Effect of 7-Methylsulfinylheptyl Isothiocyanate on the Inhibition of Melanogenesis in B16-F1 Cells
Abstract
1. Introduction
2. Results and Discussion
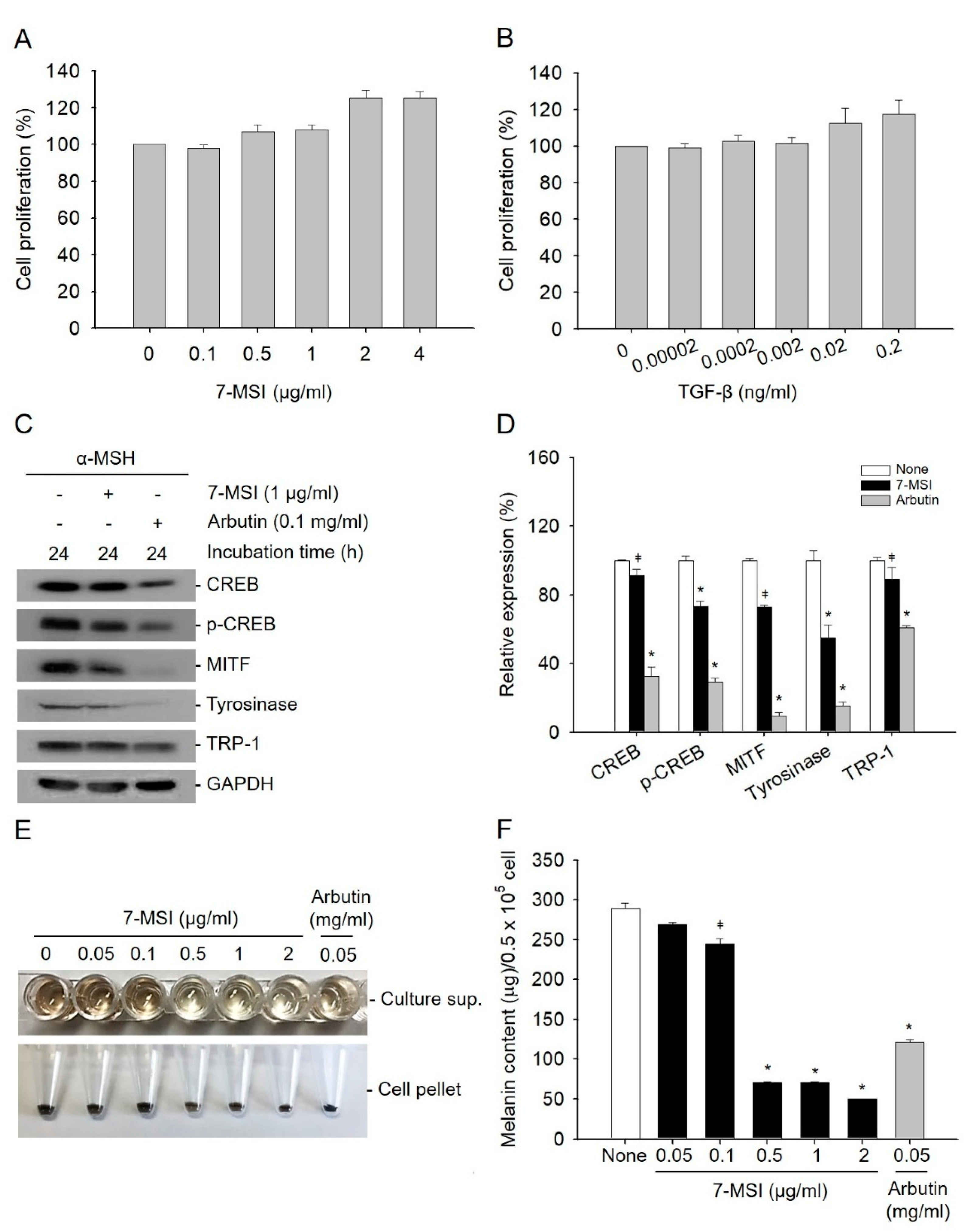
2.1. 7-MSI Suppresses Melanogenesis in B16-F1 Cells
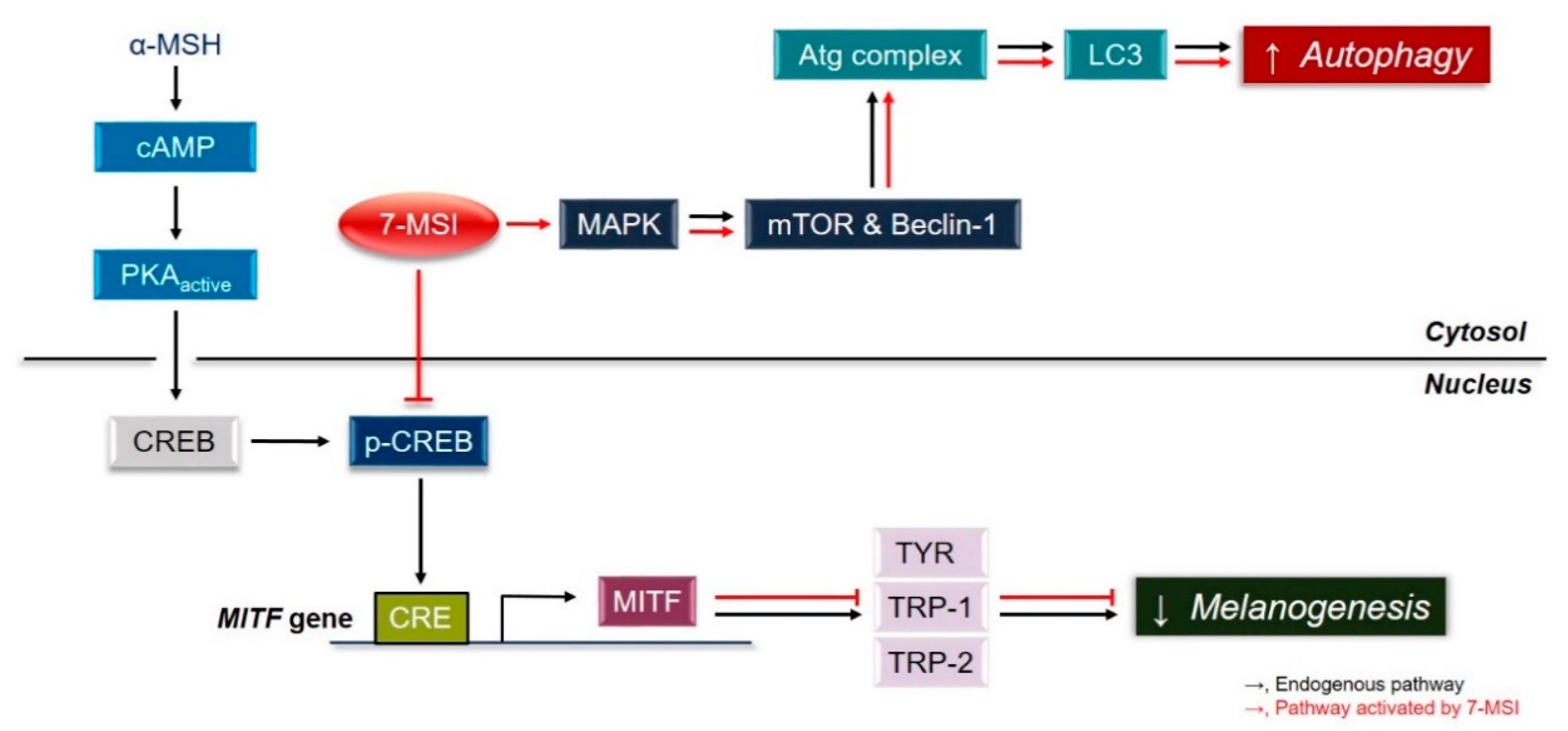
2.2. 7-MSI Activates MAPK Signaling in B16-F1 Cells
2.3. Activation of Cellular Autophagy by 7-MSI in B16-F1 Cells
3. Conclusions
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Assay
4.3. Measurement of Melanin Content
4.4. Western Blotting Assay
4.5. Confocal Microscopy Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 7-MSI | 7-methylsulfinylheptyl isothiocyanate |
| α-MSH | alpha-melanocyte-stimulating hormone |
| ATG | autophagy-related proteins |
| CREB | cAMP response element-binding protein |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ERK | extracellular-related kinase |
| LC3 | microtubule-associated protein light chain 3 |
| MAPK | mitogen-activated protein kinase |
| MC1R | melanocortin 1 receptor |
| MITF | microphthalmia-associated transcription factor |
| mTOR | mammalian target of rapamycin |
| PKA | protein kinase A |
| TGF-β | transforming growth factor-beta |
| TRP1/2 | tyrosinase-related protein-1/2 |
| UV | ultraviolet |
References
- Bouwstra, J.; Honeywell-Nguyen, P. Skin structure and mode of action of vesicles. Adv. Drug Deliv. Rev. 2002, 54, S41–S55. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Flórez-Muñoz, S.V.; Sanclemente, G.J.I. Mechanisms of skin aging. Iatreia 2017, 30, 160–170. [Google Scholar] [CrossRef]
- Stout, R.; Birch-Machin, M. Mitochondria’s role in skin ageing. Biology 2019, 8, 29. [Google Scholar] [CrossRef]
- Lim, J.W.; Ha, J.H.; Jeong, Y.J.; Park, S.N.J.P.R. Anti-melanogenesis effect of dehydroglyasperin C through the downregulation of MITF via the reduction of intracellular cAMP and acceleration of ERK activation in B16F1 melanoma cells. Pharrmacol. Rep. 2018, 70, 930–935. [Google Scholar] [CrossRef]
- Ha, S.K.; Koketsu, M.; Lee, K.; Choi, S.Y.; Park, J.-H.; Ishihara, H.; Kim, S.Y.J.B.; Bulletin, P. Inhibition of tyrosinase activity by N, N-unsubstituted selenourea derivatives. Biol. Pharm. Bull. 2005, 28, 838–840. [Google Scholar] [CrossRef]
- Lee, D.H.; Ahn, S.S.; Kim, J.-B.; Lim, Y.; Lee, Y.H.; Shin, S.Y. Downregulation of α-melanocyte-stimulating hormone-induced activation of the Pax3-MITF-tyrosinase axis by sorghum ethanolic extract in B16F10 melanoma cells. Int. J. Mol. Sci. 2018, 19, 1640. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Yun, C.-Y.; Yun, J.Y.; Park, D.; Kim, N.D.; Hwang, B.Y.; Jung, S.-H.; Park, S.K.; Kim, Y.-B.; Han, S.-B. cAMP-binding site of PKA as a molecular target of bisabolangelone against melanocyte-specific hyperpigmented disorder. J. Investig. Dermatol. 2013, 133, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Feng, J.; Fienberg, A.A.; Greengard, P. D2 dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein phosphorylation in neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 11607–11612. [Google Scholar] [CrossRef]
- Hwang, K.-S.; Yang, J.Y.; Lee, J.; Lee, Y.-R.; Kim, S.S.; Kim, G.R.; Chae, J.S.; Ahn, J.H.; Shin, D.-S.; Choi, T.-Y. A novel anti-melanogenic agent, KDZ-001, inhibits tyrosinase enzymatic activity. J. Dermatol. Sci. 2018, 89, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaga, T.; Yamauchi, K. Effect of quercetin derivatives on melanogenesis stimulation of melanoma cells. J. Wood Sci. 2015, 61, 351–363. [Google Scholar] [CrossRef]
- Ohbayashi, N.; Fukuda, M. Recent advances in understanding the molecular basis of melanogenesis in melanocytes. F1000Research 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, J.; Min, D.; Kim, J.; Kim, H.-J.; No, K.T. Tyrosinase-Targeting Gallacetophenone Inhibits Melanogenesis in Melanocytes and Human Skin-Equivalents. Int. J. Mol. Sci. 2020, 21, 3144. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jung, H.; Jang, B.; Song, H.-K.; Han, I.-O.; Oh, E.-S. D-tyrosine adds an anti-melanogenic effect to cosmetic peptides. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Qian, M.; Fang, X.; Wang, X. Autophagy and inflammation. Clin. Transl. Med. 2017, 6, 24. [Google Scholar] [CrossRef]
- Salimi, L.; Akbari, A.; Jabbari, N.; Mojarad, B.; Vahhabi, A.; Szafert, S.; Kalashani, S.A.; Soraya, H.; Nawaz, M.; Rezaie, J. Synergies in exosomes and autophagy pathways for cellular homeostasis and metastasis of tumor cells. Cell Biosci. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Lemasters, J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005, 8, 3–5. [Google Scholar] [CrossRef]
- Murase, D.; Hachiya, A.; Takano, K.; Hicks, R.; Visscher, M.O.; Kitahara, T.; Hase, T.; Takema, Y.; Yoshimori, T. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J. Investig. Dermatol. 2013, 133, 2416–2424. [Google Scholar] [CrossRef]
- Raposo, G.; Marks, M.S. Melanosomes—Dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 2007, 8, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Ganesan, A.K. The pleiotropic roles of autophagy regulators in melanogenesis. Pigment Cell Melanoma Res. 2011, 24, 595–604. [Google Scholar] [CrossRef]
- Faghiri, Z.; Bazan, N.G. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp. Eye Res. 2010, 90, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B.J.M. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef]
- Wei, R.; Zhang, X.; Cai, Y.; Liu, H.; Wang, B.; Zhao, X.; Zou, K. Busulfan Suppresses Autophagy in Mouse Spermatogonial Progenitor Cells via mTOR of AKT and p53 Signaling Pathways. Stem Cell Rev. Rep. 2020, 16, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Nowosad, A.; Jeannot, P.; Callot, C.; Creff, J.; Perchey, R.T.; Joffre, C.; Codogno, P.; Manenti, S.; Besson, A. p27 controls Ragulator and mTOR activity in amino acid-deprived cells to regulate the autophagy–lysosomal pathway and coordinate cell cycle and cell growth. Nat. Cell Biol. 2020, 22, 1076–1090. [Google Scholar] [CrossRef]
- Zhang, C.; Cuervo, A.M. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat. Med. 2008, 14, 959. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Thorburn, A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020, 27, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G.J.C. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Codogno, P.; Mehrpour, M.; Proikas-Cezanne, T. Canonical and non-canonical autophagy: Variations on a common theme of self-eating? Nat. Rev. Mol. Cell Biol. 2012, 13, 7. [Google Scholar] [CrossRef]
- Barth, S.; Glick, D.; Macleod, K.F. Autophagy: Assays and artifacts. J. Pathol. 2010, 221, 117–124. [Google Scholar] [CrossRef]
- Giménez-Xavier, P.; Francisco, R.; Platini, F.; Pérez, R.; Ambrosio, S. LC3-I conversion to LC3-II does not necessarily result in complete autophagy. Int. J. Mol. Med. 2008, 22, 781–785. [Google Scholar] [PubMed]
- Matusheski, N.V.; Jeffery, E.H. Comparision of the bioactivity of twp glucoraphanin hydrolysis products found in brocccoli, sulforaphane, and sulforaphane nitrile. J. Agric. Food Chem. 2001, 49, 5743–5749. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.; Huang, Q.; Ong, C.N.; Whiteman, M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MA-231 breast acncer cells. Toxicol. Appl. Pharmacol. 2005, 209, 105–113. [Google Scholar] [CrossRef]
- Rose, P.; Faulkner, K.; Williamson, G.; Mithen, R. 7-Methylsulfinylheptyl and 8-methylsulfinyloctyl isothiocyanates from watercress are potent inducers of phase II enzymes. Carcinogenesis 2000, 21, 1983–1988. [Google Scholar] [CrossRef]
- Lim, H.; Kim, H.J.; Jeong, H.; Park, H.-R. Anti-inflammatory effects of 1-isothiocyanato-7-(methylsulfonyl) heptane by suppressing the NFκ-B signaling pathway. Eur. J. Inflamm. 2017, 15, 57–65. [Google Scholar] [CrossRef]
- Shirasugi, I.; Kamada, M.; Matsui, T.; Sakakibara, Y.; Liu, M.C.; Suiko, M. Sulforaphane inhibited melanin synthesis by regulating turosinase gene expression in B16 mouse melanoma cells. Biosci. Biotechnol. Biochem. 2010, 74, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.L.; Park, S.Y.; Kim, Y.H.; Park, G.; Son, H.-J.; Lee, S.-J. Suppression of α-MSH and IBMX-induced melanogenesis by cordycepin via inhibition of CREB and MITF, and activation of PI3K/Akt and ERK-dependent mechanisms. Int. J. Mol. Med. 2011, 29, 119–124. [Google Scholar]
- Martin, P.; Poggi, M.C.; Chambard, J.C.; Boulukos, K.E.; Pognonec, P. Low dose cadmium poisoning results in sustained ERK phosphorylation and caspase activation. Biochem. Biophys. Res. Commun. 2006, 350, 803–807. [Google Scholar] [CrossRef]
- Yamagguchi, Y.; Hearing, V.J. The regulation of skin pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef]
- Ganesan, A.K.; Ho, H.; White, M.A. Genome-wide siRNA-based functional genomics of pugmentation identifies novel genes and pathways that impact melanogenesis in human cells. PLoS Genet. 2008, 4, e1000298. [Google Scholar] [CrossRef]
- Ohguchi, K.; Banno, Y.; Nakagawa, Y.; Akao, Y.; Nozawa, Y. Negative regulation of melanogenesis by phospholopase D1 throuhg Mtor/p70 S6 kinase 1 signaling in mouse B16 melanoma cells. J. Cell Physiol. 2005, 205, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, A.; Nakayasu, M. Quantitative measurement of melanin as tyrosinase equivalents and as weight of purified melanin. Yale J. Biol. Med. 1973, 46, 500. [Google Scholar] [PubMed]
- Cho, Y.H.; Park, J.E.; Lee, J.S. Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J. Dermatol. Sci. 2017, 88, 96–102. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, A.-J.; Park, J.E.; Cho, Y.H.; Lim, D.S.; Lee, J.S. Effect of 7-Methylsulfinylheptyl Isothiocyanate on the Inhibition of Melanogenesis in B16-F1 Cells. Life 2021, 11, 162. https://doi.org/10.3390/life11020162
Kim A-J, Park JE, Cho YH, Lim DS, Lee JS. Effect of 7-Methylsulfinylheptyl Isothiocyanate on the Inhibition of Melanogenesis in B16-F1 Cells. Life. 2021; 11(2):162. https://doi.org/10.3390/life11020162
Chicago/Turabian StyleKim, A-Ju, Jung Eun Park, Yeong Hee Cho, Do Sung Lim, and Jung Sup Lee. 2021. "Effect of 7-Methylsulfinylheptyl Isothiocyanate on the Inhibition of Melanogenesis in B16-F1 Cells" Life 11, no. 2: 162. https://doi.org/10.3390/life11020162
APA StyleKim, A.-J., Park, J. E., Cho, Y. H., Lim, D. S., & Lee, J. S. (2021). Effect of 7-Methylsulfinylheptyl Isothiocyanate on the Inhibition of Melanogenesis in B16-F1 Cells. Life, 11(2), 162. https://doi.org/10.3390/life11020162



