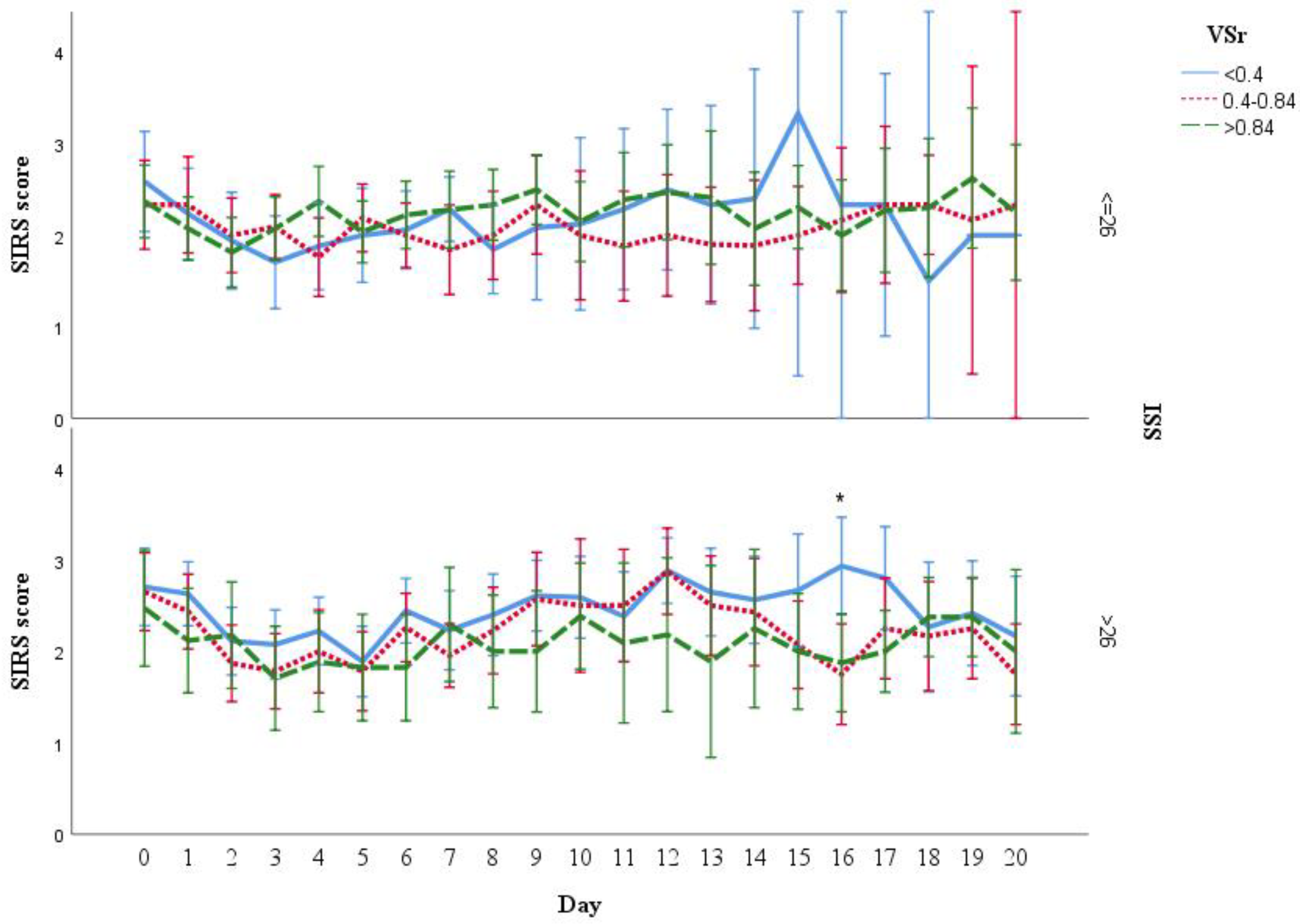
3.5. Analysis of Systemic Inflammatory Response Syndrome (SIRS) Scores and Clinical Outcomes
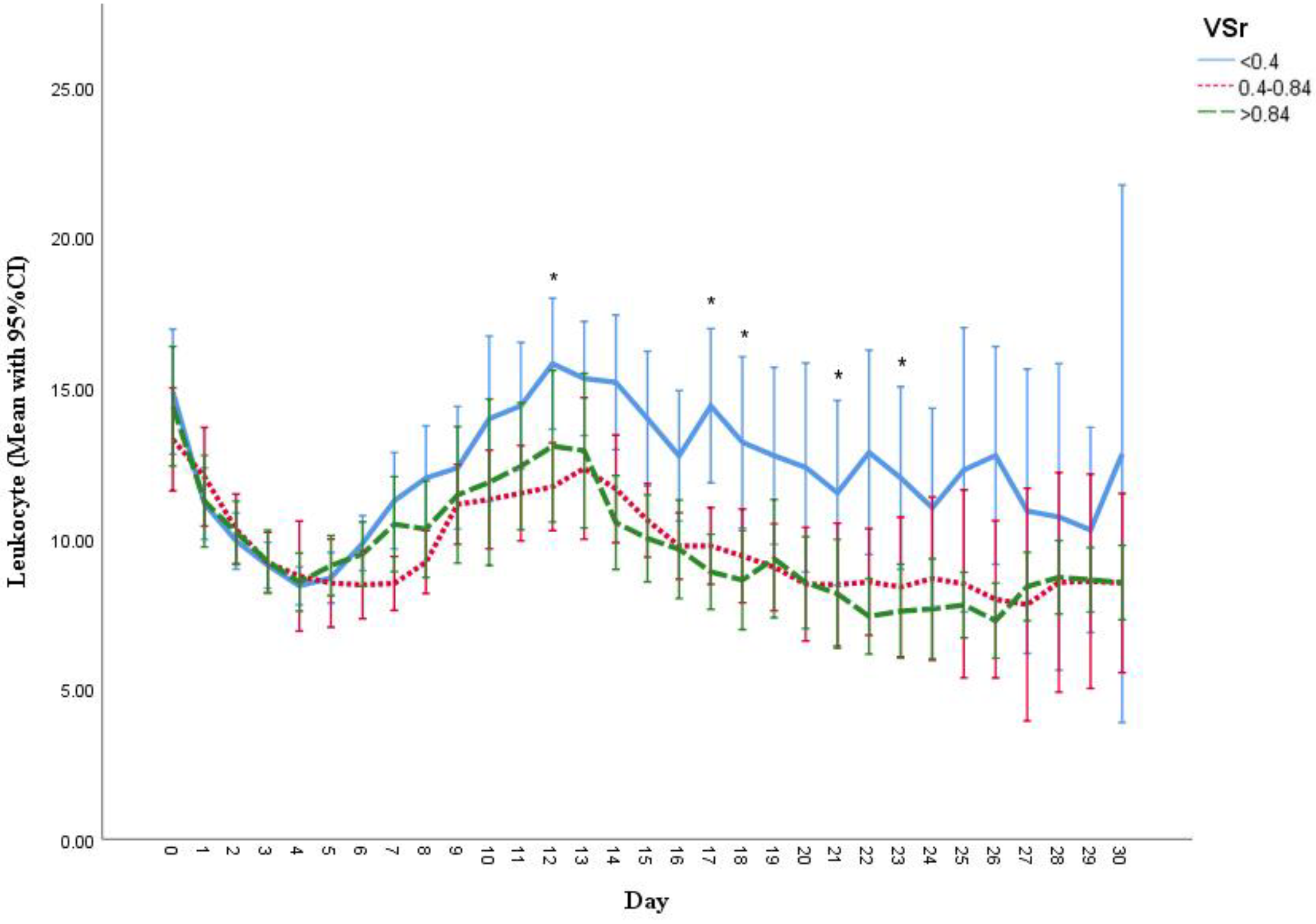
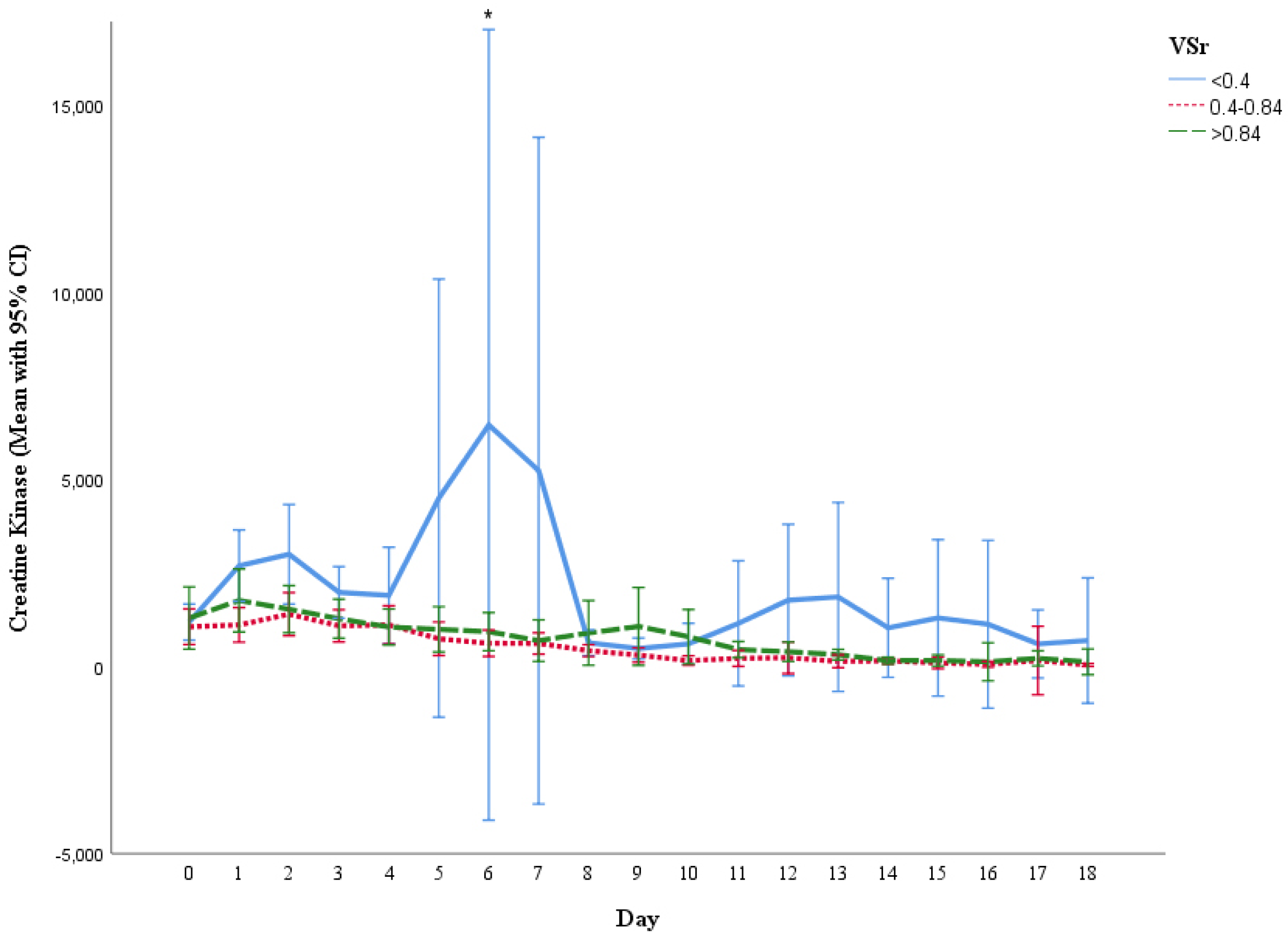
3.6. Measurement of Creatine Kinase (CK), C-Reactive Protein (CRP) and Leukocyte Count
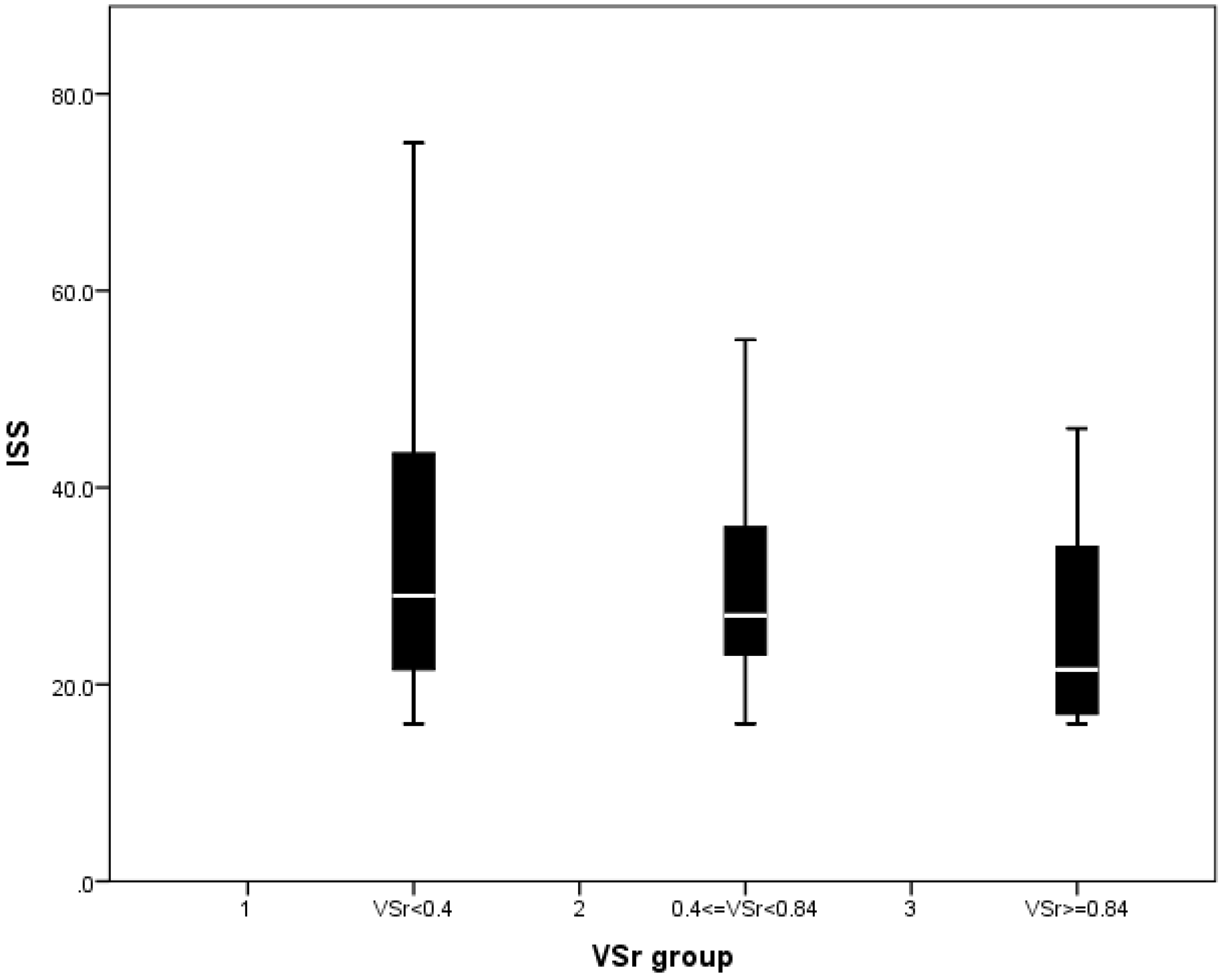
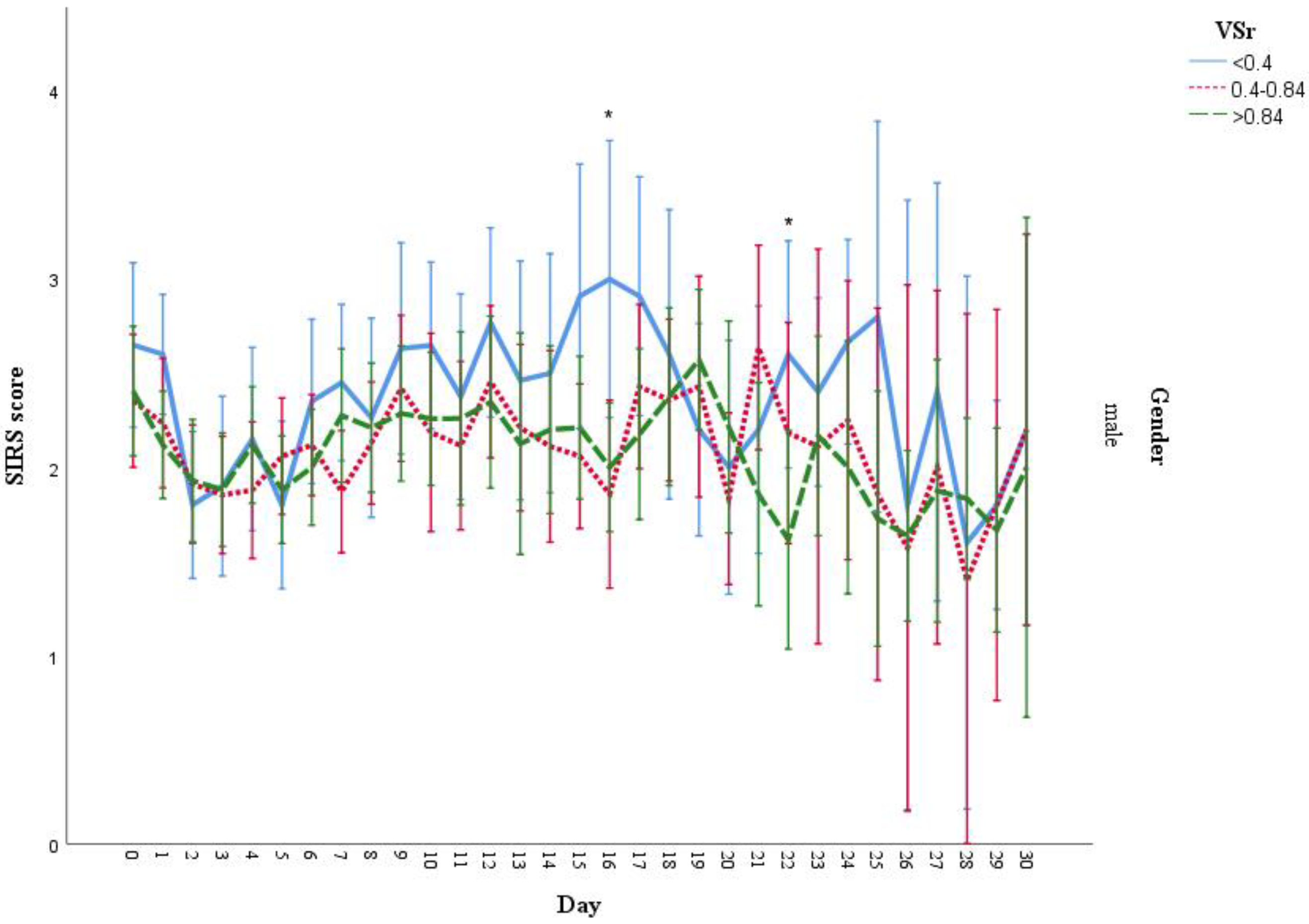
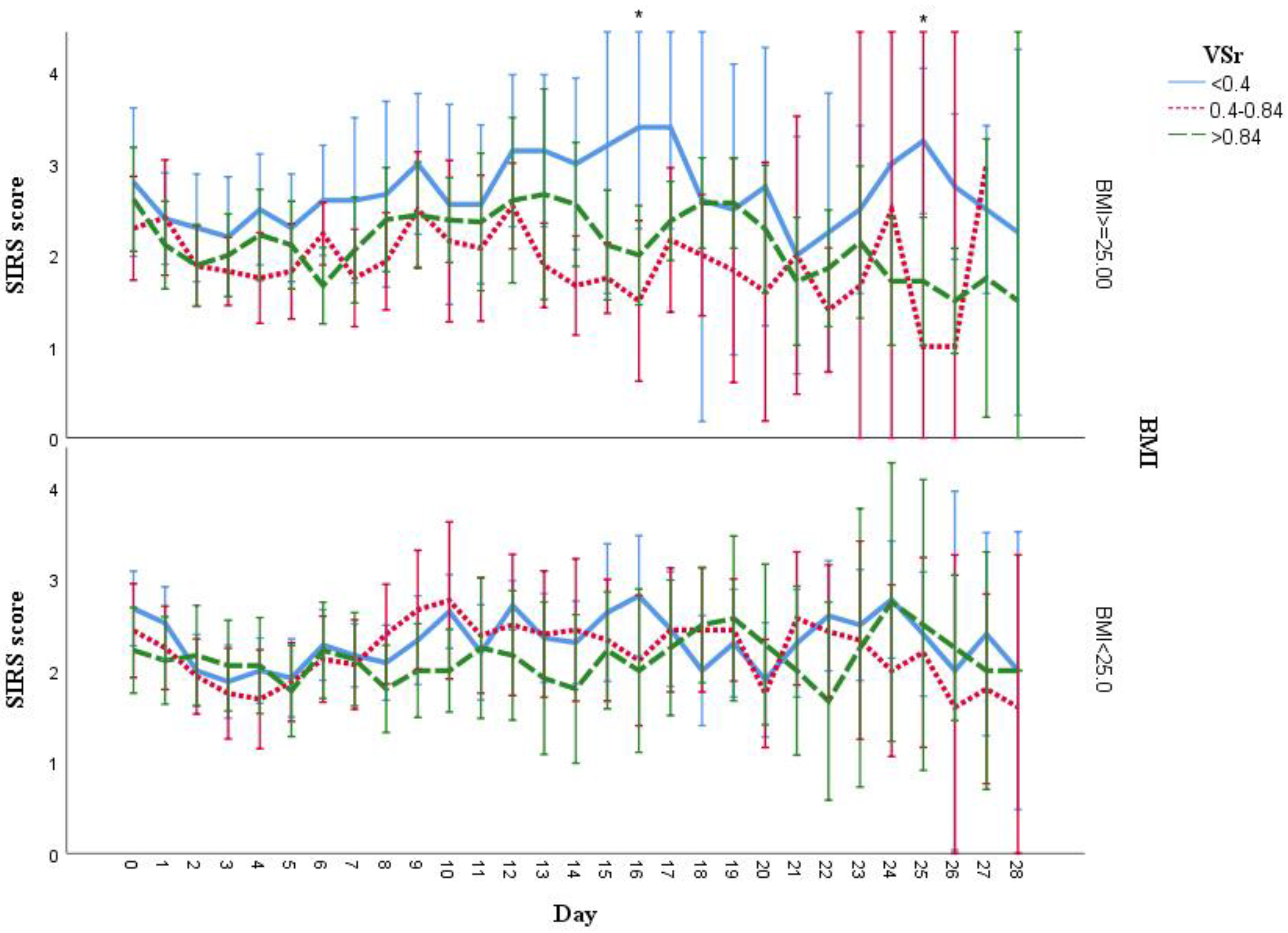
3.7. Analysis of SIRS Scores Stratified by Injury Severity Score (ISS), BMI, and Gender
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [Green Version]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Neville, A.L.; Brown, C.V.; Weng, J.; Demetriades, D.; Velmahos, G.C. Obesity is an independent risk factor of mortality in severely injured blunt trauma patients. Arch. Surg. 2004, 139, 983–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhungel, V.; Liao, J.; Raut, H.; Lilienthal, M.A.; Garcia, L.J.; Born, J.; Choi, K.C. Obesity delays functional recovery in trauma patients. J. Surg. Res. 2015, 193, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.C.; Rawles, R.B.; Jobe, J.T.; Chhabra, A.B.; Freilich, A.M. Obesity is associated with increased postoperative complications after operative management of distal humerus fractures. J. Shoulder Elb. Surg. 2015, 24, 1602–1606. [Google Scholar] [CrossRef]
- Hutagalung, R.; Marques, J.; Kobylka, K.; Zeidan, M.; Kabisch, B.; Brunkhorst, F.; Reinhart, K.; Sakr, Y. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011, 37, 1793. [Google Scholar] [CrossRef]
- Oliveros, H.; Villamor, E. Obesity and mortality in critically ill adults: A systematic review and meta-analysis. Obes. (Silver Spring) 2008, 16, 515–521. [Google Scholar] [CrossRef]
- Tremblay, A.; Bandi, V. Impact of body mass index on outcomes following critical care. Chest 2003, 123, 1202–1207. [Google Scholar] [CrossRef] [Green Version]
- Mica, L.; Vomela, J.; Keel, M.; Trentz, O. The impact of body mass index on the development of systemic inflammatory response syndrome and sepsis in patients with polytrauma. Injury 2014, 45, 253–258. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Oikonomou, E.K.; Antoniades, C.; Tousoulis, D. From the BMI paradox to the obesity paradox: The obesity-mortality association in coronary heart disease. Obes. Rev. 2016, 17, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Macdougall, C.E.; Wood, E.G.; Loschko, J.; Scagliotti, V.; Cassidy, F.C.; Robinson, M.E.; Feldhahn, N.; Castellano, L.; Voisin, M.B.; Marelli-Berg, F.; et al. Visceral Adipose Tissue Immune Homeostasis Is Regulated by the Crosstalk between Adipocytes and Dendritic Cell Subsets. Cell Metab. 2018, 27, 588–601.e584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef] [Green Version]
- Poros, B.; Irlbeck, T.; Probst, P.; Volkmann, A.; Paprottka, P.; Böcker, W.; Irlbeck, M.; Weig, T. Impact of pathologic body composition assessed by CT-based anthropometric measurements in adult patients with multiple trauma: A retrospective analysis. Eur. J. Trauma Emerg. Surg. 2019, 47, 1089–1103. [Google Scholar] [CrossRef]
- Beetz, N.L.; Maier, C.; Shnayien, S.; Trippel, T.D.; Gehle, P.; Fehrenbach, U.; Geisel, D. Artificial intelligence-based analysis of body composition in Marfan: Skeletal muscle density and psoas muscle index predict aortic enlargement. J. Cachexia Sarcopenia Muscle 2021, 12, 993–999. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Martí, A.; Marcos, A.; Martínez, J.A. Obesity and immune function relationships. Obes. Rev. 2001, 2, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Brooks, G.C.; Blaha, M.J.; Blumenthal, R.S. Relation of C-reactive protein to abdominal adiposity. Am. J. Cardiol. 2010, 106, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Corpeleijn, E.; Postmus, D.; Gansevoort, R.T.; de Jong, P.E.; Gans, R.O.; Struck, J.; Hillege, H.L.; Stolk, R.P.; Navis, G.; et al. Plasma procalcitonin is associated with obesity, insulin resistance, and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2010, 95, E26–E31. [Google Scholar] [CrossRef] [Green Version]
- Bekkelund, S.I.; Jorde, R. Lean body mass and creatine kinase are associated with reduced inflammation in obesity. Eur. J. Clin. Investig. 2017, 47, 803–811. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Gennarelli, T.A.; Wodzin, E. AIS 2005: A contemporary injury scale. Injury 2006, 37, 1083–1091. [Google Scholar] [CrossRef]
- Baker, S.P.; O’Neill, B.; Haddon, W., Jr.; Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef]
- Bochicchio, G.V.; Joshi, M.; Bochicchio, K.; Nehman, S.; Tracy, J.K.; Scalea, T.M. Impact of obesity in the critically ill trauma patient: A prospective study. J. Am. Coll. Surg. 2006, 203, 533–538. [Google Scholar] [CrossRef]
- Choban, P.S.; Weireter, L.J., Jr.; Maynes, C. Obesity and increased mortality in blunt trauma. J. Trauma 1991, 31, 1253–1257. [Google Scholar] [CrossRef]
- Glance, L.G.; Li, Y.; Osler, T.M.; Mukamel, D.B.; Dick, A.W. Impact of Obesity on Mortality and Complications in Trauma Patients. Ann. Surg. 2014, 259, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Andruszkow, H.; Veh, J.; Mommsen, P.; Zeckey, C.; Hildebrand, F.; Frink, M. Impact of the Body Mass on Complications and Outcome in Multiple Trauma Patients: What Does the Weight Weigh? Mediat. Inflamm. 2013, 2013, 345702. [Google Scholar] [CrossRef]
- Carbone, S.; Lavie, C.J.; Arena, R. Obesity and Heart Failure: Focus on the Obesity Paradox. Mayo Clin. Proc. 2017, 92, 266–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonopoulos, A.S.; Tousoulis, D. The molecular mechanisms of obesity paradox. Cardiovasc. Res. 2017, 113, 1074–1086. [Google Scholar] [CrossRef]
- Pisitsak, C.; Lee, J.G.; Boyd, J.H.; Coxson, H.O.; Russell, J.A.; Walley, K.R. Increased Ratio of Visceral to Subcutaneous Adipose Tissue in Septic Patients Is Associated With Adverse Outcome. Crit. Care Med. 2016, 44, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Collier, B.; Dossett, L.; Shipman, J.; Day, M.; Lawson, G.; Sawyer, R.; May, A. Visceral adiposity is not associated with inflammatory markers in trauma patients. J. Trauma 2010, 68, 57–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, S.L.; Mongan, A.M.; Donohoe, C.L.; Pidgeon, G.P.; Sherlock, M.; Reynolds, J.V.; Lysaght, J. Impact of visceral obesity and metabolic syndrome on the postoperative immune, inflammatory, and endocrine response following surgery for esophageal adenocarcinoma. Dis. Esophagus 2017, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhu, F.; Gong, J.; Yang, J.; Zhang, T.; Gu, L.; Zhu, W.; Guo, Z.; Li, Y.; Li, N.; et al. High Visceral to Subcutaneous Fat Ratio Is Associated with Increased Postoperative Inflammatory Response after Colorectal Resection in Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2018, 2018, 6270514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Yano, M.; Motoori, M.; Kishi, K.; Miyashiro, I.; Yamada, T.; Ohue, M.; Ohigashi, H.; Ishikawa, O.; Imaoka, S. Excess visceral fat accumulation is a risk factor for postoperative systemic inflammatory response syndrome in patients with esophageal cancer. Esophagus 2008, 5, 75–80. [Google Scholar] [CrossRef]
- Stolarczyk, E. Adipose tissue inflammation in obesity: A metabolic or immune response? Curr. Opin. Pharmacol. 2017, 37, 35–40. [Google Scholar] [CrossRef]
- Omran, F.; Christian, M. Inflammatory Signaling and Brown Fat Activity. Front. Endocrinol. 2020, 11, 156. [Google Scholar] [CrossRef]
- MacDougald, O.A.; Burant, C.F. The rapidly expanding family of adipokines. Cell Metab. 2007, 6, 159–161. [Google Scholar] [CrossRef] [Green Version]
- Shankar, K.; Kumar, D.; Gupta, S.; Varshney, S.; Rajan, S.; Srivastava, A.; Gupta, A.; Gupta, A.P.; Vishwakarma, A.L.; Gayen, J.R.; et al. Role of brown adipose tissue in modulating adipose tissue inflammation and insulin resistance in high-fat diet fed mice. Eur. J. Pharmacol. 2019, 854, 354–364. [Google Scholar] [CrossRef]
- Zuriaga, M.A.; Fuster, J.J.; Gokce, N.; Walsh, K. Humans and Mice Display Opposing Patterns of “Browning” Gene Expression in Visceral and Subcutaneous White Adipose Tissue Depots. Front. Cardiovasc. Med. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longchamp, A.; Tao, M.; Bartelt, A.; Ding, K.; Lynch, L.; Hine, C.; Corpataux, J.M.; Kristal, B.S.; Mitchell, J.R.; Ozaki, C.K. Surgical injury induces local and distant adipose tissue browning. Adipocyte 2016, 5, 163–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenwald, M.; Perdikari, A.; Rülicke, T.; Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 2013, 15, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Sidossis, L.S.; Porter, C.; Saraf, M.K.; Børsheim, E.; Radhakrishnan, R.S.; Chao, T.; Ali, A.; Chondronikola, M.; Mlcak, R.; Finnerty, C.C.; et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015, 22, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Total | VSr < 0.4 | VSr 0.4–0.84 | VSr > 0.84 | p |
|---|---|---|---|---|---|
| Number of patients | 132 | 44 | 44 | 44 | |
| Gender (male/female)—n (%) | 96/36 (72.7%, 27.3%) | 20/24 (45.5%, 54.5%) | 34/10 (77.3%, 22.7%) | 42/2 (95.5%, 4.5%) | <0.001 |
| Age (years) * | 55.4 (20.7) | 40.5 (21.3) | 59.5 (20.9) | 53.8 (12.2) | <0.001 |
| Survived—n (%) | 122 (92.4%) | 42 (31.8%) | 41 (31.1%) | 39 (29.5%) | 0.469 |
| VSr ** | 0.61 (0.36, 1.04) | 0.24 (0.13, 0.37) | 0.61 (0.51, 0.72) | 1.27 (1.03, 1.77) | <0.001 |
| AIS head | 3.0 (2.0, 4.0) | 3.0 (1.0, 4.0) | 3.0 (2.0, 4.0) | 4.0 (3.0, 4.0) | 0.170 |
| AIS face | 0 (0, 1.0) | 0 (0, 1.5) | 0 (0, 2.0) | 0 (0, 0) | 0.099 |
| AIS thorax | 3.0 (0, 4.0) | 3.0 (0, 4.0) | 3.0 (0, 4.0) | 2.0 (0, 3.0) | 0.153 |
| AIS abdomen | 0 (0, 2.0) | 0 (0, 3.0) | 0.5 (0, 2.5) | 0 (0, 2.0) | 0.396 |
| AIS extremities | 2.0 (0, 3.0) | 2.0 (2.0, 4.0) | 2.0 (0, 3.0) | 0 (0, 2.0) | <0.001 |
| AIS external | 0 (0, 0) | 0 (0, 0.5) | 0 (0, 0) | 0 (0, 0) | 0.380 |
| ISS | 27.0 (20.0, 36.0) | 29.0 (21.5, 43.5) | 28.0 (23.0, 37.0) | 21.5 (17.0, 34.0) | 0.045 |
| Glasgow coma scale (GCS) | 3.0 (3.0, 9.0) | 3.0 (3.0, 10.0) | 3.0 (3.0, 5.0) | 3.0 (3.0, 10.0) | 0.764 |
| Shock (yes/no) | 97/35 | 30/14 | 32/12 | 35/9 | 0.478 |
| Systolic pressure (mmHg) | 90.4 (17.1) | 90.3 (17.8) | 91.9 (17.6) | 88.9 (16.3) | 0.720 |
| Diastolic pressure (mmHg) | 50.1 (11.2) | 46.8 (11.9) | 51.7 (10.1) | 51.8 (11.1) | 0.058 |
| Heart rate (/min) | 105.1 (21.7) | 103.6 (18.6) | 105.7 (22.4) | 105.9 (24.2) | 0.856 |
| Haemoglobin (g/dL) | 11.2 (2.3) | 10.8 (2.2) | 11.3 (2.2) | 11.6 (2.5) | 0.313 |
| Platelet count (/nL) | 201.2 (83.3) | 211.2 (90.3) | 200.9 (89.0) | 191.4 (69.6) | 0.539 |
| Prothrombin time (%) | 63.3 (25.4) | 62.1 (25.2) | 62.2 (25.1) | 66.2 (26.9) | 0.820 |
| PH | 7.31 (0.11) | 7.32 (0.12) | 7.30 (0.10) | 7.31 (0.11) | 0.627 |
| Base excess | −4.4 (4.0) | −3.1 (3.9) | −4.7 (3.6) | −5.5 (4.1) | 0.013 |
| INR | 1.17 (1.08, 1.36) | 1.19 (1.08, 1.35) | 1.16 (1.06, 1.42) | 1.17 (1.10, 1.34) | 0.958 |
| APTT | 35.4 (30.8, 45.2) | 36.25 (30.80, 45.40) | 35.30 (30.90, 49.80) | 33.70 (30.60, 42.20) | 0.815 |
| Criteria * | Primary Criteria | Alternative Criteria |
|---|---|---|
| Leukocyte count | >12,000 or <4000/mL | 10% immature forms or bands |
| Heart rate | >90/min | N/A |
| Respiratory rate | >20/min | partial pressure of CO2 < 32mmHg |
| Body temperature | >38 °C or <36 °C | N/A |
| Total | VSr < 0.4 | VSr 0.4–0.84 | VSr > 0.84 | p | |
|---|---|---|---|---|---|
| Mean SIRS score—Mean (SD) | 2.16 (0.57) | 2.26 (0.59) | 2.11 (0.57) | 2.11 (0.56) | 0.354 |
| Max SIRS score—Mean (SD) | 3.48 (0.71) | 3.57 (0.73) | 3.39 (0.75) | 3.48 (0.66) | 0.494 |
| Days of max SIRS score(d)—Median (IQR) * | 3.0 (1.0, 4.0) | 2.0 (1.0, 4.0) | 3.0 (2.0, 4.0) | 3.0 (1.5, 4.0) | 0.387 |
| Hospitalization(d)—Median (IQR) * | 20.0 (14.0, 28.0) | 18.5 (12.0, 27.0) | 22.0 (16.0, 28.0) | 19.0 (13.0, 28.0) | 0.335 |
| ICU stay(d)—Median (IQR) * | 14.0 (10.0, 25.0) | 14.0 (10.0, 25.0) | 15.5 (11.5, 24.5) | 14.0 (10.0, 26.5) | 0.686 |
| Ventilator duration(d)—Median (IQR) * | 11.0 (5.0, 22.0) | 7.5 (4.0, 21.5) | 11.0 (7.0, 21.0) | 12.0 (5.0, 23.0) | 0.352 |
| Female | Male | p | |
|---|---|---|---|
| VAT (pixel count) | 4287.5 (2121.5, 13,278.0) | 14,460.0 (7282.0, 26,344.5) | <0.001 |
| SAT (pixel count) | 16,087.5 (9099.5, 31,848.5) | 18,618.0 (10,488.0, 24,650.0) | 0.85 |
| VSr | 0.29 (0.14, 0.51) | 0.74 (0.46, 1.19) | <0.001 |
| Muscle (pixel count) | 13,003.0 (11,837.0, 15,573.5) | 17,322.0 (15,584.5, 21,016.5) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Wittenberg, S.; Auer, T.A.; Bashkuev, M.; Gebert, P.; Fehrenbach, U.; Geisel, D.; Graef, F.; Maerdian, S.; Tsitsilonis, S. The Effect of Fat Distribution on the Inflammatory Response of Multiple Trauma Patients—A Retrospective Study. Life 2021, 11, 1243. https://doi.org/10.3390/life11111243
Chen Z, Wittenberg S, Auer TA, Bashkuev M, Gebert P, Fehrenbach U, Geisel D, Graef F, Maerdian S, Tsitsilonis S. The Effect of Fat Distribution on the Inflammatory Response of Multiple Trauma Patients—A Retrospective Study. Life. 2021; 11(11):1243. https://doi.org/10.3390/life11111243
Chicago/Turabian StyleChen, Zhaoxiong, Silvan Wittenberg, Timo Alexander Auer, Maxim Bashkuev, Pimrapat Gebert, Uli Fehrenbach, Dominik Geisel, Frank Graef, Sven Maerdian, and Serafeim Tsitsilonis. 2021. "The Effect of Fat Distribution on the Inflammatory Response of Multiple Trauma Patients—A Retrospective Study" Life 11, no. 11: 1243. https://doi.org/10.3390/life11111243






