FIB-4 First in the Diagnostic Algorithm of Metabolic-Dysfunction-Associated Fatty Liver Disease in the Era of the Global Metabodemic
Abstract
:1. Introduction
2. Which Fibrosis Stage Should We Pick up in MAFLD?
| Index | Formula | Strengths | Weaknesses |
|---|---|---|---|
| FIB-4 index [15,16] | (age [years] × AST [U/L]/(platelet count [109/L] × √ALT [U/L]) https://www.eapharma.co.jp/medicalexpert/product/livact/fib-4/calculator.html (accessed on 25 January 2021) |
|
|
| NAFLD fibrosis score [26] | −1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio–0.013 × platelet count (×109/L) − 0.66 × albumin (g/dL) http://nafldscore.com/ (accessed on 25 January 2021) |
|
|
| APRI [27] | AST to platelet ratio index |
|
|
| BARD [28] | BMI > 28 kg/m2 = 1 point AST/ALT ratio > 0.8 = 2 points Diabetes = 1 point |
|
|
| CA-fibrosis index [29] | 1.5 × type IV collagen 7S (ng/mL) + 0.0264 × AST (IU/l) |
|
|
| ELF test [22] | −7.412 + (In [HA] × 0.681) + (In [P3NP] × 0.775) + (In [TIMP1] × 0.494) |
|
|
3. The Usefulness of FIB-4 Index to Evaluating Severe Fibrosis in MAFLD
4. The Compassion between FIB-4 Index and VCTE
5. FIB-4 Index and Carcinogenesis
6. FIB-4 Index and Mortality
7. FIB-4 Index and Risk of Cardiovascular Disease
8. FIB-4 Index and Risk of Chronic Kidney Disease
9. Distribution of FIB-4 Index in MAFLD Population
10. Drawbacks of FIB-4 Index
11. Two-Step Diagnostic Algorithm Using FIB-4 Index as the First Step
12. FIB-4 Index as Milestones of Treatment in MAFLD
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AASLD | American Association for the Study of Liver Diseases |
| AF | atrial fibrillation |
| AFP | α-Fetoprotein |
| AFP-L3 | lens culinaris-agglutinin-reactive fraction of AFP |
| AGA | American Gastroenterology Association |
| AIM | apoptosis inhibitor of macrophage |
| AST | aspartate aminotransferase |
| ALD | alcoholic liver disease |
| ALT | alanine aminotransferase |
| APRI | AST to platelet ratio index |
| ARFI | acoustic radiation force impulse |
| AUROC | area under receiver operating characteristics curve |
| BMI | body mass index |
| HA | hyaluronic acid |
| PIIINP | aminoterminal propeptide of type III procollagen |
| TIMP-1 | tissue inhibitor of matrix metalloproteinase type 1 |
| CAC | coronary artery calcium |
| CAD | coronary artery disease |
| CAP | controlled attenuation parameter |
| CHF | congestive heart failure |
| CKD | chronic kidney disease |
| COI | cutoff index |
| CVD | cardiovascular disease |
| CI | confidence interval |
| CT | computed tomography |
| DILI | drug induced liver injury |
| eGFR | estimated glomerular filtration rate |
| ELF | enhanced liver fibrosis |
| ELISA | enzyme linked immunosolvent assay |
| FAST | FibroScan–AST |
| FIB-4 | Fibrosis-4 |
| GGT | gamma glutamyltransferas |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| HCC | hepatocellular carcinoma |
| HF | heart failure |
| HIV | human immunodeficiency virus |
| HR | hazard ratio |
| LSM | liver stiffness measurement |
| MAFLD | metabolism dysfunction associated fatty liver disease |
| M2BPGi | Mac-2 binding protein glycosylation isomer |
| MRE | magnetic resonance elastography |
| MRI | magnetic resonance imaging |
| NAFL | nonalcoholic fatty liver |
| NAFLD | nonalcoholic fatty liver disease |
| NASH | nonalcoholic steatohepatitis |
| NFS | NAFLD fibrosis score |
| NIT | non-invasive test |
| NPV | negative predictive value |
| OCA | obeticholic acid |
| OR | odds ratio |
| PDFF | proton density fat fraction |
| PIIINP | aminoterminal propeptide of type III procollagen |
| PNPLA3 | patatin-like phospholipase domain-containing protein 3 |
| PPV | positive predictive value |
| SLKT | simultaneous liver kidney transplantation |
| TIMP-1 | tissue inhibitor of matrix metalloproteinase type 1, |
| T2D | type 2 diabetes |
| UCAP | ultrasound-guided attenuation parameter |
| US | ultrasonography |
| VCTE | vibration-controlled transient elastography |
References
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- VanWagner, L.B.; Armstrong, M.J. Lean NAFLD: A not so benign condition? Hepatol. Commun. 2018, 2, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Negro, F.; Hallaji, S.; Younossi, Y.; Lam, B.; Srishord, M. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine 2012, 91, 319–327. [Google Scholar] [CrossRef]
- Fan, J.G.; Kim, S.U.; Wong, V.W. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017, 67, 862–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.L.; Leung, J.C.; Loong, T.C.; Wong, G.L.; Yeung, D.K.; Chan, R.S.; Chan, H.L.; Chim, A.M.; Woo, J.; Chu, W.C.; et al. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. Am. J. Gastroenterol. 2015, 110, 1306–1315. [Google Scholar] [CrossRef]
- Chen, F.; Esmaili, S.; Rogers, G.B.; Bugianesi, E.; Petta, S.; Marchesini, G.; Bayoumi, A.; Metwally, M.; Azardaryany, M.K.; Coulter, S.; et al. Lean NAFLD: A Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatology 2020, 71, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Nishioji, K.; Mochizuki, N.; Kobayashi, M.; Kamaguchi, M.; Sumida, Y.; Nishimura, T.; Yamaguchi, K.; Kadotani, H.; Itoh, Y. The Impact of PNPLA3 rs738409 Genetic Polymorphism and Weight Gain ≥10 kg after Age 20 on Non-Alcoholic Fatty Liver Disease in Non-Obese Japanese Individuals. PLoS ONE 2015, 10, e0140427. [Google Scholar] [CrossRef] [PubMed]
- Fracanzani, A.L.; Petta, S.; Lombardi, R.; Pisano, G.; Russello, M.; Consonni, D.; Di Marco, V.; Cammà, C.; Mensi, L.; Dongiovanni, P.; et al. Liver and Cardiovascular Damage in Patients With Lean Nonalcoholic Fatty Liver Disease, and Association with Visceral Obesity. Clin. Gastroenterol. Hepatol. 2017, 15, 1604–1611. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Eguchi, Y.; Wong, G.; Akhtar, O.; Sumida, Y. Non-invasive diagnosis of nonalcoholic steatohepatitis (NASH) and advanced fibrosis in Japan: A targeted literature review. Hepatol. Res. 2020, in press. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J.; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [Green Version]
- Sumida, Y.; Yoneda, M.; Hyogo, H.; Itoh, Y.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD); et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012, 12, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020, 158, 1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumida, Y.; Yoneda, M.; Seko, Y.; Ishiba, H.; Hara, T.; Toyoda, H.; Yasuda, S.; Kumada, T.; Hayashi, H.; Kobayashi, T.; et al. Japan Study Group of NAFLD (JSG-NAFLD). Surveillance of Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. Diagnostics 2020, 10, 579. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Chalasani, N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol. 2018, 68, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Vali, Y.; Lee, J.; Boursier, J.; Spijker, R.; Löffler, J.; Verheij, J.; Brosnan, M.J.; Böcskei, Z.; Anstee, Q.M.; LITMUS Systematic Review Team; et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 2020, 73, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Gailer, R.; Tanwar, S.; Trembling, P.; Parkes, J.; Rodger, A.; Suri, D.; Thorburn, D.; Sennett, K.; Morgan, S.; et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J. Hepatol. 2019, 71, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inadomi, C.; Takahashi, H.; Ogawa, Y.; Inadomi, C.; Takahashi, H.; Ogawa, Y.; Oeda, S.; Imajo, K.; Kubotsu, Y.; Tanaka, K.; et al. Accuracy of the Enhanced Liver Fibrosis test, and combination of the Enhanced Liver Fibrosis and non-invasive tests for the diagnosis of advanced liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2020, 50, 682–692. [Google Scholar] [CrossRef]
- Crossan, C.; Majumdar, A.; Srivastava, A.; Thorburn, D.; Rosenberg, W.; Pinzani, M.; Longworth, L.; Tsochatzis, E.A. Referral pathways for patients with NAFLD based on non-invasive fibrosis tests: Diagnostic accuracy and cost analysis. Liver Int. 2019, 39, 2052–2060. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Peleg, N.; Issachar, A.; Sneh-Arbib, O.; Shlomai, A. AST to platelet ratio index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig. Liver Dis. 2017, 49, 1133–1138. [Google Scholar] [CrossRef]
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef]
- Okanoue, T.; Ebise, H.; Kai, T.; Mizuno, M.; Shima, T.; Ichihara, J.; Aoki, M. A simple scoring system using type IV collagen 7S and aspartate aminotransferase for diagnosing nonalcoholic steatohepatitis and related fibrosis. J. Gastroenterol. 2018, 53, 129–139. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2017, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Beste, L.A.; Green, P.K.; Singal, A.G.; Tapper, E.B.; Waljee, A.K.; Sterling, R.K.; Feld, J.J.; Kaplan, D.E.; Taddei, T.H.; et al. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology 2019, 157, 1264–1278. [Google Scholar] [CrossRef] [Green Version]
- Tseng, T.C.; Liu, C.J.; Su, T.H.; Yang, W.T.; Chen, C.L.; Yang, H.C.; Kuo, S.F.; Liu, C.H.; Chen, P.J.; Chen, D.S.; et al. Fibrosis-4 index predicts cirrhosis risk and liver-related mortality in 2075 patients with chronic HBV infection. Aliment. Pharmacol. Ther. 2018, 47, 1480–1489. [Google Scholar] [CrossRef] [Green Version]
- Tseng, T.C.; Liu, C.J.; Su, T.H.; Yang, W.T.; Chen, C.L.; Yang, H.C.; Wang, C.C.; Kuo, S.F.; Liu, C.H.; Chen, P.J.; et al. Fibrosis-4 Index Helps Identify HBV Carriers With the Lowest Risk of Hepatocellular Carcinoma. Am. J. Gastroenterol. 2017, 112, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Nishijima, N.; Enomoto, H.; Sakamoto, A.; Nasu, A.; Komekado, H.; Nishimura, T.; Kita, R.; Kimura, T.; Iijima, H.; et al. Comparison of FIB-4 index and aspartate aminotransferase to platelet ratio index on carcinogenesis in chronic hepatitis B treated with entecavir. J. Cancer 2017, 8, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.A.; Gifford, E.J.; Glass, L.M.; Turner, M.J.; Han, B.; Moylan, C.A.; Choi, S.; Suzuki, A.; Provenzale, D.; Hunt, C.M. Identifying Nonalcoholic Fatty Liver Disease Advanced Fibrosis in the Veterans Health Administration. Dig. Dis. Sci. 2018, 63, 2259–2266. [Google Scholar] [CrossRef]
- McPherson, S.; Stewart, S.F.; Henderson, E.; Burt, A.D.; Day, C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef] [Green Version]
- De Carli, M.A.; de Carli, L.A.; Correa, M.B.; Junqueira GJr Tovo, C.V.; Coral, G.P. Performance of noninvasive scores for the diagnosis of advanced liver fibrosis in morbidly obese with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2020, 32, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Kumada, T.; Toyoda, H.; Tada, T.; Ito, T.; Kage, M.; Okanoue, T.; Kudo, M. Ability of Cytokeratin-18 Fragments and FIB-4 Index to Diagnose Overall and Mild Fibrosis Nonalcoholic Steatohepatitis in Japanese Nonalcoholic Fatty Liver Disease Patients. Dig. Dis. 2017, 35, 521–530. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Anstee, Q.M.; Henderson, E.; Day, C.P.; Burt, A.D. Are simple noninvasive scoring systems for fibrosis reliable in patients with NAFLD and normal ALT levels? Eur. J. Gastroenterol. Hepatol. 2013, 25, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, S.; Zhang, J.; Dong, M.; Wang, Y.; Wang, M.; Xin, Y. Proportion of NAFLD patients with normal ALT value in overall NAFLD patients: A systematic review and meta-analysis. BMC Gastroenterol. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneda, M.; Imajo, K.; Eguchi, Y.; Fujii, H.; Sumida, Y.; Hyogo, H.; Ono, M.; Suzuki, Y.; Kawaguchi, T.; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD); et al. Noninvasive scoring systems in patients with nonalcoholic fatty liver disease with normal alanine aminotransferase levels. J. Gastroenterol. 2013, 48, 1051–1060. [Google Scholar] [CrossRef]
- Honda, Y.; Yoneda, M.; Imajo, K.; Nakajima, A. Elastography Techniques for the Assessment of Liver Fibrosis in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 4039. [Google Scholar] [CrossRef]
- Kwok, R.; Tse, Y.K.; Wong, G.L.; Ha, Y.; Lee, A.U.; Ngu, M.C.; Chan, H.L.; Wong, V.W. Systematic review with meta-analysis: Non-invasive assessment of non-alcoholic fatty liver disease--the role of transient elastography and plasma cytokeratin-18 fragments. Aliment. Pharmacol. Ther. 2014, 39, 254–269. [Google Scholar] [CrossRef]
- Petta, S.; Wai-Sun Wong, V.; Bugianesi, E.; Fracanzani, A.L.; Cammà, C.; Hiriart, J.B.; Lai-Hung Wong, G.; Vergniol, J.; Wing-Hung Chan, A.; Giannetti, A.; et al. Impact of Obesity and Alanine Aminotransferase Levels on the Diagnostic Accuracy for Advanced Liver Fibrosis of Noninvasive Tools in Patients With Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2019, 114, 916–928. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281. [Google Scholar] [CrossRef] [Green Version]
- Davyduke, T.; Tandon, P.; Al-Karaghouli, M.; Abraldes, J.G.; Ma, M.M. Impact of Implementing a “FIB-4 First” Strategy on a Pathway for Patients with NAFLD Referred From Primary Care. Hepatol. Commun. 2019, 3, 1322–1333. [Google Scholar] [CrossRef] [Green Version]
- Sumida, Y.; Shima, T.; Mitsumoto, Y.; Katayama, T.; Umemura, A.; Yamaguchi, K.; Itoh, Y.; Yoneda, M.; Okanoue, T. Epidemiology, Pathogenesis, and Diagnostic Strategy of Diabetic Liver Disease in Japan. Int. J. Mol. Sci. 2020, 21, 4337. [Google Scholar] [CrossRef]
- Yoneda, M.; Imajo, K.; Takahashi, H.; Ogawa, Y.; Eguchi, Y.; Sumida, Y.; Yoneda, M.; Kawanaka, M.; Saito, S.; Tokushige, K.; et al. Clinical strategy of diagnosing and following patients with nonalcoholic fatty liver disease based on invasive and noninvasive methods. J. Gastroenterol. 2018, 53, 181. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.K.; Treeprasertsuk, S.; Goh, G.B.; Fan, J.G.; Song, M.J.; Charatcharoenwitthaya, P.; Duseja, A.; Dan, Y.Y.; Imajo, K.; Nakajima, A.; et al. Optimizing Use of Nonalcoholic Fatty Liver Disease Fibrosis Score, Fibrosis-4 Score, and Liver Stiffness Measurement to Identify Patients with Advanced Fibrosis. Clin. Gastroenterol. Hepatol. 2019, 17, 2570–2580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, R.; Yang, X. FIB-4 index serves as a noninvasive prognostic biomarker in patients with hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore) 2018, 97, e13696. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.A.; Lee, H.C.; Choe, J.; Kim, M.J.; Lee, M.J.; Chang, H.S.; Bae, I.Y.; Kim, H.K.; An, J.; Shim, J.H.; et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2018, 68, 104–146. [Google Scholar] [CrossRef]
- Peleg, N.; Sneh Arbib, O.; Issachar, A.; Cohen-Naftaly, M.; Braun, M.; Shlomai, A. Noninvasive scoring systems predict hepatic and extra-hepatic cancers in patients with nonalcoholic fatty liver disease. PLoS ONE 2018, 13, e0202393. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ishigami, M.; Ishizu, Y.; Kuzuya, T.; Honda, T.; Hayashi, K.; Nishimura, D.; Toyoda, H.; Kumada, T.; Goto, H.; et al. Utility and limitations of noninvasive fibrosis markers for predicting prognosis in biopsy-proven Japanese non-alcoholic fatty liver disease patients. J. Gastroenterol. Hepatol. 2019, 34, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angulo, P.; Bugianesi, E.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Barrera, F.; Haflidadottir, S.; Day, C.P.; George, J. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 782–789. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kim, W.R.; Kim, H.J.; Therneau, T.M. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013, 57, 1357–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unalp-Arida, A.; Ruhl, C.E. Liver fibrosis scores predict liver disease mortality in the United States population. Hepatology 2017, 66, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Stål, P.; Hultcrantz, R.; Kechagias, S. Accuracy of Noninvasive Scoring Systems in Assessing Risk of Death and Liver-Related Endpoints in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 1148–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertot, L.C.; Jeffrey, G.P.; de Boer, B.; MacQuillan, G.; Garas, G.; Chin, J.; Huang, Y.; Adams, L.A. Diabetes impacts prediction of cirrhosis and prognosis by non-invasive fibrosis models in non-alcoholic fatty liver disease. Liver Int. 2018, 38, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Mizuno, K.; Sone, Y.; Akita, T.; Tanaka, J. Progression of liver fibrosis is associated with non-liver-related mortality in patients with nonalcoholic fatty liver disease. Hepatol. Commun. 2017, 1, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.U.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Park, Y.B.; Han, K.H.; Lee, S.W. Fibrosis-4 index at diagnosis can predict all-cause mortality in patients with rheumatoid arthritis: A retrospective monocentric study. Mod. Rheumatol. 2020, 30, 70–77. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.Y.; Jung, S.M.; Song, J.J.; Park, Y.B.; Lee, S.W. Fibrosis-4 index at diagnosis is associated with all-cause mortality in patients with microscopic polyangiitis and granulomatosis with polyangiitis. BMC Gastroenterol. 2019, 19, 90. [Google Scholar] [CrossRef] [Green Version]
- Yong, S.H.; Leem, A.Y.; Kim, Y.S.; Park, M.S.; Chang, J.; Kim, S.U.; Jung, J.Y. Hepatic Fibrosis Assessed Using Fibrosis-4 Index Is Predictive of All-Cause Mortality in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 831–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xun, Y.H.; Guo, J.C.; Lou, G.Q.; Jiang, Y.M.; Zhuang, Z.J.; Zhu, M.F.; Luo, Y.; Ma, X.J.; Liu, J.; Bian, D.X.; et al. Non-alcoholic fatty liver disease (NAFLD) fibrosis score predicts 6.6-year overall mortality of Chinese patients with NAFLD. Clin. Exp. Pharmacol. Physiol. 2014, 41, 643–649. [Google Scholar] [PubMed]
- Sebastiani, G.; Alshaalan, R.; Wong, P.; Rubino, M.; Salman, A.; Metrakos, P.; Deschenes, M.; Ghali, P. Prognostic Value of Non-Invasive Fibrosis and Steatosis Tools, Hepatic Venous Pressure Gradient (HVPG) and Histology in Nonalcoholic Steatohepatitis. PLoS ONE 2015, 10, e0128774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, J.P.; Pitts, A.; Younossi, Z.M. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J. Hepatol. 2008, 49, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Park, G.M.; Lee, J.Y.; Lee, B.U.; Park, J.H.; Kim, B.G.; Jung, S.W.; Jeong, I.D.; Bang, S.J.; Shin, J.W.; et al. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: An observational cohort study. J. Hepatol. 2018, 68, 1018–1024. [Google Scholar] [CrossRef]
- Käräjämäki, A.J.; Pätsi, O.P.; Savolainen, M.; Kesäniemi, Y.A.; Huikuri, H.; Ukkola, O. Non-Alcoholic Fatty Liver Disease as a Predictor of Atrial Fibrillation in Middle-Aged Population (OPERA Study). PLoS ONE 2015, 10, e0142937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Pernigo, M.; Bergamini, C.; Bonapace, S.; Lipari, P.; Pichiri, I.; Bertolini, L.; Valbusa, F.; Barbieri, E.; Zoppini, G.; et al. Nonalcoholic Fatty Liver Disease Is Independently Associated with Early Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. PLoS ONE 2015, 10, e0135329. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.E.; Lee, J.H.; Lee, H.; Kim, M.K.; Yim, J.Y.; Choi, S.Y.; Kim, Y.J.; Yoon, J.H.; Kim, D. Nonalcoholic fatty liver disease and advanced fibrosis are associated with left ventricular diastolic dysfunction. Atherosclerosis 2018, 272, 137–144. [Google Scholar] [CrossRef]
- Baratta, F.; Pastori, D.; Angelico, F.; Balla, A.; Paganini, A.M.; Cocomello, N.; Ferro, D.; Violi, F.; Sanyal, A.J.; Del Ben, M. Nonalcoholic Fatty Liver Disease and Fibrosis Associated With Increased Risk of Cardiovascular Events in a Prospective Study. Clin. Gastroenterol. Hepatol. 2020, 18, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Okumura, Y.; Nagashima, K.; Fukamachi, D.; Yokoyama, K.; Matsumoto, N.; Tachibana, E.; Kuronuma, K.; Oiwa, K.; Matsumoto, M.; et al. Impact of the Fibrosis-4 Index on Risk Stratification of Cardiovascular Events and Mortality in Patients with Atrial Fibrillation: Findings from a Japanese Multicenter Registry. J. Clin. Med. 2020, 9, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Li, Q.; Li, D.; Chen, X.; Liu, Z.; Hu, G.; Wang, J.; Ling, W. Association between liver fibrosis scores and the risk of mortality among patients with coronary artery disease. Atherosclerosis 2020, 299, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ishiba, H.; Sumida, Y.; Kataoka, S.; Kuroda, M.; Akabame, S.; Tomiyasu, K.; Tanaka, M.; Arai, M.; Taketani, H.; Seko, Y.; et al. Association of coronary artery calcification with liver fibrosis in Japanese patients with non-alcoholic fatty liver disease. Hepatol. Res. 2016, 46, 1107–1117. [Google Scholar] [CrossRef]
- Song, D.S.; Chang, U.I.; Kang, S.G.; Song, S.W.; Yang, J.M. Noninvasive Serum Fibrosis Markers are Associated with Coronary Artery Calcification in Patients with Nonalcoholic Fatty Liver Disease. Gut Liver 2019, 13, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshihisa, A.; Kanno, Y.; Watanabe, S.; Yokokawa, T.; Abe, S.; Misaka, T.; Sato, T.; Suzuki, S.; Oikawa, M.; et al. Liver stiffness assessed by Fibrosis-4 index predicts mortality in patients with heart failure. Open Heart 2017, 4, e000598. [Google Scholar] [CrossRef] [PubMed]
- So-Armah, K.A.; Lim, J.K.; Lo Re, V.; Tate, J.P.; Chang, C.H.; Butt, A.A.; Gibert, C.L.; Rimland, D.; Marconi, V.C.; Goetz, M.B.; et al. Veterans Aging Cohort Study Project Team. FIB-4 stage of liver fibrosis predicts incident heart failure among HIV-infected and uninfected patients. Hepatology 2017, 66, 1286–1295. [Google Scholar] [CrossRef] [Green Version]
- Singal, A.K.; Hasanin, M.; Kaif, M.; Wiesner, R.; Kuo, Y.F. Nonalcoholic Steatohepatitis is the Most Rapidly Growing Indication for Simultaneous Liver Kidney Transplantation in the United States. Transplantation 2016, 100, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Tabibian, J.H.; Ekstedt, M.; Kechagias, S.; Hamaguchi, M.; Hultcrantz, R.; Hagström, H.; Yoon, S.K.; Charatcharoenwitthaya, P.; et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001680. [Google Scholar] [CrossRef] [Green Version]
- Sumida, Y.; Yoneda, M.; Toyoda, H.; Yasuda, S.; Tada, T.; Hayashi, H.; Nishigaki, Y.; Suzuki, Y.; Naiki, T.; Morishita, A.; et al. Japan Study Group of NAFLD (JSG-NAFLD). Common Drug Pipelines for the Treatment of Diabetic Nephropathy and Hepatopathy: Can We Kill Two Birds with One Stone? Int. J. Mol. Sci. 2020, 21, E4939. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Cohney, S.; De Michieli, F.; Pinach, S.; Saba, F.; Gambino, R. Fatty Liver and Chronic Kidney Disease: Novel Mechanistic Insights and Therapeutic Opportunities. Diabetes Care 2016, 39, 1830–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijarnpreecha, K.; Thongprayoon, C.; Scribani, M.; Ungprasert, P.; Cheungpasitporn, W. Noninvasive fibrosis markers and chronic kidney disease among adults with nonalcoholic fatty liver in USA. Eur. J. Gastroenterol. Hepatol. 2018, 30, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Arase, Y.; Suzuki, F.; Kobayashi, M.; Suzuki, Y.; Kawamura, Y.; Matsumoto, N.; Akuta, N.; Kobayashi, M.; Sezaki, H.; Saito, S.; et al. The development of chronic kidney disease in Japanese patients with non-alcoholic fatty liver disease. Intern. Med. 2011, 50, 1081–1087. [Google Scholar] [CrossRef] [Green Version]
- Seko, Y.; Yano, K.; Takahashi, A.; Okishio, S.; Kataoka, S.; Okuda, K.; Mizuno, N.; Takemura, M.; Taketani, H.; Umemura, A.; et al. FIB-4 Index and Diabetes Mellitus Are Associated with Chronic Kidney Disease in Japanese Patients with Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2019, 21, 171. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Taliento, A.; Zusi, C.; Baselli, G.; Prati, D.; Granata, S.; Zaza, G.; Colecchia, A.; Maffeis, C.; Byrne, C.D.; et al. PNPLA3 I148M gene variant and chronic kidney disease in type 2 diabetic patients with NAFLD: Clinical and experimental findings. Liver Int. 2020, 40, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.Q.; Zheng, K.I.; Xu, G.; Ma, H.L.; Zhang, H.Y.; Pan, X.Y.; Zhu, P.W.; Wang, X.D.; Targher, G.; Byrne, C.D.; et al. PNPLA3 rs738409 is associated with renal glomerular and tubular injury in NAFLD patients with persistently normal ALT levels. Liver Int. 2020, 40, 107–119. [Google Scholar] [CrossRef]
- Wada, T.; Zeniya, M. Background of the FIB-4 index in Japanese non-alcoholic fatty liver disease. Intern. Med. 2015, 54, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Sumida, Y.; Sakuragi, S.; Hibino, S.; Furutani, M. Diatribution of FIB4 index in Japanese Nonalcoholic fatty liver disease population: A multi-center study. Ningen Dock Int. 2015, 2, 32–34. [Google Scholar]
- McPherson, S.; Hardy, T.; Dufour, J.F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.; et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiba, H.; Sumida, Y.; Tanaka, S.; Yoneda, M.; Hyogo, H.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Yoneda, M.; et al. Japan Study Group of Non-Alcoholic Fatty Liver Disease (JSG-NAFLD). The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: A multi-center study. J. Gastroenterol. 2018, 53, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Pitisuttithum, P.; Chan, W.K.; Piyachaturawat, P.; Imajo, K.; Nakajima, A.; Seki, Y.; Kasama, K.; Kakizaki, S.; Fan, J.G.; Song, M.J.; et al. Predictors of advanced fibrosis in elderly patients with biopsy-confirmed nonalcoholic fatty liver disease: The GOASIA study. BMC Gastroenterol. 2020, 20, 88. [Google Scholar] [CrossRef] [PubMed]
- Blond, E.; Disse, E.; Cuerq, C.; Drai, J.; Valette, P.J.; Laville, M.; Thivolet, C.; Simon, C.; Caussy, C. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease in severely obese people: Do they lead to over-referral? Diabetologia 2017, 60, 1218–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, S.K.; Kim, W.; Kim, D.; Kim, J.H.; Oh, S.; Lee, K.L.; Chang, M.S.; Jung, Y.J.; So, Y.H.; Lee, M.S.; et al. Steatosis severity affects the diagnostic performances of noninvasive fibrosis tests in nonalcoholic fatty liver disease. Liver Int. 2018, 38, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ishiba, Y.; Sumida, Y.; Tanaka, S.; Yoneda, M.; Hyogo, H.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Japan Study Group of Non-Alcoholic Fatty Liver Disease (JSG-NAFLD); et al. Type IV collagen 7S is the most accurate test for identifying advanced fibrosis in non-alcoholic fatty liver disease with type 2 diabetes. Hepatol. Commun. 2020, in press. [Google Scholar] [CrossRef]
- Singh, A.; Gosai, F.; Siddiqui, M.T.; Gupta, M.; Lopez, R.; Lawitz, E.; Poordad, F.; Carey, W.; McCullough, A.; Alkhouri, N. Accuracy of Noninvasive Fibrosis Scores to Detect Advanced Fibrosis in Patients With Type-2 Diabetes With Biopsy-proven Nonalcoholic Fatty Liver Disease. J. Clin. Gastroenterol. 2020, in press. [Google Scholar] [CrossRef]
- Shah, S.; Dhami-Shah, H.; Kamble, S.; Shukla, A. FIB-4 cut-off of 1.3 may be inappropriate in a primary care referral pathway for patients with non-alcoholic fatty liver disease. J. Hepatol. 2020, 73, 216–217. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M.; Ogawa, Y.; Yoneda, M.; Okanoue, T.; Nakajima, A.; Japan Study Group of NAFLD (JSG-NAFLD). Current and new pharmacotherapy options for non-alcoholic steatohepatitis. Expert Opin. Pharmacother. 2020, 21, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef] [Green Version]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.H.; Wong, V.W.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Oeda, S.; Takahashi, H.; Imajo, K.; Seko, Y.; Kobayashi, T.; Ogawa, Y.; Moriguchi, M.; Yoneda, M.; Anzai, K.; Irie, H.; et al. Diagnostic accuracy of FibroScan-AST score to identify non-alcoholic steatohepatitis with significant activity and fibrosis in Japanese patients with non-alcoholic fatty liver disease: Comparison between M and XL probes. Hepatol. Res. 2020, 50, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, N.; Alkhouri, N.; Brown, K.A.; Noureddin, M. Driving NASH forward using the FAST score but obey the traffic lights. Hepatology 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Miyake, T.; Kuno, A.; Imai, Y.; Sawai, Y.; Hino, K.; Hara, Y.; Hige, S.; Sakamoto, M.; Yamada, G.; et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J. Gastroenterol. 2015, 50, 776–784. [Google Scholar] [CrossRef]
- Ogawa, Y.; Honda, Y.; Kessoku, T.; Tomeno, W.; Imajo, K.; Yoneda, M.; Kawanaka, M.; Kirikoshi, H.; Ono, M.; Taguri, M.; et al. Wisteria floribunda agglutinin-positive Mac-2-binding protein and type 4 collagen 7S: Useful markers for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2018, 33, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jun, D.W.; Park, H.; Kang, B.K.; Sumida, Y. Sequential Combination of FIB-4 Followed by M2BPGi Enhanced Diagnostic Performance for Advanced Hepatic Fibrosis in an Average Risk Population. J. Clin. Med. 2020, 9, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, S.J.; Leeming, D.J.; Eslam, M.; Hashem, A.M.; Nielsen, M.J.; Krag, A.; Karsdal, M.A.; Grove, J.I.; Neil Guha, I.; Kawaguchi, T.; et al. ADAPT: An Algorithm Incorporating PRO-C3 Accurately Identifies Patients With NAFLD and Advanced Fibrosis. Hepatology 2019, 69, 1075–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimori, N.; Umemura, T.; Kimura, T.; Tanaka, N.; Sugiura, A.; Yamazaki, T.; Joshita, S.; Komatsu, M.; Usami, Y.; Sano, K.; et al. Serum autotaxin levels are correlated with hepatic fibrosis and ballooning in patients with non-alcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Imajo, K.; Kobayashi, T.; Kessoku, T.; Ogawa, Y.; Tomeno, W.; Yoneda, M.; Kobayashi, N.; Saito, S.; Nakajima, A. Autotaxin is a valuable biomarker for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2019, 49, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Liu, S.; Du, S.; Zhang, Q.; Xiao, J.; Dong, Q.; Xin, Y. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: A meta-analysis. Eur. Radiol. 2019, 29, 3564–3573. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Kobayashi, N.; Sone, Y.; Oguri, T.; Kamiyama, N. Utility of Attenuation Coefficient Measurement Using an Ultrasound-Guided Attenuation Parameter for Evaluation of Hepatic Steatosis: Comparison With MRI-Determined Proton Density Fat Fraction. AJR Am. J. Roentgenol. 2019, 212, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Yamada, G.; Vuppalanchi, R.; Van Natta, M.; Loomba, R.; Guy, C.; Brandman, D.; Tonascia, J.; Chalasani, N.; Neuschwander-Tetri, B.; et al. Diagnostic Accuracy of Noninvasive Fibrosis Models to Detect Change in Fibrosis Stage. Clin. Gastroenterol. Hepatol. 2019, 17, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Abdelmalek, M.F.; Loomba, R.; Kowdley, K.V.; McCullough, A.J.; Dasarathy, S.; Neuschwander-Tetri, B.A.; Terrault, N.; Ferguson, B.; Shringarpure, R.; et al. Relationship between three commonly used non-invasive fibrosis biomarkers and improvement in fibrosis stage in patients with non-alcoholic steatohepatitis. Liver Int. 2019, 39, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, E.; Takamura, T.; Sakurai, M.; Mizukoshi, E.; Zen, Y.; Takeshita, Y.; Kurita, S.; Arai, K.; Yamashita, T.; Sasaki, M.; et al. Histological course of nonalcoholic fatty liver disease in Japanese patients: Tight glycemic control, rather than weight reduction, ameliorates liver fibrosis. Diabetes Care 2010, 33, 284–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Yamaguchi, K.; Moriguchi, M.; et al. Serum alanine aminotransferase predicts the histological course of non-alcoholic steatohepatitis in Japanese patients. Hepatol. Res. 2015, 45, E53-61. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H.; Van Natta, M.L.; Kleiner, D.E.; Clark, J.M.; Kowdley, K.V.; Loomba, R.; Neuschwander-Tetri, B.A.; Sanyal, A.J.; Tonascia, J.; Non-alcoholic Steatohepatitis Clinical Research Network (NASH CRN). Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2013, 38, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilar-Gomez, E.; Yasells-Garcia, A.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Villa-Jimenez, O.; Friedman, S.L.; Diago, M.; et al. Development and validation of a noninvasive prediction model for nonalcoholic steatohepatitis resolution after lifestyle intervention. Hepatology 2016, 63, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Jain, A.K.; Deppe, R.; Yates, K.; Comerford, M.; Masuoka, H.C.; Neuschwander-Tetri, B.A.; Loomba, R.; Brunt, E.M.; Kleiner, D.E.; et al. Relationship between changes in serum levels of keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2014, 12, e1–e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayakumar, S.; Middleton, M.S.; Lawitz, E.J.; Mantry, P.S.; Caldwell, S.H.; Arnold, H.; Mae Diehl, A.; Ghalib, R.; Elkhashab, M.; Abdelmalek, M.F.; et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J. Hepatol. 2019, 70, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Terrault, N.; Chalasani, N.P.; Abdelmalek, M.F.; McCullough, A.J.; Shringarpure, R.; Ferguson, B.; Lee, L.; et al. Factors Associated With Histologic Response in Adult Patients With Nonalcoholic Steatohepatitis. Gastroenterology 2019, 156, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Jong, S.; Gola, A.; Srivastava, A.; Jong, S.; Gola, A.; Gailer, R.; Morgan, S.; Sennett, K.; Tanwar, S.; et al. Cost-comparison analysis of FIB-4, ELF and fibroscan in community pathways for non-alcoholic fatty liver disease. BMC Gastroenterol. 2019, 19, 122. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.J.; Banh, X.; Horsfall, L.U.; Hayward, K.L.; Hossain, F.; Johnson, T.; Stuart, K.A.; Brown, N.N.; Saad, N.; Clouston, A.; et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: Limited awareness of surrogate markers of fibrosis. Intern. Med. J. 2018, 48, 144–151. [Google Scholar] [CrossRef] [PubMed]
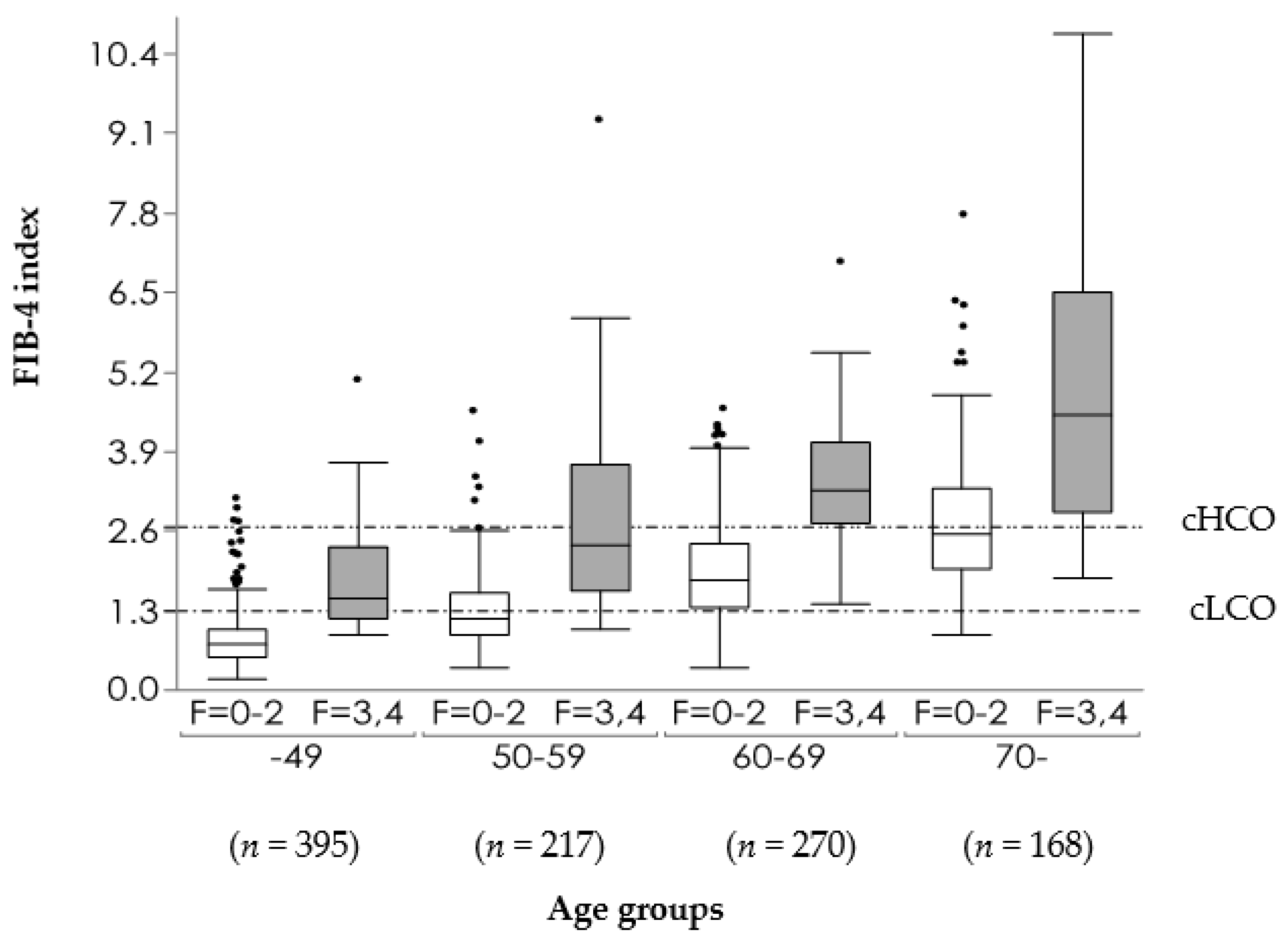

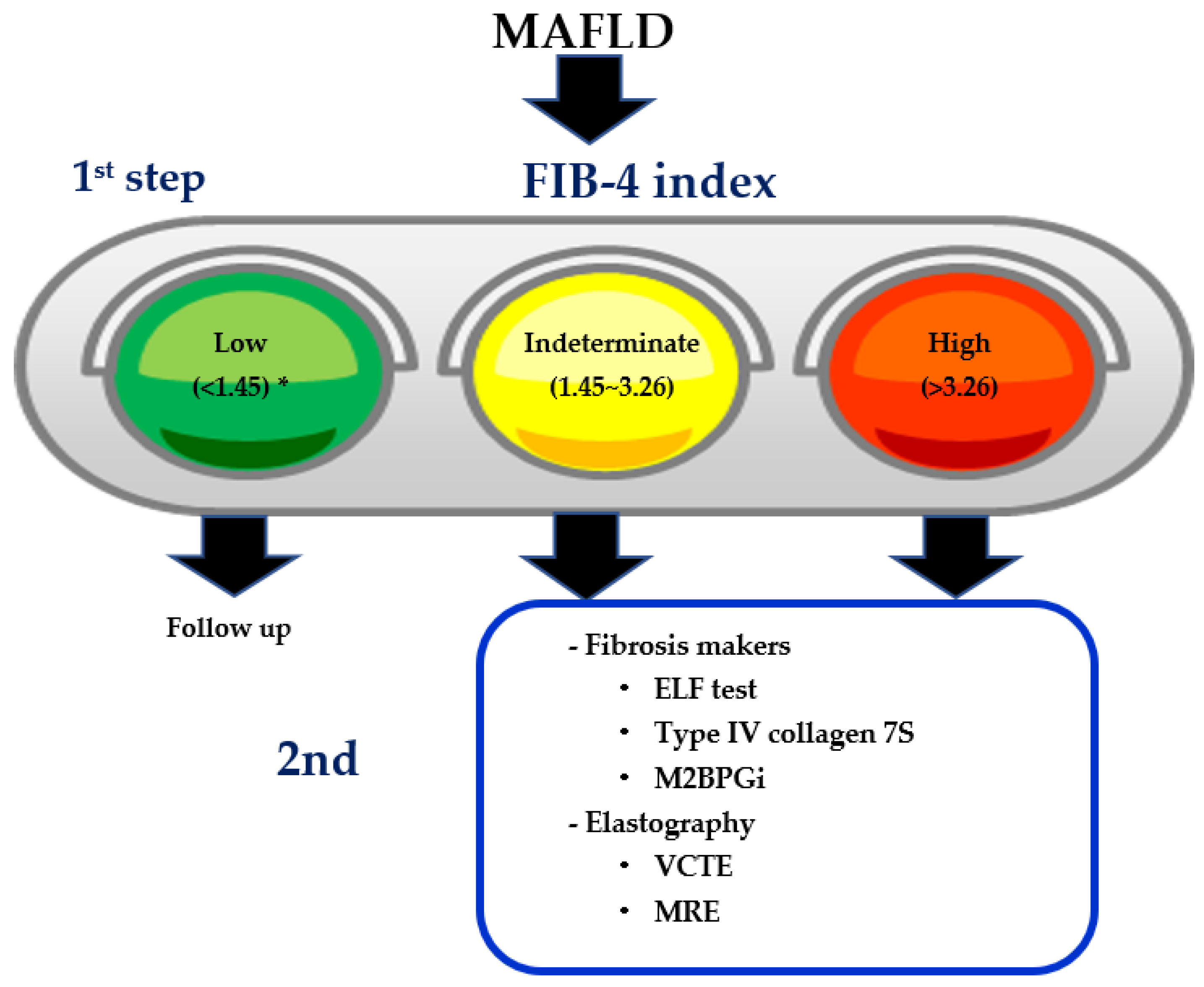
| Cutoff Values | No. of Studies (No. of Patients) | Summary Sensitivity, %, Mean (Range) | Summary Specificity, %, Mean (Range) | Summary PPV, %, Mean (Range) | Summary NPV, %, Mean (Range) |
|---|---|---|---|---|---|
| APRI | |||||
| 0.452–0.50 | 5 (729) | 72.9 (50.0–87.4) | 67.7 (43.1–91.0) | 44.8 (22.9–71.0) | 89.4 (84.9–95.0) |
| 0.54–0.98 | 7 (1,351) | 68.6 (61.0–76.2) | 72.7 (59.4–86.0) | 61.4 (46.9–76.2) | 77.6 (59.4–94.0) |
| 1.00 | 4 (1101) | 43.2 (27.0–67.0) | 86.1 (81.0–89.0) | 33.5 (26.0–40.0) | 89.8 (84.0–95.0) |
| 1.50 | 4 (682) | 32.9 (6.3–70.0) | 90.5 (74.5–97.0) | 55.5 (40.0–72.1) | 79.1 (73.2–87.2) |
| FIB-4 index | |||||
| 1.24–1.45 | 10 (2759) | 77.8 (63.0–90.0) | 71.2 (55.5–88.0) | 40.3 (24.0–50.6) | 92.7 (88.0–98.0) |
| 1.51–2.24 | 8 (1533) | 77.0 (70.6–89.5) | 79.2 (67.1–93.6) | 66.4 (37.4–85.7) | 83.9 (58.6–97.2) |
| 2.67 | 6 (1910) | 31.9 (12.0–63.2) | 95.7 (88.3–98.7) | 66.0 (51.1–80.0) | 85.0 (79.4–92.6) |
| 3.25 | 6 (1890) | 37.3 (5.0–56.0) | 95.8 (89.0–100) | 72.5 (37.0–100) | 87.3 (78.5–94.0) |
| 5.31–10.62 | 4 (543) | 67.5 (50.0–100) | 80.8 (54.0–100) | 90.0 (80.0–100) | 85.1 (80.0–90.2) |
| BARD | |||||
| 1.5 | 1 (242) | 83.0 | 59.0 | 34.0 | 93.0 |
| 2 | 14 (3057) | 75.2 (41.7–100) | 61.6 (32.5–88.9) | 38.3 (15.0–79.8) | 88.7 (49.6–100) |
| 3–4 | 5 (736) | 59.4 (33.3–85.2) | 75.1 (59.9–91.8) | 55.2 (24.0–69.2) | 81.0 (71.4–90.1) |
| NFS | |||||
| (−26.93)–(−2.16) | 2 (106) | 80.5 (78.0–83.0) | 69.5 (69.0–70.0) | None | None |
| −1.455 | 10 (3057) | 72.9 (22.7–96.0) | 73.8 (42.9–100) | 50.4 (24.0–100) | 91.8 (81.3–98.1) |
| (−1.31)–(0.156) | 5 (963) | 78.2 (69.0–86.4) | 71.7 (60.0–83.0) | 58.4 (34.0–80.8) | 82.1 (54.1–95.0) |
| 0.67–0.676 | 14 (3896) | 43.1 (8.3–100) | 88.4 (25.0–100) | 66.9 (26.0–100) | 88.5 (78.6–100) |
| 0.735 | 1 (235) | 68.4 | 88.3 | 53.0 | 93.5 |
| Subjects | N | Nation | Dx | Observation Period | Over-all Mortality /Morbidity | Liver-Related Mortality/Morbidity | Liver Event | HCC | CVD Mortality | Extrahepatic Cancer |
|---|---|---|---|---|---|---|---|---|---|---|
| NAFLD [62] | 646 | Sweden | Biopsy | 19.9 ±8.7 years | FIB-4 ○ | FIB-4 ○ | ||||
| NFS ○ | NFS ○ | |||||||||
| Viral hepatitis-negative adults [61] | 14,841 | USA | General population | Median 19.3 years (IRQ, 17.5–21.1) years | APRI ○ | APRI ○ | FIB-4 ○ | APRI ○ | ||
| FIB-4 ○ | FIB-4 ○ | |||||||||
| NFS ○ | NFS ○ | |||||||||
| Forns score ○ | Forns score○ | |||||||||
| NAFLD [57] | 153 | Israel | Biopsy | 100 months (mean) | FIB-4 ○ | FIB-4 ○ | FIB-4 ○ | |||
| NFS ○ | NFS ○ | NFS ○ | ||||||||
| APRI × | APRI ○ | APRI ○ | ||||||||
| NAFLD [68] | 180 | China | US | 6.6 (range 0.5–14.8) years | NFS ◎ | |||||
| FIB-4 ○ | ||||||||||
| APRI× | ||||||||||
| BARD× | ||||||||||
| NAFLD [58] | 646 | Japan | Biopsy | FIB-4 ○ | FIB-4 ○ | FIB-4 × | ||||
| NAFLD [64] | 4073 | Japan | US | NFS ○ | NFS ○ | |||||
| NAFLD with diabetes [63] | 284 | Australia | US | 51.4 (range 6.1–146). months | NFS × | |||||
| FIB-4 × | ||||||||||
| APRI × | ||||||||||
| NAFLD [60] | 11,154 | US | US | 14.5 years | FIB-4 ○ | FIB-4 ○ | ||||
| NFS ○ | NFS ○ | |||||||||
| APRI ○ | APRI ○ | |||||||||
| NASH [69] | 148 | Canada | biopsy | Median: 5 years (IQR: 3–8) | FIB-4 ○ | |||||
| NFS ○ | ||||||||||
| APRI ○ | ||||||||||
| NAFLD [59] | 153 | US | biopsy | Median 104.8 (range, 3–317) months | NFS ◎ | |||||
| FIB-4 ○ | ||||||||||
| APRI ○ |
| Over-Referral | Under-Referral | |
|---|---|---|
| FIB-4 index low COI | 1.3 | 1.45 |
| GP | Work ↓ | Work ↑ |
| Hepatologists | Work ↑ | Work ↓ |
| Unnecessary liver biopsy | May increase | May reduce |
| HCC early detection | Possible? | May delay diagnosis? |
| Heath economic costs | High? | Low? |
| Author | Subjects | Outcomes | Parameter Correlated with Pathological Improvement |
|---|---|---|---|
| Hamaguchi [121] | MAFLD (n = 39) | Hepatic fibrosis | ⊿HbA1c reduction |
| Seko [122] | Steatohepatitis (n = 52) | NAS Hepatic fibrosis | ⊿ALT reduction ≥ 30% from baseline |
| Hoofnagle [123] | Steatohepatitis (n = 139) without DM PIVENS trial | NAS Hepatic fibrosis | ⊿ALT reduction ≥ 30% from baseline or post-treatment ALT ≤ 40 IU/L |
| Vilar-Gomez [124] | Steatohepatitis (n = 261) | NASH resolution w/o worsening fibrosis | ⊿BW reduction, absence of T2D ALT normalization, younger age, NAS < 5 |
| Vuppalanchi | Adult steatohepatitis (n = 231) Pediatric MAFLD (n = 152) | Histological improvement | ⊿CK18 reduction (inferior to ⊿ALT reduction) |
| Siddiqui [119] | MAFLD (n = 292) | Hepatic fibrosis | ⊿FIB-4 index, ⊿NFS, ⊿APRI |
| Jayakumar [111] | Steatohepatitis, stage 2–3 (n = 54) Selonsertib (Phase 2) | Hepatic fibrosis | ⊿MRE |
| Hepatic steatosis | MRI-PDFF > 25% reduction | ||
| Chalasani [120] | Steatohepatitis (n = 200) FLINT trial (Phase 2) Placebo vs. OCA 72wk | Hepatic fibrosis | ⊿FIB-4 index ⊿APRI (⊿NFS: no correlation) |
| Loomba | NAS ≥ 2 points reduction without worsening fibrosis | OCA(+), pretreatment NAS > 5, TG ≤ 154 mg/dL, INR < 1, AST < 49 IU/L, ⊿ALT at 24wk (>17 IU/L) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumida, Y.; Yoneda, M.; Tokushige, K.; Kawanaka, M.; Fujii, H.; Yoneda, M.; Imajo, K.; Takahashi, H.; Eguchi, Y.; Ono, M.; et al. FIB-4 First in the Diagnostic Algorithm of Metabolic-Dysfunction-Associated Fatty Liver Disease in the Era of the Global Metabodemic. Life 2021, 11, 143. https://doi.org/10.3390/life11020143
Sumida Y, Yoneda M, Tokushige K, Kawanaka M, Fujii H, Yoneda M, Imajo K, Takahashi H, Eguchi Y, Ono M, et al. FIB-4 First in the Diagnostic Algorithm of Metabolic-Dysfunction-Associated Fatty Liver Disease in the Era of the Global Metabodemic. Life. 2021; 11(2):143. https://doi.org/10.3390/life11020143
Chicago/Turabian StyleSumida, Yoshio, Masashi Yoneda, Katsutoshi Tokushige, Miwa Kawanaka, Hideki Fujii, Masato Yoneda, Kento Imajo, Hirokazu Takahashi, Yuichiro Eguchi, Masafumi Ono, and et al. 2021. "FIB-4 First in the Diagnostic Algorithm of Metabolic-Dysfunction-Associated Fatty Liver Disease in the Era of the Global Metabodemic" Life 11, no. 2: 143. https://doi.org/10.3390/life11020143
APA StyleSumida, Y., Yoneda, M., Tokushige, K., Kawanaka, M., Fujii, H., Yoneda, M., Imajo, K., Takahashi, H., Eguchi, Y., Ono, M., Nozaki, Y., Hyogo, H., Koseki, M., Yoshida, Y., Kawaguchi, T., Kamada, Y., Okanoue, T., Nakajima, A., & Japan Study Group of NAFLD (JSG-NAFLD). (2021). FIB-4 First in the Diagnostic Algorithm of Metabolic-Dysfunction-Associated Fatty Liver Disease in the Era of the Global Metabodemic. Life, 11(2), 143. https://doi.org/10.3390/life11020143











