Obesity-Related Metabolic Dysfunction in Dairy Cows and Horses: Comparison to Human Metabolic Syndrome
Abstract
1. Introduction
2. Human Metabolic Syndrome (MetS)
3. Dyslipidemia and Fatty Liver Disease in Animals
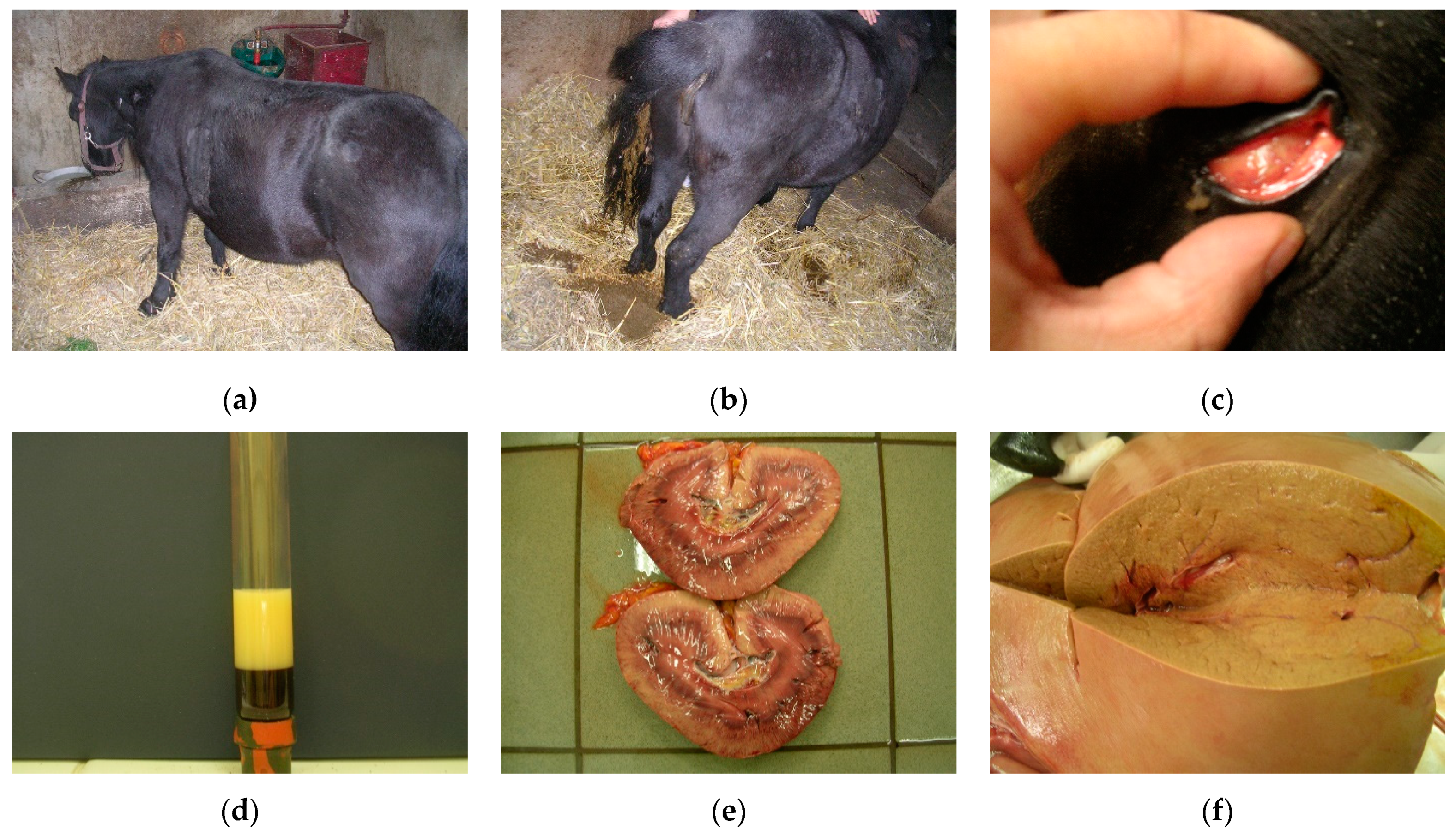
3.1. Fat Cow Syndrome in Dairy Cows
3.1.1. Clinical Signs
3.1.2. Diagnosis
3.1.3. Prevention and Treatment
3.2. Comparison between FCS and MetS
4. Equine Metabolic Syndrome
4.1. Hyperlipidemias and Hepatic Lipidosis
4.1.1. Clinical Signs and Diagnosis
4.1.2. Prevention and Treatment
4.2. Comparison between EMS and MetS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Drewnowski, A.; Buszkiewicz, J.; Aggarwal, A.; Rose, C.; Gupta, S.; Bradshaw, A. Obesity and the built environment: A reappraisal. Obesity 2020, 28, 22–30. [Google Scholar] [CrossRef]
- McCafferty, B.J.; Hill, J.O.; Gunn, A.J. Obesity: Scope, lifestyle interventions, and medical management. Tech. Vasc. Interv. Radiol. 2020, 23, 100653. [Google Scholar] [CrossRef]
- Grundy, S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Keen, J.; McGowan, C. Equine metabolic syndrome. Vet. Rec. 2015, 177, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Lee, M.-J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 2013, 34, 1–11. [Google Scholar] [CrossRef]
- Shin, S.W.; Lee, S.J. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 2236–2238. [Google Scholar] [CrossRef]
- Wong, N.D.; Pio, J.R.; Franklin, S.S.; L’Italien, G.J.; Kamath, T.V.; Williams, G.R. Preventing coronary events by optimal control of blood pressure and lipids in patients with the metabolic syndrome. Am. J. Cardiol. 2003, 91, 1421–1426. [Google Scholar] [CrossRef]
- Cătoi, A.F.; Pârvu, A.; Mureşan, A.; Busetto, L. Metabolic mechanisms in obesity and type 2 diabetes: Insights from bariatric/metabolic surgery. Obes. Facts 2015, 8, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Lorenzatti, A.J.; Toth, P.P. New perspectives on atherogenic dyslipidaemia and cardiovascular disease. Eur. Cardiol. Rev. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Taylor, R. Type 2 Diabetes: Etiology and reversibility. Diabetes Care 2013, 36, 1047–1055. [Google Scholar] [CrossRef]
- De Koster, J.; Opsomer, G. Are modern dairy cows suffering from modern diseases? Vlaams Diergeneeskd. Tijdschr. 2012, 81, 71–80. [Google Scholar] [CrossRef]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.K. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Pollard, D.; Wylie, C.E.; Verheyen, K.L.P.; Newton, J.R. Identification of modifiable factors associated with owner-reported equine laminitis in Britain using a web-based cohort study approach. BMC Vet. Res. 2019, 15, 59. [Google Scholar] [CrossRef]
- Tóth, F.; Frank, N.; Chameroy, K.A.; Bostont, R.C. Effects of endotoxaemia and carbohydrate overload on glucose and insulin dynamics and the development of laminitis in horses. Equine Vet. J. 2009, 41, 852–858. [Google Scholar] [CrossRef]
- Longland, A.C.; Byrd, B.M. Pasture nonstructural carbohydrates and equine laminitis. J. Nutr. 2006, 136, 2099–2102. [Google Scholar] [CrossRef]
- Wylie, C.E.; Collins, S.N.; Verheyen, K.L.; Newton, J.R. Risk factors for equine laminitis: A case-control study conducted in veterinary-registered horses and ponies in Great Britain between 2009 and 2011. Vet. J. 2013, 198, 57–69. [Google Scholar] [CrossRef]
- Menzies-Gow, N.J.; Harris, P.A.; Elliott, J. Prospective cohort study evaluating risk factors for the development of pasture-associated laminitis in the United Kingdom. Equine Vet. J. 2017, 49, 300–306. [Google Scholar] [CrossRef]
- Coleman, M.C.; Belknap, J.K.; Eades, S.C.; Galantino-Homer, H.L.; Hunt, R.J.; Geor, R.J.; McCue, M.E.; McIlwraith, C.W.; Moore, R.M.; Peroni, J.F.; et al. Case-control study of risk factors for pasture-and Endocr.inopathy-associated laminitis in North American horses. J. Am. Vet. Med. Assoc. 2018, 253, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Kylin, E. Studien ueber das Hypertonie-Hyperglyka “mie-Hyperurika” miesyndrom. Zent. Inn. Med. 1923, 44, 105–127. [Google Scholar]
- Vague, J. La differenciation sexuelle-facteur determinant des formes de l’obesite. Presse Med. 1947, 30, 339–340. [Google Scholar]
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.M. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch. Intern. Med. 1989, 149, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Willerson, J.T.; Ridker, P.M. Inflammation as a Cardiovascular Risk Factor. Circulation 2004, 109, II-2–II-10. [Google Scholar] [CrossRef]
- Manchanayake, J.; Chitturi, S.; Nolan, C.; Farrell, G.C. Postprandial hyperinsulinemia is universal in non-diabetic patients with nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2011, 26, 510–516. [Google Scholar] [CrossRef]
- Hsieh, J.; Hayashi, A.A.; Webb, J.; Adeli, K. Postprandial dyslipidemia in insulin resistance: Mechanisms and role of intestinal insulin sensitivity. Atheroscler. Suppl. 2008, 9, 7–13. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Balkau, B.; Charles, M.A. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet. Med. 1999, 16, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian syndrome: Is the metabolic syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Zhang, Y.L.; Hernandez-Ono, A. Metabolic syndrome: Focus on dyslipidemia. Obesity 2006, 14 (Suppl. S1), 41S–49S. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, M.; Schaper, F. 21-Dyslipidemia in the metabolic syndrome. In The Metabolic Syndrome at the Beginning of the XXI Century; Serrano Ríos, M., Caro, J.F., Carraro, R., Gutiérrez Fuentes, J.A., Eds.; Elsevier España: Madrid, Spain, 2005; pp. 347–358. [Google Scholar] [CrossRef]
- Morrow, D.A. Fat cow syndrome. J. Dairy Sci. 1976, 59, 1625–1629. [Google Scholar] [CrossRef]
- Shi, K.; Li, R.; Xu, Z.; Zhang, Q. Identification of crucial genetic factors, such as PPARγ, that regulate the pathogenesis of fatty liver disease in dairy cows is imperative for the sustainable development of dairy industry. Animals 2020, 10, 639. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, P.R.; Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Ramesha, K.P.; Sejian, V.; Rajendran, D.; Varghese, M.R. Metabolic and immunological changes in transition dairy cows: A review. Vet. World 2017, 10, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.L.; Eness, P.G. Bovine fatty liver disease. Iowa State Univ. Vet. 1984, 46, 7. [Google Scholar]
- Farid, A.S.; Honkawa, K.; Fath, E.M.; Nonaka, N.; Horii, Y. Serum paraoxonase-1 as biomarker for improved diagnosis of fatty liver in dairy cows. BMC Vet. Res. 2013, 9, 73. [Google Scholar] [CrossRef]
- Bobe, G.; Young, J.W.; Beitz, D.C. Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- Grummer, R.R. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. J. Anim. Sci. 1995, 73, 2820–2833. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Yin, Z.Y.; Lin, X.Y.; Yan, Z.G.; Wang, Z.H. Effects of feeding fatty acid calcium and the interaction of forage quality on production performance and biochemical indexes in early lactation cow. J. Anim. Physiol. Anim. Nutr. 2015, 99, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Katoh, N. Relevance of apolipoproteins in the development of fatty liver and fatty liver-related peripartum diseases in dairy cows. J. Vet. Med. Sci. 2002, 64, 293–307. [Google Scholar] [CrossRef] [PubMed]
- De Koster, J.D.; Opsomer, G. Insulin Resistance in Dairy Cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Jorritsma, R.; Jorritsma, H.; Schukken, Y.H.; Wentink, G.H. Relationships between fatty liver and fertility and some periparturient diseases in commercial Dutch dairy herds. Theriogenology 2000, 54, 1065–1074. [Google Scholar] [CrossRef]
- Oikawa, S.; Katoh, N. Decreases in serum apolipoprotein B-100 and A-I concentrations in cows with milk fever and downer cows. Can. J. Vet. Res. 2002, 66, 31–34. [Google Scholar] [PubMed]
- White, H.M. The role of TCA cycle anaplerosis in ketosis and fatty liver in periparturient dairy cows. Animals 2015, 5, 793–802. [Google Scholar] [CrossRef]
- Sweeney, R.W.; Divers, T.J.; Whitlock, R.H.; Acland, H.M.; Tulleners, E.P.; Palmer, J.E. Hepatic failure in dairy cattle following mastitis or metritis. J. Vet. Intern. Med. 1988, 2, 80–84. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.; Nydam, D.V.; Oetzel, G.R.; Overton, T.R.; Ospina, P.A. Elevated non-esterified fatty acids and β-hydroxybutyrate and their association with transition dairy cow performance. Vet. J. 2013, 198, 560–570. [Google Scholar] [CrossRef]
- Ruoff, J.; Bertulat, S.; Burfeind, O.; Heuwieser, W. Associations of β-hydroxybutyrate, cholesterol, triglycerides and high-density lipoproteins to non-esterified fatty acids pre- and postpartum. J. Dairy Res. 2016, 83, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.R. Nutritional and management strategies for the prevention of fatty liver in dairy cattle. Vet. J. 2008, 176, 10–20. [Google Scholar] [CrossRef]
- Gerloff, B.J. Dry cow management for the prevention of ketosis and fatty liver in dairy cows. Vet. Clin. N. Am. Equine Pract. 2000, 16, 283–292. [Google Scholar] [CrossRef]
- Hippen, A.R.; She, P.; Young, J.W.; Beitz, D.C.; Lindberg, G.L.; Richardson, L.F.; Tucker, R.W. Alleviation of fatty liver in dairy cows with 14-day intravenous infusions of glucagon. J. Dairy Sci. 1999, 82, 1139–1152. [Google Scholar] [CrossRef]
- Nafikov, R.A.; Ametaj, B.N.; Bobe, G.; Koehler, K.J.; Young, J.W.; Beitz, D.C. Prevention of fatty liver in transition dairy cows by subcutaneous injections of glucagon. J. Dairy Sci. 2006, 89, 1533–1545. [Google Scholar] [CrossRef]
- Schäff, C.; Börner, S.; Hacke, S.; Kautzsch, U.; Sauerwein, H.; Spachmann, S.K.; Schweigel-Röntgen, M.; Hammon, H.M.; Kuhla, B. Increased muscle fatty acid oxidation in dairy cows with intensive body fat mobilization during early lactation. J. Dairy Sci. 2013, 96, 6449–6460. [Google Scholar] [CrossRef] [PubMed]
- Schultz, L.H. Management and nutritional aspects of ketosis. J. Dairy Sci. 1971, 54, 962–973. [Google Scholar] [CrossRef]
- Hayirli, A.; Bertics, S.J.; Grummer, R.R. Effects of slow-release insulin on production, liver triglyceride, and metabolic profiles of Holsteins in early lactation. J. Dairy Sci. 2002, 85, 2180–2191. [Google Scholar] [CrossRef]
- Studer, V.A.; Grummer, R.R.; Bertics, S.J.; Reynolds, C.K. Effect of prepartum propylene glycol administration on periparturient fatty liver in dairy cows. J. Dairy Sci. 1993, 76, 2931–2939. [Google Scholar] [CrossRef]
- Chauhan, S.; Celi, P.; Ponnampalam, E.; Leury, B.; Liu, F.; Dunshea, F. Antioxidant dynamics in the live animal and implications for ruminant health and product (meat/milk) quality: Role of vitamin E and selenium. Anim. Prod. Sci. 2014, 54, 1525–1536. [Google Scholar] [CrossRef]
- Akins, M.S.; Bertics, S.J.; Socha, M.T.; Shaver, R.D. Effects of cobalt supplementation and vitamin B12 injections on lactation performance and metabolism of Holstein dairy cows. J. Dairy Sci. 2013, 96, 1755–1768. [Google Scholar] [CrossRef]
- Basoglu, A.; Sevinc, M.; Birdane, F.M.; Boydak, M. Efficacy of sodium borate in the prevention of fatty liver in dairy cows. J. Vet. Intern. Med. 2002, 16, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.F.; Del Río, N.S.; Caraviello, D.Z.; Bertics, S.J.; Ramos, M.H.; Grummer, R.R. Supplemental choline for prevention and alleviation of fatty liver in dairy cattle. J. Dairy Sci. 2007, 90, 2413–2418. [Google Scholar] [CrossRef]
- Grummer, R.R.; Hoffman, P.C.; Luck, M.L.; Bertics, S.J. Effect of prepartum and postpartum dietary energy on growth and lactation of primiparous cows. J. Dairy Sci. 1995, 78, 172–180. [Google Scholar] [CrossRef]
- Karkoodi, K.; Tamizrad, K. Effect of niacin supplementation on performance and blood parameters of Holstein cows. S. Afr. J. Anim. Sci. 2009, 39, 349–354. [Google Scholar] [CrossRef]
- Skaar, T.C.; Grummer, R.R.; Dentine, M.R.; Stauffacher, R.H. Seasonal effects of prepartum and postpartum fat and niacin feeding on lactation performance and lipid metabolism. J. Dairy Sci. 1989, 72, 2028–2038. [Google Scholar] [CrossRef]
- Weber, C.; Schäff, C.T.; Kautzsch, U.; Börner, S.; Erdmann, S.; Görs, S.; Röntgen, M.; Sauerwein, H.; Bruckmaier, R.M.; Metges, C.C.; et al. Insulin-dependent glucose metabolism in dairy cows with variable fat mobilization around calving. J. Dairy Sci. 2016, 99, 6665–6679. [Google Scholar] [CrossRef]
- Holtenius, P.; Holtenius, K. A model to estimate insulin sensitivity in dairy cows. Acta Vet. Scand. 2007, 49, 29. [Google Scholar] [CrossRef] [PubMed]
- Ingvartsen, K.L.; Boisclair, Y.R. Leptin and the regulation of food intake, energy homeostasis and immunity with special focus on periparturient ruminants. Domest. Anim. Endocrinol. 2001, 21, 215–250. [Google Scholar] [CrossRef]
- Komatsu, T.; Itoh, F.; Mikawa, S.; Hodate, K. Gene expression of resistin in adipose tissue and mammary gland of lactating and non-lactating cows. J. Endocrinol. 2003, 178, R1–R5. [Google Scholar] [CrossRef][Green Version]
- McCann, J.P.; Reimers, T.J. Insulin response to glucose in estrous and diestrous obese and lean heifers. J. Anim. Sci. 1985, 61, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Lemor, A.; Hosseini, A.; Sauerwein, H.; Mielenz, M. Transition period-related changes in the abundance of the mRNAs of adiponectin and its receptors, of visfatin, and of fatty acid binding receptors in adipose tissue of high-yielding dairy cows. Domest. Anim. Endocrinol. 2009, 37, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sadri, H.; Bruckmaier, R.M.; Rahmani, H.R.; Ghorbani, G.R.; Morel, I.; Van Dorland, H.A. Gene expression of tumour necrosis factor and insulin signalling-related factors in subcutaneous adipose tissue during the dry period and in early lactation in dairy cows. J. Anim. Physiol. Anim. Nutr. 2010, 94, e194–e202. [Google Scholar] [CrossRef] [PubMed]
- Lacetera, N.; Scalia, D.; Bernabucci, U.; Ronchi, B.; Pirazzi, D.; Nardone, A. Lymphocyte functions in overconditioned cows around parturition. J. Dairy Sci. 2005, 88, 2010–2016. [Google Scholar] [CrossRef]
- Opsomer, G.; Wensing, T.; Laevens, H.; Coryn, M.; de Kruif, A. Insulin resistance: The link between metabolic Disorders and cystic ovarian disease in high yielding dairy cows? Anim. Reprod. Sci. 1999, 56, 211–222. [Google Scholar] [CrossRef]
- Semple, R.K.; Sleigh, A.; Murgatroyd, P.R.; Adams, C.A.; Bluck, L.; Jackson, S.; Vottero, A.; Kanabar, D.; Charlton-Menys, V.; Durrington, P.; et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J. Clin. Investig. 2009, 119, 315–322. [Google Scholar] [CrossRef]
- Xu, E.; Schwab, M.; Marette, A. Role of protein tyrosine phosphatases in the modulation of insulin signaling and their implication in the pathogenesis of obesity-linked insulin resistance. Rev. Endocr. Metab. Disord. 2013, 15, 79–97. [Google Scholar] [CrossRef]
- Herdt, T.H. Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis and fatty liver. Vet. Clin. N. Am. Food Anim. Pract 2000, 16, 215–230. [Google Scholar] [CrossRef]
- Fürll, B.; Hadrich, G.; Fürll, M. Metabolic syndrome in cows: TNF-α concentrations before and after parturition in healthy and ill cows. In Proceedings of the Ruminal Physiology Conference, Clermont-Ferrand, France, 6–9 September 2009. [Google Scholar]
- Chatrath, H.; Vuppalanchi, R.; Chalasani, N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin. Liver Dis. 2012, 32, 22–29. [Google Scholar] [CrossRef]
- Katsiki, N.; MikhailiDis, D.P.; Mantzoros, C.S. Non-alcoholic fatty liver Disease and dyslipidemia: An update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Folnožić, I.; Turk, R.; Đuričić, D.; Vince, S.; Pleadin, J.; Flegar-Meštrić, Z.; Valpotić, H.; Dobranić, T.; Gračner, D.; Samardžija, M. Influence of Body Condition on Serum Metabolic Indicators of Lipid Mobilization and Oxidative Stress in Dairy Cows During the Transition Period. Reprod. Domest. Anim. 2015, 50, 910–917. [Google Scholar] [CrossRef]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E462–E468. [Google Scholar] [CrossRef]
- Newton, J.L. Systemic symptoms in non-alcoholic fatty liver Disease. Dig. Dis. 2010, 28, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.L.; Jones, D.E.; Henderson, E.; Kane, L.; Wilton, K.; Burt, A.D.; Day, C.P. Fatigue in non-alcoholic fatty liver Disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver Disease severity or insulin resistance. Gut 2008, 57, 807–813. [Google Scholar] [CrossRef]
- Johnson, P.J. The equine Metabolic syndrome peripheral Cushing’s syndrome. Vet. Clin. N. Am. Equine Pract. 2002, 18, 271–293. [Google Scholar] [CrossRef]
- Frank, N.; Geor, R.J.; Bailey, S.R.; Durham, A.E.; Johnson, P.J. Equine Metabolic syndrome. J. Vet. Intern. Med. 2010, 24, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.E.; Frank, N.; McGowan, C.M.; Menzies-Gow, N.J.; Roelfsema, E.; Vervuert, I.; Feige, K.; Fey, K. ECEIM consensus statement on equine Metabolic syndrome. J. Vet. Intern. Med. 2019, 33, 335–349. [Google Scholar] [CrossRef]
- Jacob, S.I.; Geor, R.J.; Weber, P.S.D.; Harris, P.A.; McCue, M.E. Effect of age and dietary carbohydrate profiles on glucose and insulin dynamics in horses. Equine Vet. J. 2017, 50, 249–254. [Google Scholar] [CrossRef]
- Laat, M.A.d.; McGree, J.M.; Sillence, M.N. Equine hyperinsulinemia: Investigation of the enteroinsular axis during insulin dysregulation. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E61–E72. [Google Scholar] [CrossRef]
- Frank, N. Equine Metabolic syndrome. Vet. Clin. N. Am. Equine Pract. 2011, 27, 73–92. [Google Scholar] [CrossRef] [PubMed]
- TÓTh, F.; Frank, N.; Martin-JimÉNez, T.; Elliott, S.B.; Geor, R.J.; Boston, R.C. Measurement of C-peptide concentrations and responses to somatostatin, glucose infusion, and insulin resistance in horses. Equine Vet. J. 2010, 42, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Asplin, K.E.; Sillence, M.N.; Pollitt, C.C.; McGowan, C.M. Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet. J. 2007, 174, 530–535. [Google Scholar] [CrossRef] [PubMed]
- de Laat, M.A.; McGowan, C.M.; Sillence, M.N.; Pollitt, C.C. Hyperinsulinemic laminitis. Vet. Clin. N. Am. Equine Pract. 2010, 26, 257–264. [Google Scholar] [CrossRef] [PubMed]
- de Laat, M.A.; Sillence, M.N.; McGowan, C.M.; Pollitt, C.C. Continuous intravenous infusion of glucose induces endogenous hyperinsulinaemia and lamellar histopathology in Standardbred horses. Vet. J. 2012, 191, 317–322. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, H.C., III. Equine hyperlipidemias. Vet. Clin. N. Am. Equine Pract. 2011, 27, 59–72. [Google Scholar] [CrossRef]
- Dunkel, B.; McKenzie, H.C., III. Severe hypertriglyceridaemia in clinically ill horses: Diagnosis, treatment and outcome. Equine Vet. J. 2003, 35, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Naylor, J.M.; Kronfeld, D.S.; Acland, H. Hyperlipemia in horses: Effects of undernutrition and Disease. Am. J. Vet. Res. 1980, 41, 899–905. [Google Scholar]
- Field, J. Hyperlipaemia in a Quarterhorse. Comp. Cont. Educ. Pract. 1987, 10, 218–221. [Google Scholar]
- McAuliffe, S. Knottenbelt and Pascoe’s Color Atlas of Diseases and Disorders of the Horse E-Book, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Mogg, T.D.; Palmer, J.E. Hyperlipidemia, hyperlipemia, and hepatic lipidosis in American miniature horses: 23 cases (1990–1994). J. Am. Vet. Med. Assoc. 1995, 207, 604–607. [Google Scholar]
- Moore, B.R.; Abood, S.K.; Hinchcliff, K.W. Hyperlipemia in 9 miniature horses and miniature donkeys. J. Vet. Intern. Med. 1994, 8, 376–381. [Google Scholar] [CrossRef]
- Reid, S.W.; Mohammed, H.O. Survival analysis approach to risk factors associated with hyperlipemia in donkeys. J. Am. Vet. Med. Assoc. 1996, 209, 1449–1452. [Google Scholar]
- Jeffcott, L.B.; Field, J.R.; McLean, J.G.; O’Dea, K. Glucose tolerance and insulin sensitivity in ponies and Standardbred horses. Equine Vet. J. 1986, 18, 97–101. [Google Scholar] [CrossRef]
- Watson, T.D.; Murphy, D.; Love, S. Equine hyperlipaemia in the United Kingdom: Clinical features and blood biochemistry of 18 cases. Vet. Rec. 1992, 131, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S. Hyperglycemia/hyperinsulinemia after feeding a meal of grain to young horses with osteochondritis Dissecans (OCD) lesions. Pferdeheilkunde Equine Med. 1996, 12, 320–322. [Google Scholar] [CrossRef]
- Foreman, J.H. Hyperlipemia and Hepatic Lipidosis in Large Animals. Available online: https://www.msdvetmanual.com/Dig.estive-system/hepatic-Disease-in-large-animals/hyperlipemia-and-hepatic-lipidosis-in-large-animals#v3265716 (accessed on 12 September 2021).
- Watson, T.D.; Burns, L.; Packard, C.J.; Shepherd, J. Effects of pregnancy and lactation on plasma lipid and lipoprotein concentrations, lipoprotein composition and post-heparin lipase activities in Shetland pony mares. J. Reprod Fertil. 1993, 97, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.O. Congenital hyperlipaemia in a Shetland pony foal. Equine Vet. J. 1986, 18, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.I.; Edwards, A.L.; Symonds, C.J.; Beck, P.L. Hypertriglyceridemia-induced pancreatitis: A case-based review. World J. Gastroenterol. 2006, 12, 7197–7202. [Google Scholar] [CrossRef] [PubMed]
- Breidenbach, A.; Fuhrmann, H.; Deegen, E.; Lindholm, A.; Sallmann, H.P. Studies on equine lipid Metabolism. Lipolytic activities of plasma and tissue lipases in large horses and ponies. J. Vet. Med. Ser. A 1999, 46, 39–48. [Google Scholar] [CrossRef]
- Van Weyenberg, S.; Hesta, M.; Buyse, J.; Janssens, G.P. The effect of weight loss by energy restriction on Metabolic profile and glucose tolerance in ponies. J. Anim. Physiol. Anim. Nutr. 2008, 92, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Geor, R.J.; Harris, P. Dietary management of obesity and insulin resistance: Countering risk for laminitis. Vet. Clin. N. Am. Equine Pract. 2009, 25, 51–65. [Google Scholar] [CrossRef]
- Hughes, K.J.; Hodgson, D.R.; Dart, A.J. Equine hyperlipaemia: A review. Aust. Vet. J. 2004, 82, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, O.; Deegen, E.; Fuhrmann, H.; Dühlmeier, R.; Sallmann, H.P. Effects of fat feeding and energy level on plasma Metabolites and hormones in Shetland ponies. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2001, 48, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Bamford, N.J.; Potter, S.J.; Baskerville, C.L.; Harris, P.A.; Bailey, S.R. Effect of increased adiposity on insulin sensitivity and adipokine concentrations in different equine breeds adapted to cereal-rich or fat-rich meals. Vet. J. 2016, 214, 14–20. [Google Scholar] [CrossRef]
- Pratt, S.E.; Geor, R.J.; McCutcheon, L.J. Effects of dietary energy source and physical conditioning on insulin sensitivity and glucose tolerance in Standardbred horses. Equine Vet. J. 2006, 38, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.W.; Burk, A.O.; Hartsock, T.G.; Petersen, E.D.; Whitley, N.C.; Treiber, K.H.; Boston, R.C. Insulin sensitivity in Thoroughbred geldings: Effect of weight gain, diet, and exercise on insulin sensitivity in Thoroughbred geldings. J. Equine Vet. Sci. 2008, 28, 728–738. [Google Scholar] [CrossRef]
- Chameroy, K.A.; Frank, N.; Elliott, S.B.; Boston, R.C. Comparison of plasma active glucagon-like peptide 1 concentrations in normal horses and those with equine Metabolic syndrome and in horses placed on a high-grain diet. J. Equine Vet. Sci. 2016, 40, 16–25. [Google Scholar] [CrossRef]
- Krause, J.B.; McKenzie, H.C., III. Parenteral nutrition in foals: A retrospective study of 45 cases (2000–2004). Equine Vet. J. 2007, 39, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Magdesian, K.G. Parenteral nutrition in the mature horse. Equine Vet. Educ. 2010, 22, 364–371. [Google Scholar] [CrossRef]
- Durham, A.E.; Rendle, D.I.; Newton, J.E. The effect of metformin on measurements of insulin sensitivity and beta cell response in 18 horses and ponies with insulin resistance. Equine Vet. J. 2008, 40, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.; Sommardahl, C.S.; Eiler, H.; Webb, L.L.; Denhart, J.W.; Boston, R.C. Effects of oral administration of levothyroxine sodium on concentrations of plasma lipids, concentration and composition of very-low-density lipoproteins, and glucose dynamics in healthy adult mares. Am. J. Vet. Res. 2005, 66, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Rendle, D.I.; Rutledge, F.; Hughes, K.J.; Heller, J.; Durham, A.E. Effects of metformin hydrochloride on blood glucose and insulin responses to oral dextrose in horses. Equine Vet. J. 2013, 45, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Tinworth, K.D.; Boston, R.C.; Harris, P.A.; Sillence, M.N.; Raidal, S.L.; Noble, G.K. The effect of oral metformin on insulin sensitivity in insulin-resistant ponies. Vet. J. 2012, 191, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Waitt, L.H.; Cebra, C.K. Characterization of hypertriglyceridemia and response to treatment with insulin in horses, ponies, and donkeys: 44 cases (1995–2005). J. Am. Vet. Med. Assoc. 2009, 234, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.P. Heparin treatment for aevere hypertriglyceridemia in diabetic ketoacidosis. Arch. Intern. Med. 2009, 169, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Vick, M.M.; Adams, A.A.; Murphy, B.A.; Sessions, D.R.; Horohov, D.W.; Cook, R.F.; Shelton, B.J.; Fitzgerald, B.P. Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse. J. Anim. Sci. 2007, 85, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Vick, M.M.; Murphy, B.A.; Sessions, D.R.; Reedy, S.E.; Kennedy, E.L.; Horohov, D.W.; Cook, R.F.; Fitzgerald, B.P. Effects of systemic inflammation on insulin sensitivity in horses and inflammatory cytokine expression in adipose tissue. Am. J. Vet. Res. 2008, 69, 130–139. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Stehouwer, C.D.; Emeis, J.J.; Coppack, S.W. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef]
- Treiber, K.; Carter, R.; Gay, L.; Williams, C.; Geor, R. Inflammatory and redox status of ponies with a history of pasture-associated laminitis. Vet. Immunol. Immunopathol. 2009, 129, 216–220. [Google Scholar] [CrossRef] [PubMed]
- de Laat, M.A.; Clement, C.K.; McGowan, C.M.; Sillence, M.N.; Pollitt, C.C.; Lacombe, V.A. Toll-like receptor and pro-inflammatory cytokine expression during prolonged hyperinsulinaemia in horses: Implications for laminitis. Vet. Immunol. Immunopathol. 2014, 157, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, M.P.; Lamon-Fava, S.; Fielding, R.A. Skeletal muscle lipid deposition and insulin resistance: Effect of dietary fatty acids and exercise. Am. J. Clin. Nutr. 2007, 85, 662–677. [Google Scholar] [CrossRef]
- Eckardt, K.; Taube, A.; Eckel, J. Obesity-associated insulin resistance in skeletal muscle: Role of lipid accumulation and physical inactivity. Rev. Endocr. Metab. Disord. 2011, 12, 163–172. [Google Scholar] [CrossRef]
- Einstein, F.H.; Huffman, D.M.; Fishman, S.; Jerschow, E.; Heo, H.J.; Atzmon, G.; Schechter, C.; Barzilai, N.; Muzumdar, R.H. Aging per se Increases the Susceptibility to Free Fatty Acid–Induced Insulin Resistance. J. Gerontol. Ser. A 2010, 65A, 800–808. [Google Scholar] [CrossRef]
- Treiber, K.H.; Kronfeld, D.S.; Hess, T.M.; Byrd, B.M.; Splan, R.K.; Staniar, W.B. Evaluation of genetic and Metabolic predispositions and nutritional risk factors for pasture-associated laminitis in ponies. J. Am. Vet. Med. Assoc. 2006, 228, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Geelen, S.N.; Jansen, W.L.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Breukink, H.J.; Beynen, A.C. Fat feeding increases equine heparin-released lipoprotein lipase activity. J. Vet. Intern. Med. 2001, 15, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.; Elliott, S.B.; Brandt, L.E.; Keisler, D.H. Physical characteristics, blood hormone concentrations, and plasma lipid concentrations in obese horses with insulin resistance. J. Am. Vet. Med. Assoc. 2006, 228, 1383–1390. [Google Scholar] [CrossRef]
- Carr, M.C.; Brunzell, J.D. Abdominal obesity and dyslipidemia in the Metabolic syndrome: Importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery Disease risk. J. Clin. Endocrinol. Metab. 2004, 89, 2601–2607. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.D.; Packard, C.J.; Shepherd, J. Plasma lipid transport in the horse (Equus caballus). Comp. Biochem. Physiol. B Comp. Biochem. 1993, 106, 27–34. [Google Scholar] [CrossRef]
- McCue, M.E.; Geor, R.J.; Schultz, N. Equine Metabolic syndrome: A complex Disease influenced by genetics and the environment. J. Equine Vet. Sci. 2015, 35, 367–375. [Google Scholar] [CrossRef]
- Perreault, M.; Zulyniak, M.A.; Badoud, F.; Stephenson, S.; Badawi, A.; Buchholz, A.; Mutch, D.M. A Distinct fatty acid profile underlies the reduced inflammatory state of Metabolically healthy obese individuals. PLoS ONE 2014, 9, e88539. [Google Scholar] [CrossRef]
- Calori, G.; Lattuada, G.; Piemonti, L.; Garancini, M.P.; Ragogna, F.; Villa, M.; Mannino, S.; Crosignani, P.; Bosi, E.; Luzi, L.; et al. Prevalence, Metabolic features, and prognosis of Metabolically healthy obese Italian individuals: The Cremona study. Diabetes Care 2010, 34, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M. Metabolically healthy obesity: Definitions, determinants and clinical implications. Rev. Endocr. Metab. Disord. 2013, 14, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Engelsen, C.D.; Gorter, K.J.; Salomé, P.L.; Rutten, G.E. Development of Metabolic syndrome components in adults with a healthy obese phenotype: A 3-year follow-up. Obesity 2013, 21, 1025–1030. [Google Scholar] [CrossRef]
- Schlaich, M.; Straznicky, N.; Lambert, E.; Lambert, G. Metabolic syndrome: A sympathetic Disease? Lancet Diabetes Endocrinol. 2015, 3, 148–157. [Google Scholar] [CrossRef]
- Carter, R.A.; Geor, R.J.; Burton Staniar, W.; Cubitt, T.A.; Harris, P.A. Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies. Vet. J. 2009, 179, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.A.; Treiber, K.H.; Geor, R.J.; Douglass, L.; Harris, P.A. Prediction of incipient pasture-associated laminitis from hyperinsulinaemia, hyperleptinaemia and generalised and localised obesity in a cohort of ponies. Equine Vet. J. 2009, 41, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer—A review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Papanas, N.; Ziegler, D. Risk factors and comorbidities in diabetic neuropathy: An update. Rev. Diabet. Stud. 2015, 12, 48–62. [Google Scholar] [CrossRef]
- Jones, E.; Viñuela-Fernandez, I.; Eager, R.A.; Delaney, A.; Anderson, H.; Patel, A.; Robertson, D.C.; Allchorne, A.; Sirinathsinghji, E.C.; Milne, E.M.; et al. Neuropathic changes in equine laminitis pain. Pain 2007, 132, 321–331. [Google Scholar] [CrossRef]
- Zamboulis, D.E.; Senior, M.; Clegg, P.D.; Milner, P.I. Expression of purinergic P2X receptor subtypes 1, 2, 3 and 7 in equine laminitis. Vet. J. 2013, 198, 472–478. [Google Scholar] [CrossRef]
- Driessen, B.; Bauquier, S.H.; Zarucco, L. Neuropathic pain management in chronic laminitis. Vet. Clin. N. Am. Equine Pract. 2010, 26, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Chang-Chen, K.J.; Mullur, R.; Bernal-Mizrachi, E. Beta-cell failure as a complication of diabetes. Rev. Endocr. Metab. Disord. 2008, 9, 329–343. [Google Scholar] [CrossRef]
- Rajaie, S.; Azadbakht, L.; Khazaei, M.; Sherbafchi, M.; Esmaillzadeh, A. Moderate replacement of carbohydrates by dietary fats affects features of Metabolic syndrome: A randomized crossover clinical trial. Nutrition 2014, 30, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Micha, R.; Wu, J.H.Y.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: A systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2013, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Ragno, V.M.; Zello, G.A.; Klein, C.D.; Montgomery, J.B. From Table to Stable: A Comparative Review of Selected Aspects of Human and Equine Metabolic Syndrome. J. Equine Vet. Sci. 2019, 79, 131–138. [Google Scholar] [CrossRef]
- Carr, E.A. Enteral/Parenteral Nutrition in Foals and Adult Horses Practical Guidelines for the Practitioner. Vet. Clin. N. Am. Equine Pract. 2018, 34, 169–180. [Google Scholar] [CrossRef]
- Golenz, M.R.; Knight, D.A.; Yvorchuk-St Jean, K.E. Use of a human enteral feeding preparation for treatment of hyperlipemia and nutritional support during healing of an esophageal laceration in a miniature horse. J. Am. Vet. Med. Assoc. 1992, 200, 951–953. [Google Scholar]
- Magdesian, K.G. Nutrition for critical gastrointestinal illness: Feeding horses with diarrhea or colic. Vet. Clin. N. Am. Equine Pract. 2003, 19, 617–644. [Google Scholar] [CrossRef]
- Lewis, S.J.; Andersen, H.K.; Thomas, S. Early Enteral Nutrition Within 24 h of Intestinal Surgery Versus Later Commencement of Feeding: A Systematic review and Meta-analysis. J. Gastrointest. Surg. 2008, 13, 569. [Google Scholar] [CrossRef]
- Borghouts, L.B.; Keizer, H.A. Exercise and insulin sensitivity: A review. Int. J. Sports Med. 2000, 21, 1–12. [Google Scholar] [CrossRef]
- Durham, A.E.; Hughes, K.J.; Cottle, H.J.; Rendle, D.I.; Boston, R.C. Type 2 diabetes mellitus with pancreatic β cell dysfunction in 3 horses confirmed with minimal model analysis. Equine Vet. J. 2009, 41, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, B.; Gulanick, M.; Lamendola, C. Risk factors for type 2 diabetes mellitus. J. Cardiovasc. Nurs. 2002, 16, 17–23. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.E. Endocrine Disease in Aged Horses. Vet. Clin. N. Am. Equine Pract. 2016, 32, 301–315. [Google Scholar] [CrossRef]
- Durham, A.E. Therapeutics for Equine Endocrine Disorders. Vet. Clin. N. Am. Equine Pract. 2017, 33, 127–139. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daradics, Z.; Crecan, C.M.; Rus, M.A.; Morar, I.A.; Mircean, M.V.; Cătoi, A.F.; Cecan, A.D.; Cătoi, C. Obesity-Related Metabolic Dysfunction in Dairy Cows and Horses: Comparison to Human Metabolic Syndrome. Life 2021, 11, 1406. https://doi.org/10.3390/life11121406
Daradics Z, Crecan CM, Rus MA, Morar IA, Mircean MV, Cătoi AF, Cecan AD, Cătoi C. Obesity-Related Metabolic Dysfunction in Dairy Cows and Horses: Comparison to Human Metabolic Syndrome. Life. 2021; 11(12):1406. https://doi.org/10.3390/life11121406
Chicago/Turabian StyleDaradics, Zsofia, Cristian M. Crecan, Mirela A. Rus, Iancu A. Morar, Mircea V. Mircean, Adriana Florinela Cătoi, Andra Diana Cecan, and Cornel Cătoi. 2021. "Obesity-Related Metabolic Dysfunction in Dairy Cows and Horses: Comparison to Human Metabolic Syndrome" Life 11, no. 12: 1406. https://doi.org/10.3390/life11121406
APA StyleDaradics, Z., Crecan, C. M., Rus, M. A., Morar, I. A., Mircean, M. V., Cătoi, A. F., Cecan, A. D., & Cătoi, C. (2021). Obesity-Related Metabolic Dysfunction in Dairy Cows and Horses: Comparison to Human Metabolic Syndrome. Life, 11(12), 1406. https://doi.org/10.3390/life11121406






