Abstract
Background: Because of the important role in regulating the immune system, increasing evidence suggests a possible implication of gut microbiota in Chronic spontaneous urticaria (CSU). Although the oral cavity is the first site of contact between microbiota and the immune system, the association between salivary microbiota and CSU has not yet been reported. Objective: This case-control study aimed to compare differences in salivary microbiota between CSU patients and healthy controls (HC). Twenty-three participants—13 patients with CSU and 10 HC were enrolled; salivary microbiota was determined by molecular approach targeting 16S ribosomal RNA. Terminal restriction fragment length polymorphism (T-RFLP) analysis was performed. Results: Alpha diversity of salivary microbiota in CSU patients was significantly reduced compared to HC, resulting in alteration of the community composition. Species richness determined via the Shannon index was significantly reduced in the CSU group. Conclusion: Dysbiosis of salivary microbiota may contribute to a dysregulated immune system in the development of CSU. To our knowledge, this was the first study that reported an alteration in salivary microbiota composition in CSU patients.
1. Introduction
Chronic urticaria (CU) is defined as a continuous or intermittent occurrence of wheals, angioedema, or both for more than 6 weeks [1]. The prevalence of CU is increasing worldwide, with an overall point prevalence of 0.7%, ranging from 0.1% to 1.5% in adults [2,3]. Children population is affected in a similar proportion, with prevalence ranging from 0.1% to 3% [4]. Women are affected nearly twice as often as men, with the peak age between 20 and 40 years [5].
Based on the relevance of triggering factors, CU is classified into two categories–chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CInd) [1]. Approximately 80% of CU cases are CSU with no identified triggering stimuli or specific allergens [6]. Debilitating symptoms and often unsuccessful treatment affect patients’ performance at work and school and significantly impair quality of life [2]. The course of the disease can be self-limiting, although, in 10–25% of patients, it lasts longer than 5 years [7].
Although many advances have been made in identifying etiopathogenetic mechanisms of CSU, the etiology and pathogenesis are complex and remain largely unclear. The abnormal activation of mast cells and basophils is the key process in CSU development [8]. Autoimmunity type I (IgE autoantibodies to IL-24, thyroperoxidase, double-stranded DNA, and other autoallergens) or type IIb (IgG antibodies to the patient’s own IgE or its high-affinity receptor-FcεRI) are involved in a large proportion of CSU cases [8], but also other factors have been elucidated: infections, pseudoallergic reactions, coagulation, stress, vitamin D [9]. Several recent studies have been identified altered gut microbiota as a possible underlying cause of CSU [6,10,11,12,13,14].
The oral cavity is a significant gateway to the human body. It comprises different microbiological habitats (saliva, buccal mucosa, palatal mucosa, tongue dorsum, dental surfaces) that form the salivary or oral microbiota [15]. The microbial community of the oral cavity is the second most complex of the human body after the gut microbiome. To date, over 700 oral bacterial species have been identified in the human oral microbiome database (HOMD) [16]. The microorganisms residing in the oral cavity, and their inter-relationships, are essential components in changing the balance between health and disease. Microorganisms colonizing one area of the oral cavity have a significant probability of spreading on contiguous epithelial surfaces to neighboring sites [15]. Saliva can be rapidly collected from subjects and immediately preserved, allowing accurate profiling of taxonomic changes in response to different treatments or disease states. It is also convenient for research since the use of saliva samples is inexpensive and non-invasive. Therefore, salivary microbiota has become one of the most studied microbiota to date. Many recent studies have reported alterations in salivary microbiota for various diseases, including obesity [17], rheumatoid arthritis [18], type 2 diabetes mellitus [19], atherosclerosis [20], etc. Also, it is important to mention increasing evidence suggesting that some oral bacteria may play a physiological role in digesting gluten in patients with celiac disease; enzymes produced by oral bacteria are capable to modify immunologically important gliadin peptides and the way they present to the gut immune system [21].
Even though the oral cavity is the first site of contact and interaction between many microorganisms and the immune system, an association between salivary microbiota and CSU has not yet been reported. Thus, this study aimed to identify and compare differences in salivary microbiota composition between patients with CSU and healthy subjects.
2. Results
2.1. Demographic and Clinical Characteristics of Participants
The study included 23 participants, 13 patients with CSU, and 10 healthy controls (HC) (Table 1). There were no significant differences in gender and age between the observed groups.

Table 1.
Baseline characteristics of CSU patients and HC.
Clinical and laboratory parameters of CSU patients are provided in Table 2.

Table 2.
Clinical and laboratory parameters of CSU patients.
At the time of enrollment in the study, all patients were under treatment with 2nd-generation H1-antihistamines. Up-dosing antihistamines (up to 4 times the standard dose) was required in 85% of patients. None of the patients had been treated with oral corticosteroids, biologics, or immunosuppressants. Disease activity was assessed by UAS (Urticaria activity score) test (Table 3).

Table 3.
UAS score.
2.2. Composition of Salivary Microbiota in CSU Patients and HC
Terminal restriction fragment length polymorphism (T-RFLP) analysis identified 96 Operational Taxonomic Units (OTUs) in HhaI, and 102 OTUs in MspI-digested amplified 16S rRNA samples. More specifically, among 96 HhaI-digested OTUs, 79 OTUs were identified in the control group, and 69 OTUs were identified in the CSU group. From 102 OTUs digested by MspI, 91 OTUs were identified in the control group, whereas 77 OTUs were identified in the CSU group. Based on the results of HhaI/MspI-digested T-RF patterns, microbial diversity in CSU patients’ saliva samples was reduced compared to HC. Lower biodiversity was observed in datasets acquired from T-RFLP analysis of HhaI (p = 0.007, t-test) and MspI (p = 0.028, t-test) digested 16S rRNA fragments. The Shannon diversity index was significantly higher in the HC group compared to the CSU group (Figure 1a,b).
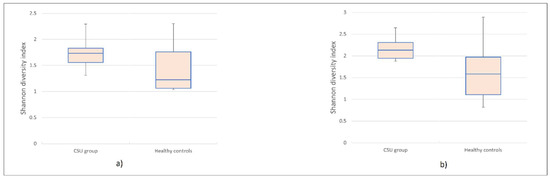
Figure 1.
Boxplot was used to display the differences in alpha diversity visually. The Shannon index was significantly lower in Chronic Spontaneous Urticaria (CSU) group compared to the HC group. (a) HhaI-digestion. (b) MspI-digestion.
Dendrogram constructed by using a minimal variance algorithm did not reveal any major cluster characteristic for either group in neither HhaI nor MspI digested T-RF patterns (shown in Figure 2a,b). This study found no correlation between the severity of urticaria (evaluated by the UAS score) and microbial diversity. The post-hoc analysis did not identify any statistically significant difference in OTU abundance between the CSU and HC groups. The dominant phylum in the CSU group (according to the HhaI and MspI-digested T-RF patterns) was Proteobacteria, while Firmicutes were more abundant in the HC group. There was no significant difference at the phylum level in Firmicutes, Bacteroidetes, and Proteobacteria between the groups. OTUs representing the genus Fretibacterium were observed only in the control group. OTUs representing the genus Pseudomonas were more abundant in the CSU group. OTUs representing the genera Bacteroides, Haemophilus, Bifidobacterium, Capnocytophaga, Fretibacterium, Porphyromonas were more abundant in the HC group than in the CSU group.
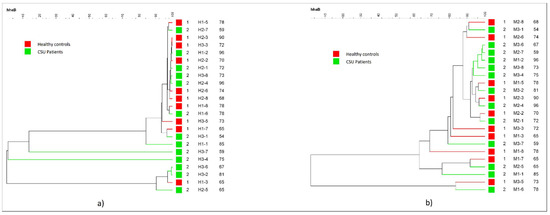
Figure 2.
Dendogram of the salivary microbiota profiles of Chronic Spontaneous Urticaria (CSU) patients and HC. (a) HhaI-digestion. (b) MspI-digestion.
3. Discussion
This case-control study aimed to identify and compare salivary microbial composition between CSU patients and HC. Our major findings revealed that alpha diversity of salivary microbiota in CSU patients was significantly reduced compared to HC. The Shannon index was significantly lower in the CSU group than in HC, which is highly consistent with other microbiome studies in CSU patients [6,11,22]. Beta diversity revealed that microbial composition was comparable between groups, without significant differences. Loss of microbial diversity leads to dysbiosis, which should be considered as an important underlying cause of immune-mediated diseases.
Due to the lack of previous studies in this field, we compared our results with recent reports of the connection between gut microbiota and CSU and the association between salivary microbiota and other immune-mediated diseases. There were a few overlaps between the salivary microbial composition in our CSU group and previously published reports about the implication of gut microbiota in CU/CSU. Based on our research, the dominant phylum in the CSU group was Proteobacteria, in contrast to the oral microbial composition of healthy individuals, where the most abundant microbes on the phylum level are Firmicutes [16]. However, increased Proteobacteria were observed in recent studies on gut dysbiosis in CU/CSU patients [10,14]. Increased Proteobacteria in the gut drastically enhance the permeability of the normally sterile mucus inner layer to the more penetrable region. That results in the infiltration of bacteria into the intestinal inner layer close to the epithelium [23]. Furthermore, Proteobacteria were increased in patients with asthma and allergic diseases; it was reported that Proteobacteria might cause allergies by upregulating Th17-related genes [24,25]. Increased Th17 cells were detected in CSU patients, suggesting that CSU development can be associated with Th17 cells. [26]. Our study showed a lower abundance of OTUs representing the genus Bacteroides in the CSU group, which is consistent with the study of Wang et al. [11] about the composition of the gut microbiome in CSU. Recently, it has been reported that unsaturated fatty acids, well-known for their anti-inflammatory properties [27,28], also have a stimulating effect on the growth of Bacteroides [29]. Wang et al. reported that the reduction in unsaturated fatty acids exacerbated inflammatory responses and triggered CSU development [11]. The abundance of Bifidobacterium was decreased in the CSU group compared to HC. The lower amount of Bifidobacterium was also reported in studies by Wang et al. on CSU patients [11], and Rezazadeh et al. on CU patients [30]. Some species of Bifidobacterium have anti-inflammatory effects; some strains are used as probiotics to improve dysbiosis and therefore alleviate inflammatory response [31]. A possible mechanism is by inducing regulatory T (Treg) cells [32,33]. Treg cells further induce the secretion of anti-inflammatory mediators [34], and therefore, can reduce inflammation. Reduced number and function of Treg in CU patients were shown in some studies [34,35]. OTUs representing the genus Haemophilus were more abundant in the control group than in the CSU group. For comparison, a study by Zhang et al. reported depletion of the Haemophilus species in saliva, dental plaque, and fecal samples in patients with rheumatoid arthritis, which was partly normalized after treatment of rheumatoid arthritis [18].
Gut and oral environments are infinitely complex, and the microbiota of these environments plays an important role in maintaining homeostasis. With reduced exposure of the gut microbiota to the immune system, a significant increase in the incidence and prevalence of allergic as well as autoimmune and inflammatory disorders has been reported. [36]. Although the current literature largely suggests gut dysbiosis as the most critical in the development of immune imbalance, there is a growing number of research suggesting the capability of oral bacteria to disrupt the gut microbial composition and induce chronic inflammation, and therefore trigger or exacerbate certain diseases [37,38,39].
Salivary bacteria are continuously swallowed, can spread and colonize other areas, and thus, may colonize, shape, and influence the gut microbiota [21]. An association between the oral and gut microbiota has been observed in rheumatoid arthritis, gastrointestinal cancer, and inflammatory bowel disease [18,39,40]. We speculate that there is a possible correlation between salivary and gut microbiota in CSU also. Therefore, there is an unmet need for studying microbiome at different sites along the digestive tract to better understand dysbiosis and its impact on the immune system.
Our study has several limitations. A small number of subjects are included, which probably resulted in nonsignificant statistical trends in the abundance of bacteria. We performed not so powerful molecular method which could detect bacteria at the species level; we only characterized the microbiota from one body site. Also, many clinical, demographic, and environmental factors (age, gender, dietary intake, lifestyle habits) can affect the salivary microbiota; so more extensive research is required to confirm whether microbial dysbiosis is associated exclusively with disease or other environmental factors are included. Although T-RFLP may not be as sufficient at the species-level classification of bacteria as next-generation sequencing (NGS), it is a reliable technique and a good tool for comparing microbial communities and assessing diversity in complex microbial communities [41].
To our knowledge, this was the first study that found changes in salivary microbiota in CSU patients. However, this study is in the exploratory stage. Larger and sufficiently powered studies are needed to analyze genomic, structural, and functional differences of salivary and gut microbiota and investigate the correlation between these microbial sites.
In conclusion, our study revealed changes in the composition of salivary microbiota in CSU patients. Dysbiosis of salivary microbiota may contribute to the dysregulated immune system in the development of CSU.
Further clinical studies on the salivary microbiome are needed to investigate the usefulness of salivary analysis for potential clinical and practical implications in CSU management.
4. Materials and Methods
4.1. Study Design and Sample Collection
Twenty-three participants (13 patients with CSU and 10 healthy controls) were enrolled in the study at the Department of Dermatology and Venereology, Sestre milosrdnice University Hospital Centre, Zagreb, Croatia. The study was approved by the Research Ethics Committee of Sestre milosrdnice’s University Hospital Centre, Zagreb (Approval No. EP-8247/19-10; 9 May 2019). Written informed consent was obtained from all the participants.
Inclusion criteria were: patients aged 18 or older with diagnosed CSU according to EAACI/GA LEN/EDF/WAO guidelines [1]. Exclusion criteria were: taking systemic antibiotics in the last three months, chronic smoking or alcohol consumption, periodontitis, history of rheumatoid arthritis, metabolic diseases (diabetes type I and II, obesity), gastrointestinal diseases (celiac disease, ulcerative colitis, Chron disease), malignant diseases and pregnancy. A dermatovenerologist examined all patients with CSU. The dynamic and severity of the disease were evaluated via the Urticaria Activity Score questionnaire (UAS) [1]. Saliva samples were collected from all 23 participants, who were asked not to drink or eat for at least 2 h before sampling. Saliva samples were collected according to a standard technique: 5 mL of spontaneous, whole, unstimulated saliva was collected from each participant into a 50 mL sterile DNA-free conical tube and stored at −80 °C. According to the manufacturer’s instructions, total bacterial DNA extraction from 250 uL of saliva samples was performed using the ZymoBIOMICS DNA Miniprep Kit (Zymo Research, Irvine, CA, USA).
4.2. PCR Amplification and T-RFLP Analysis
Polymerase chain reaction (PCR) amplification of a total 16S rDNA and T-RFLP analysis were performed according to Andoh et al. [42]. 6′-carboxyfluorescein (6-FAM) labeled 27-F primer (6-FAM-5′-AGAGTTTGATCCTGGCTCAG-3′), and 1492R (5′-GGTTACCTTGTTACGACTT-3′) (Thermo Fisher Scientific, Waltham, MA, USA) were used to amplify total 16S rDNA from the human saliva samples [42,43]. The PCR amplification of a total of 16S rDNA was performed in triplicates, in a total volume of 50 µL as described previously [44,45]. Purification of Amplified DNA was performed by QIAquick PCR Purification Kit (Qiagen, Hilden, Germany). 120 ng of purified PCR product was digested separately by HhaI and MspI enzymes (Thermo Fisher Scientific, Waltham, MA, USA) [42]. Restriction products were purified by ethanol/sodium acetate/EDTA precipitation and resuspended in deionized formamide to a final concentration of 10 ng/µL (Thermo Fisher Scientific, Waltham, MA, USA) [28]. T-RFLP analysis service (Macrogen Europe BV, Amsterdam, The Netherlands) on ABI PRISM 3730XLs (Thermo Fisher Scientific, Waltham, MA, USA), using 1200LIZ size standard, was used to obtain T-RFLP profiles of every sample. GeneMapper 3.7 software (Thermo Fisher Scientific, Waltham, MA, USA) was used for fragment size estimation. All T-RFs sized 50–900 bp with a peak height over 25 fluorescence units were included in further analysis. T-REX software (http://trex.biohpc.org/, accessed date November 2020) was used for the alignment of terminal restriction fragments (T-RFs) [46]. A binning threshold of 2 bp was used to assign T-RFs to OTUs [47].
The OTUs were quantified as the percentage values of individual OTU areas per total OTU area. This was expressed as the percentage of the area of the under peak curve (AUC) [48]. In silico assignment of OTUs to bacterial taxa listed in the Oral Micriome CORE Database (version 13 October 2017) was performed by PAT+ tool as a part of MiCA3 (http://mica.ibest.uidaho.edu/pat.php, accessed date November 2020) [49].
4.3. Statistical Analysis
Categorical data were analyzed by chi-square test. The age difference between groups was compared by using a student’s t-test. The Shannon-Wiener diversity index was calculated to compare diversity between CSU and HC groups, using the relative abundance of OTUs. The statistical difference between the indices was further calculated using the student’s t-test. Correlation between UAS score and Shannon-Wiener diversity index in the CSU group was assessed by calculating Pearson’s correlation coefficient. Dendrograms representing calculated similarity distances were generated using Pearson’s similarity coefficient analysis and the unweighted pair-group methods with arithmetic means (UPGMA). Dendrograms based on the HhaI or MspI T-RFLP patterns were generated by BioNumerics software (Applied Maths, Sint-Martens-Latem, Belgium). Statistical analysis was performed using SPSS 19.0 (Chicago, IL, USA), p values less than 0.05 were considered statistically significant.
Author Contributions
D.Ć. and L.L.-M. designed the study. I.F. and I.B. collected samples. M.J. performed the data analysis. D.Ć. drafted the manuscript. L.L.-M., A.G.G. and A.T.A. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Committee of Sestre milosrdnice University Hospital Centre (Approval No. EP-8247/19-10; 9 May 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the personal data of the study participants.
Acknowledgments
The authors appreciate the support of the Department of Dermatology and Venereology, Sestre milosrdnice, University Hospital Centre, Zagreb and Department of Clinical Microbiology, University Hospital for Infectious Diseases, Zagreb for providing clinic access and performing the experiments and data analysis.
Conflicts of Interest
The authors report no conflict of interest in relation to this work.
References
- Zuberbier, T.; Aberer, W.; Asero, R.; Bindslev-Jensen, C.; Brzoza, Z.; Canonica, G.W.; Church, M.K.; Ensina, L.F.; Giménez-Arnau, A.; Godse, K.; et al. The EAACI/GA(2)LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of Urticaria. The 2017 Revision and Update. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef]
- Gonçalo, M.; Gimenéz-Arnau, A.; Al-Ahmad, M.; Ben-Shoshan, M.; Bernstein, J.; Ensina, L.; Fomina, D.; Galvàn, C.; Godse, K.; Grattan, C.; et al. The global burden of chronic urticaria for the patient and society. Br. J. Dermatol. 2021, 184, 226–236. [Google Scholar] [CrossRef]
- Maurer, M.; Staubach, P.; Raap, U.; Richter-Huhn, G.; Baier-Ebert, M.; Chapman-Rothe, N. ATTENTUS, a German online survey of patients with chronic urticaria highlighting the burden of disease, unmet needs and real-life clinical practice. Br. J. Dermatol. 2016, 174, 892–894. [Google Scholar] [CrossRef]
- Poddighe, D. The prevalence of chronic spontaneous urticaria (CSU) in the pediatric population. J. Am. Acad. Dermatol. 2019, 81, e149. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.; Weller, K.; Bindslev-Jensen, C.; Giménez-Arnau, A.; Bousquet, P.J.; Bousquet, J.; Canonica, G.W.; Church, M.K.; Godse, K.V.; Grattan, C.E.H.; et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report1. Allergy 2010, 66, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yi, W.; He, L.; Luo, S.; Wang, J.; Jiang, L.; Long, H.; Zhao, M.; Lu, Q. Abnormalities in Gut Microbiota and Metabolism in Patients with Chronic Spontaneous Urticaria. Front. Immunol. 2021, 12, 691304. [Google Scholar] [CrossRef] [PubMed]
- Rabelo-Filardi, R.; de Oliveira, R.D.; Campos, R.A. Parameters Associated with Chronic Spontaneous Urticaria Duration and Severity: A Systematic Review. Int. Arch. Allergy Immunol. 2013, 161, 197–204. [Google Scholar] [CrossRef]
- Bracken, S.J.; Abraham, S.; MacLeod, A.S. Autoimmune Theories of Chronic Spontaneous Urticaria. Front. Immunol. 2019, 10, 627. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Zuberbier, T. Urticaria and the gut. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Y.; Guo, Y.; Sun, J.; Shen, W.; Yuan, M.; Zhang, S.; He, P.; Jiao, X. Altered Gut Microbiota Diversity and Composition in Chronic Urticaria. Dis. Markers 2019, 2019, 6417471. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Guo, S.; He, H.; Gong, L.; Cui, H. Gut Microbiome and Serum Metabolome Analyses Identify Unsaturated Fatty Acids and Butanoate Metabolism Induced by Gut Microbiota in Patients with Chronic Spontaneous Urticaria. Front. Cell. Infect. Microbiol. 2020, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Nabizadeh, E.; Jazani, N.H.; Bagheri, M.; Shahabi, S. Association of altered gut microbiota composition with chronic urticaria. Ann. Allergy Asthma Immunol. 2017, 119, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Refaat, M.; Talaat, S.; Elgendy, A.; Hendy, D. The Relationship between Gut Microbiota and Chronic Spontaneous Urticaria. J. Allergy Clin. Immunol. 2020, 145, AB199. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Chu, Z.; Shi, L.; Geng, S.; Guo, K. Gut Microbiome Alterations and Functional Prediction in Chronic Spontaneous Urticaria Patients. J. Microbiol. Biotechnol. 2021, 31, 747–755. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Yu, W.-H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database 2010, 2010, baq013. [Google Scholar] [CrossRef]
- Wu, Y.; Chi, X.; Zhang, Q.; Chen, F.; Deng, X. Characterization of the salivary microbiome in people with obesity. PeerJ 2018, 6, e4458. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Tam, J.; Hoffmann, T.; Fischer, S.; Bornstein, S.; Graessler, J.; Noack, B. Obesity alters composition and diversity of the oral microbiota in patients with type 2 diabetes mellitus independently of glycemic control. PLoS ONE 2018, 13, e0204724. [Google Scholar] [CrossRef]
- Fåk, F.; Tremaroli, V.; Bergström, G.; Bäckhed, F. Oral microbiota in patients with atherosclerosis. Atherosclerosis 2015, 243, 573–578. [Google Scholar] [CrossRef]
- Poddighe, D.; Kushugulova, A. Salivary Microbiome in Pediatric and Adult Celiac Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625162. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Peng, C.; Jing, D.; Xiao, Y.; Zhu, W.; Zhao, S.; Zhang, J.; Chen, X.; Li, J. Biomarkers of Gut Microbiota in Chronic Spontaneous Urticaria and Symptomatic Dermographism. Front. Cell. Infect. Microbiol. 2021, 11, 1111. [Google Scholar] [CrossRef]
- The Human Microbiome Project Consortium. Structure, function, and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e8578. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.J.; Nelson, C.E.; Brodie, E.L.; DeSantis, T.Z.; Baek, M.S.; Liu, J.; Woyke, T.; Allgaier, M.; Bristow, J.; Wiener-Kronish, J.P.; et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J. Allergy Clin. Immunol. 2011, 127, 372.e3–381.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moy, A.P.; Murali, M.; Nazarian, R.M. Identification of a Th2 and Th17 Skewed Immune Phenotype in Chronic Urticaria with Th22 Reduction Dependent on Autoimmunity and Thyroid Disease Markers. J. Cutan. Pathol. 2016, 43, 372–378. [Google Scholar] [CrossRef]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56, S14–S19. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Tafti, Z.S.M.; Moshiri, A.; Marvasti, F.E.; Tarashi, S.; Khalili, S.F.S.; Motahhary, A.; Fateh, A.; Vaziri, F.; Badi, S.A.; Siadat, S.D. The effect of saturated and unsaturated fatty acids on the production of outer membrane vesicles from Bacteroides fragilis and Bacteroides thetaiotaomicron. Gastroenterol. Hepatol. Bed Bench 2019, 12, 155–162. [Google Scholar]
- Rezazadeh, A.; Shahabi, S.; Bagheri, M.; Nabizadeh, E.; Jazani, N.H. The protective effect of Lactobacillus and Bifidobacterium as the gut microbiota members against chronic urticaria. Int. Immunopharmacol. 2018, 59, 168–173. [Google Scholar] [CrossRef]
- Kim, M.; Byun, J.; Yoon, Y.; Yum, D.; Chung, M.; Lee, J. A probiotic combination attenuates experimental colitis through inhibition of innate cytokine production. Benef. Microbes 2017, 8, 231–241. [Google Scholar] [CrossRef]
- Zuo, L.; Yuan, K.-T.; Yu, L.; Meng, Q.-H.; Chung, P.C.-K.; Yang, D.-H. Bifidobacterium infantisattenuates colitis by regulating T cell subset responses. World J. Gastroenterol. 2014, 20, 18316–18329. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-J.; Yu, Z.; Yang, F.-L.; Lv, D.; Hung, S.; Zhang, J.; Lin, P.; Liu, S.-X.; Zhang, N.; Bachert, C. Effects of Bifidobacterium Breve Feeding Strategy and Delivery Modes on Experimental Allergic Rhinitis Mice. PLoS ONE 2015, 10, e0140018. [Google Scholar] [CrossRef] [Green Version]
- Arshi, S.; Babaie, D.; Nabavi, M.; Tebianian, M.; Ghalehbaghi, B.; Jalali, F.; Ahmadvand, A.; Gholami, R. Circulating level of CD4+ CD25+ FOXP3+ T cells in patients with chronic urticaria. Int. J. Dermatol. 2014, 53, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Chiang, B.-L.; Liu, H.E.; Leu, S.-J.; Lee, Y.-L. Defective functions of circulating CD4+CD25+ and CD4+CD25− T cells in patients with chronic ordinary urticaria. J. Dermatol. Sci. 2008, 51, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W.; Raison, C.L.; Lowry, C.A. Microbiota, Immunoregulatory Old Friends and Psychiatric Disorders. Adv. Exp. Med. Biol. 2014, 817, 319–356. [Google Scholar] [CrossRef]
- Espina, M.D.T.; Gabarrini, G.; Harmsen, H.J.M.; Westra, J.; Van Winkelhoff, A.J.; Van Dijl, J.M. Talk to your gut: The oral-gut microbiome axis and its immunomodulatory role in the etiology of rheumatoid arthritis. FEMS Microbiol. Rev. 2019, 43, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic colonization of oral bacteria in the intestine drives T H 1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O‘Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2017, 67, 1454–1463. [Google Scholar] [CrossRef] [Green Version]
- Kitts, C.L. Terminal restriction fragment patterns: A tool for comparing microbial communities and assessing community dynamics. Curr. Issues Intest. Microbiol. 2001, 2, 17–25. [Google Scholar] [PubMed]
- Andoh, A.; Kuzuoka, H.; Tsujikawa, T.; Nakamura, S.; Hirai, F.; Suzuki, Y.; Matsui, T.; Fujiyama, Y.; Matsumoto, T. Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn’s disease. J. Gastroenterol. 2012, 47, 1298–1307. [Google Scholar] [CrossRef]
- Abe, K.; Takahashi, A.; Fujita, M.; Imaizumi, H.; Hayashi, M.; Okai, K.; Ohira, H. Dysbiosis of oral microbiota and its association with salivary immunological biomarkers in autoimmune liver disease. PLoS ONE 2018, 13, e0198757. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Sakamoto, M.; Hayashi, H.; Benno, Y. Novel phylogenetic assignment database for terminal-restriction fragment length polymorphism analysis of human colonic microbiota. J. Microbiol. Methods 2005, 61, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Sila, S.; Jelić, M.; Trivić, I.; Andrašević, A.T.; Hojsak, I.; Kolaček, S. Altered Gut Microbiota Is Present in Newly Diagnosed Pediatric Patients With Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Culman, S.W.; Bukowski, R.; Gauch, H.G.; Cadillo-Quiroz, H.; Buckley, D.H. T-REX: Software for the processing and analysis of T-RFLP data. BMC Bioinform. 2009, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Hullar, M.A.; Lampe, J.W. Optimization of terminal restriction fragment polymorphism (TRFLP) analysis of human gut microbiota. J. Microbiol. Methods 2007, 68, 303–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andoh, A.; Imaeda, H.; Aomatsu, T.; Inatomi, O.; Bamba, S.; Sasaki, M.; Saito, Y.; Tsujikawa, T.; Fujiyama, Y. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn’s disease using terminal restriction fragment length polymorphism analysis. J. Gastroenterol. 2011, 46, 479–486. [Google Scholar] [CrossRef]
- Griffen, A.L.; Beall, C.; Firestone, N.D.; Gross, E.L.; DiFranco, J.M.; Hardman, J.H.; Vriesendorp, B.; Faust, R.A.; Janies, D.A.; Leys, E.J. CORE: A Phylogenetically-Curated 16S rDNA Database of the Core Oral Microbiome. PLoS ONE 2011, 6, e19051. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).