Protein Receptors Evolved from Homologous Cohesion Modules That Self-Associated and Are Encoded by Interactive Networked Genes
Abstract
1. Introduction
2. Methods
2.1. Compilation of Receptor and Non-Receptor Gene Lists
2.2. Analysis of Amino Acid Sequence Homology in Whole Genome Searches
2.3. Characterization of Receptor Gene Environment in the Genome
2.4. Evaluation of Gene–Gene Interactions
2.5. Statistical Analysis
3. Results
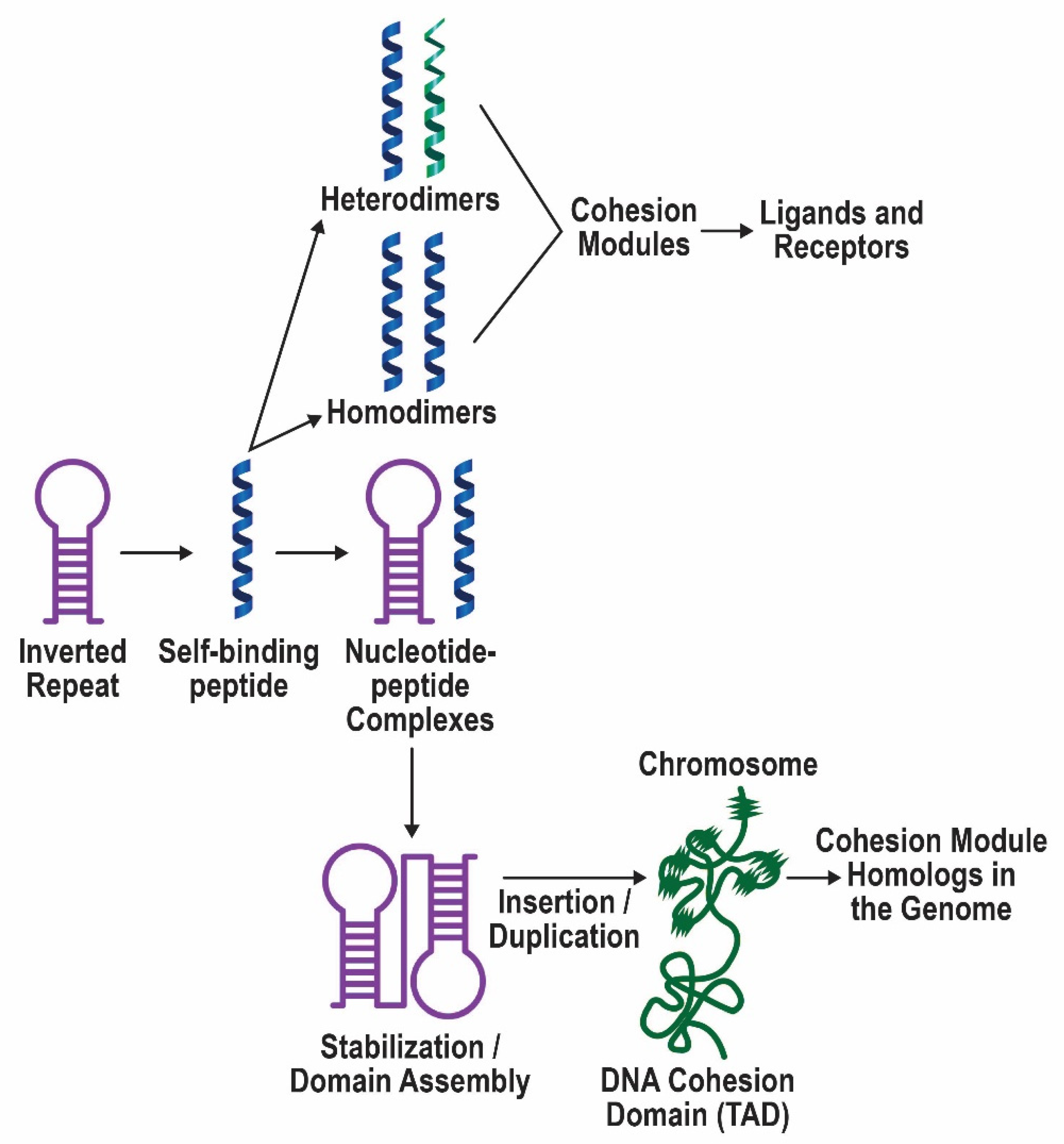
3.1. Summary of the Model and Supporting Evidence
3.2. Greater Number of Homologous Hits with Receptor Sequence Query
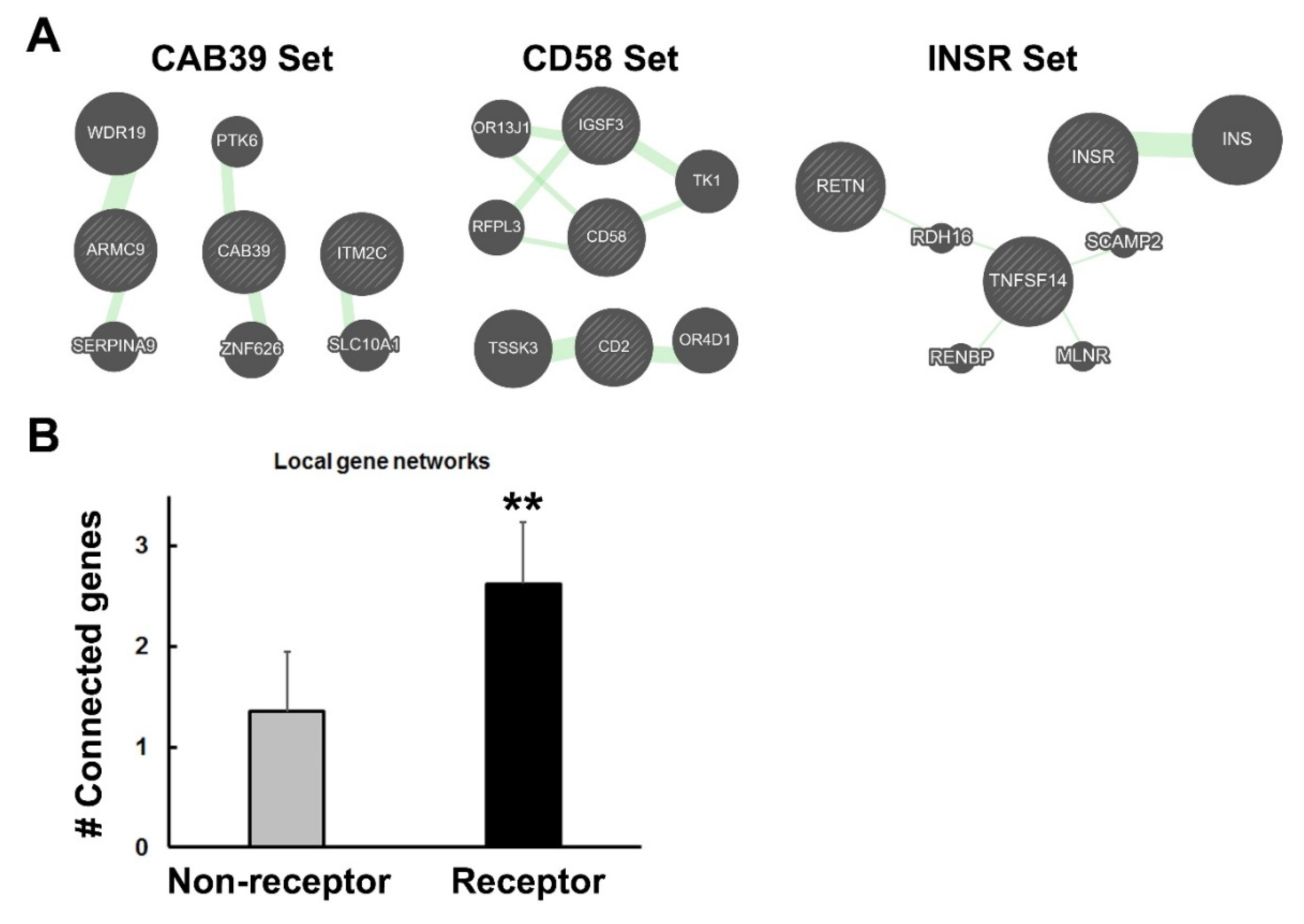
3.3. More Gene–Gene Interactions among Receptor Genes
3.4. Identification of Syntenic Blocks Containing Receptor Genes
3.5. Increase in Genetic Interactions among Members of Receptor Gene Blocks
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dwyer, D.S. Amino acid sequence homology between ligands and their receptors: Potential identification of binding sites. Life Sci. 1989, 45, 421–429. [Google Scholar] [CrossRef]
- Moyle, W.R.; Campbell, R.K.; Myers, R.V.; Bernard, M.P.; Han, Y.; Wang, X. Co-evolution of ligand-receptor pairs. Nature 1994, 368, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.S. Assembly of exons from unitary transposable genetic elements: Implications for the evolution of protein-protein interactions. J. Theor. Biol. 1998, 194, 11–27. [Google Scholar] [CrossRef]
- Root-Bernstein, R. Molecular complementarity III. Peptide complementarity as a basis for peptide receptor evolution: A bioinformatic case study of insulin, glucagon and gastrin. J. Theor. Biol. 2002, 218, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Darlison, M.G.; Richter, D. Multiple genes for neuropeptides and their receptors: Co-evolution and physiology. Trends Neurosci. 1999, 22, 81–88. [Google Scholar] [CrossRef]
- Markov, G.V.; Paris, M.; Bertrand, S.; Laudet, V. The evolution of the ligand/receptor couple: A long road from comparative endocrinology to comparative genomics. Mol. Cell. Endocrinol. 2008, 293, 5–16. [Google Scholar] [CrossRef]
- Root-Bernstein, R. Peptide self-aggregation and peptide complementarity as bases for the evolution of peptide receptors: A review. J. Mol. Recognit. 2005, 18, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.S.; Ardizzone, T.D. Mimicry of dimerization by synthetic peptides designed to target homologous regions of proteins. Proteomics 2003, 3, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Thornton, J.M. Principles of protein-protein interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, D.; Rahat, O.; Cohen, M.; Neuvirth, H.; Schreiber, G. The molecular architecture of protein-protein binding sites. Curr. Opin. Struct. Biol. 2007, 17, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Archakov, A.I.; Govorun, V.M.; Dubanov, A.V.; Veselovsky, A.V.; Lewi, P.; Janssen, P. Protein-protein interactions as a target for drugs in proteomics. Proteomics 2003, 3, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Aloy, P. Novel peptide-mediated interactions derived from high-resolution 3-dimensional structures. PLoS Comput. Biol. 2010, 6, 1000789. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Hui, S.; Jain, S.; Law, B.; Bader, G.D. Domain-mediated protein interaction prediction: From genome to network. FEBS Lett. 2012, 586, 2751–2763. [Google Scholar] [CrossRef]
- Richardson, S.R.; Morell, S.; Faulkner, G.J. L1 retrotransposons and somatic mosaicism in the brain. Annu. Rev. Genet. 2014, 48, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Blalock, J.E.; Smith, E.M. Hydropathic anti-complementarity of amino acids based on the genetic code. Biochem. Biophys. Res. Commun. 1984, 121, 203–207. [Google Scholar] [CrossRef]
- Blalock, J.E.; Bost, K.L. Binding of peptides that are specified by complementary RNAs. Biochem. J. 1986, 234, 679–683. [Google Scholar] [CrossRef]
- Eichler, E. Recent duplication, domain accretion and the dynamic mutation of the human genome. Trends Genet. 2001, 17, 661–669. [Google Scholar] [CrossRef]
- Orengo, C.A.; Thornton, J.M. Protein families and their evolution—A structural perspective. Annu. Rev. Biochem. 2005, 74, 867–900. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Betran, E.; Thornton, K.; Wang, W. The origin of new genes: Glimpses from the young and old. Nat. Rev. Genet. 2003, 4, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.F.; Eskiw, C.; Cook, P.R. Transcription factories. Biochem. Soc. Trans. 2008, 36, 585–589. [Google Scholar] [CrossRef]
- Maass, P.G.; Barutcu, A.R.; Rinn, J.L. Interchromosomal interactions: A genomic love story of kissing chromosomes. J. Cell Biol. 2018, 218, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Achuthan, P.; Akanni, W.; Allen, J.; Amode, M.R.; Armean, I.M.; Bennett, R.; Bhai, J.; Billis, K.; Boddu, S.; et al. Ensembl 2019. Nucleic Acids Res. 2019, 47, D745–D751. [Google Scholar] [CrossRef] [PubMed]
- Kasap, M.; Rajani, V.; Rajani, J.; Dwyer, D.S. Surprising conservation of schizophrenia risk genes in lower organisms reflects their essential function and the evolution of genetic liability. Schizophr. Res. 2018, 202, 120–128. [Google Scholar] [CrossRef]
- Franklin, C.; Dwyer, D.S. Candidate risk genes for bipolar disorder are highly conserved during evolution and highly interconnected. Bipolar Disord. 2021, 23, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.M.; Collis, C.M. Mobile gene cassettes and integrons: Capture and spread of genes by site-specific recombination. Mol. Microbiol. 1995, 15, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.G. Shared strategies in gene organization among prokaryotes and eukaryotes. Cell 2002, 110, 407–413. [Google Scholar] [CrossRef]
- Kikuta, H.; Laplante, M.; Navratilova, P.; Komisarczuk, A.Z.; Engström, P.G.; Fredman, D.; Akalin, A.; Caccamo, M.; Sealy, I.; Howe, K.; et al. Genomic regulatory blocks encompass multiple neighboring genes and maintain conserved synteny in vertebrates. Genome Res. 2007, 17, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, F.; Zugck, C.; Rauch, G.-J.; Ivandic, B.; Weichenhan, D.; Müller-Bardorff, M.; Meder, B.; El Mokhtari, N.E.; Regitz-Zagrosek, V.; Hetzer, R.; et al. HBEGF, SRA1, and IK: Three cosegregating genes as determinants of cardiomyopathy. Genome Res. 2009, 19, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Wolfe, K.H. Birth of a metabolic gene cluster in yeast by adaptive gene relocation. Nat. Genet. 2005, 17, 777–782. [Google Scholar] [CrossRef]
- Mota, N.R.; Araujo-Jnr, E.V.; Rodrigues Paixäo-Côrtes, V.; Bortolini, M.C.; Dotto Bau, C.H. Linking dopamine neurotransmission and neurogenesis: The evolutionary history of the NTAD (NCAM1-TTC12-ANKK1-DRD2) gene cluster. Genet. Mol. Biol. 2012, 35 (Suppl. S4), 912–918. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, K.; Franz, M.; Rodriguez, H.; Montojo, J.; Lopes, C.T.; Bader, G.D.; Morris, Q. GeneMANIA prediction server 2013 update. Nucleic Acids Res. 2013, 41, W115–W122. [Google Scholar] [CrossRef]
- Lin, A.; Wang, R.T.; Ahn, S.; Park, C.C.; Smith, D.J. A genome-wide map of human genetic interactions inferred from radiation hybrid genotypes. Genome Res. 2010, 20, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, S.-X.; Bren, N.; Cheng, K.; Gomoto, R.; Chen, L.; Sine, S.M. Complex between α-bungarotoxin and an α7 nicotinic receptor ligand-binding domain chimera. Biochem. J. 2013, 454, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Uchikawa, E.; Choi, E.; Shang, G.; Yu, H.; Bai, X. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. eLife 2019, 8, e48630. [Google Scholar] [CrossRef]
- Sall, S.; Thompson, W.; Santos, A.; Dwyer, D.S. Analysis of major depression risk genes reveals evolutionary conservation, shared phenotypes, and extensive genetic interactions. Front. Psychiatry 2021, 12, 698029. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.M.; Miner, J.L. Role of resistin in insulin sensitivity in rodents and humans. Curr. Protein Pept. Sci. 2009, 10, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Emamalipour, M.; Seidi, K.; Jahanban-Esfahlan, A.; Jahanban-Esfahlan, R. Implications of resistin in type 2 diabetes mellitus and coronary artery disease: Impairing insulin function and inducing pro-inflammatory cytokines. J. Cell. Physiol. 2019, 234, 21758–21769. [Google Scholar] [CrossRef]
- Halvorsen, B.; Santilli, F.; Scholz, H.; Sahraoui, A.; Gulseth, H.L.; Wium, C.; Lattanzio, S.; Formoso, G.; Di Fulvio, P.; Otterdal, K.; et al. LIGHT/TNFSF14 is increased in patients with type 2 diabetes mellitus and promotes islet cell dysfunction and endothelial cell inflammation in vitro. Diabetologia 2016, 59, 2134–2144. [Google Scholar] [CrossRef]
- Seed, B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. Nature 1987, 329, 840–842. [Google Scholar] [CrossRef] [PubMed]
- Fennelly, J.A.; Tiwari, B.; Davis, S.J.; Evans, E.J. CD2F-10: A new member of the CD2 subset of the immunoglobulin superfamily. Immunogenetics 2001, 53, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Sameshima, S.; Nakao, M.; Somamoto, T. Diversity of CD2 subfamily receptors in cyprinid fishes. Results Immunol. 2012, 2, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.D.; Erba, E.B.; Robinson, C.V.; Teichmann, S.A. Assembly reflects evolution of protein complexes. Nature 2008, 453, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Gelernter, J.; Yu, Y.; Weiss, R.; Brady, K.; Panhuysen, C.; Yang, B.Z.; Kranzler, H.R.; Farrer, L. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two American populations. Hum. Mol. Genet. 2006, 15, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Makgoba, M.W.; Sanders, M.E.; Shaw, S. The CD2-LFA-3 and LFA-1-ICAM pathways: Relevance to T-cell recognition. Immunol. Today 1989, 10, 417–422. [Google Scholar] [CrossRef][Green Version]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A.A. Cutting edge: Molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J. Immunol. 2002, 169, 5392–5395. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.S.; Dobbelstein, C. Insulin binds to glucagon forming a complex that is hyper-antigenic and inducing complementary antibodies having an idiotype-antiidiotype relationship. Autoimmunity 2001, 33, 153–169. [Google Scholar] [CrossRef]
- Greenspan, R.J. The flexible genome. Nat. Rev. Genet. 2001, 2, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.S. Genomic chaos begets psychiatric disorder. Complex Psychiatry 2020, 6, 20–29. [Google Scholar] [CrossRef]
- Root-Bernstein, R.S.; Holsworth, D.D. Antisense peptides: A critical mini-review. J. Theor. Biol. 1998, 190, 107–119. [Google Scholar] [CrossRef]
- Jaeger, L.; Westhof, E.; Leontis, N.B. TectoRNA: Modular assembly units for the construction of RNA nano-objects. Nucleic Acids Res. 2001, 29, 455–463. [Google Scholar] [CrossRef]
- Lieberman-Aiden, E.; van Berkum, N.; Williams, L.; Imakaev, M.; Ragoczy, T.; Telling, A.; Amit, I.; Lajoie, B.R.; Sabo, P.J.; Dorschner, M.D.; et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009, 326, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012, 485, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, K.; Wirtz, J.; Rauscher, M.; Wiehe, T. A common genomic code for chromatin architecture and recombination landscape. PLoS ONE 2019, 14, e0213278. [Google Scholar] [CrossRef] [PubMed]
- Eigen, M. Molecular self-organization in the early stages of evolution. Q. Rev. Biophys. 1971, 4, 149–212. [Google Scholar] [CrossRef] [PubMed]




| Query Protein | Homologous Hits | Homology (E Value) | Domain Match |
|---|---|---|---|
| NCAM1 | Hemicentin-1 | 4e−41 | Ig |
| DCC netrin receptor | 4e−29 | Ig, Fn | |
| Titin | 6e−29 | Ig | |
| TENM4 | Tenascin XB | 4e−45 | Laminin, EGF |
| Tenascin R | 3e−22 | Laminin, EGF | |
| Integrin subunit β-4 | 6e−12 | Laminin | |
| ESR2 | Retinoid X receptor γ | 8e−49 | NR-DBD, NR-LBD |
| Androgen receptor | 5e−26 | NR-DBD, NR-LBD | |
| Vitamin D receptor | 5e−18 | NR-DBD | |
| ITIH5 | Inter-alpha-trypsin inhibitor heavy chain-3 | 8e−121 | ITIH heavy chain |
| Von Willebrand factor A domain containing-3A | 7e−7 | vWFA-like | |
| Ca++ voltage-gated channel subunit α2δ4 | 0.001 | vWFA-like |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dwyer, D.S. Protein Receptors Evolved from Homologous Cohesion Modules That Self-Associated and Are Encoded by Interactive Networked Genes. Life 2021, 11, 1335. https://doi.org/10.3390/life11121335
Dwyer DS. Protein Receptors Evolved from Homologous Cohesion Modules That Self-Associated and Are Encoded by Interactive Networked Genes. Life. 2021; 11(12):1335. https://doi.org/10.3390/life11121335
Chicago/Turabian StyleDwyer, Donard S. 2021. "Protein Receptors Evolved from Homologous Cohesion Modules That Self-Associated and Are Encoded by Interactive Networked Genes" Life 11, no. 12: 1335. https://doi.org/10.3390/life11121335
APA StyleDwyer, D. S. (2021). Protein Receptors Evolved from Homologous Cohesion Modules That Self-Associated and Are Encoded by Interactive Networked Genes. Life, 11(12), 1335. https://doi.org/10.3390/life11121335





