Management of Infants with Brief Resolved Unexplained Events (BRUE) and Apparent Life-Threatening Events (ALTE): A RAND/UCLA Appropriateness Approach
Abstract
:1. Introduction
2. Materials and Methods
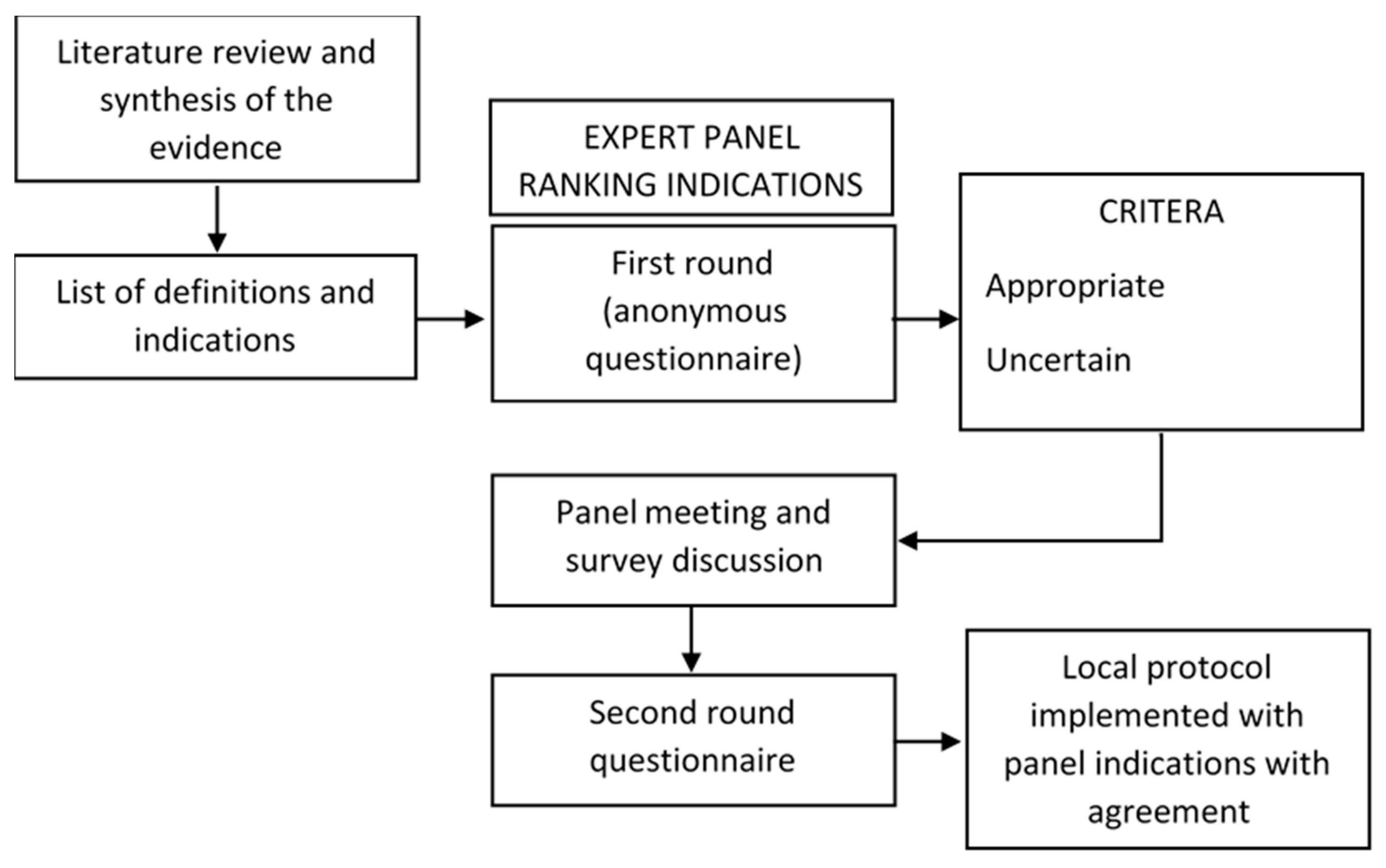
2.1. RAND/UCLA Appropriateness Method
2.2. Literature Search
2.3. Questionnaire Development
2.4. Panel Selection
2.5. First Round and Data Analysis
2.6. Consensus Meeting and Second Round: Definition of Disagreement/Agreement
2.7. Protocol
3. Results
3.1. Study Participants
3.2. Scenario 1—Prevention of ALTE/BRUE
3.3. Scenario 2—Emergency Room (ER) Evaluation
3.4. Scenario 3—Hospital Admission
3.5. Scenario 4—Management of Patients Who Do Not Need Hospitalization
3.6. Scenario 5—Management of BRUE/ALTE Cases Associated with Gastrointestinal Symptoms
3.7. Scenario 6. First-Line Tests to Perform in Hospitalized Patients with ALTE
3.8. Scenario 7—Investigations for Infectious Disease in BRUE/ALTE Patients
3.9. Scenario 8—Indication to Polysomnography
3.10. Scenario 9—When to Suspect a Metabolic Disease
3.11. Scenario 10—Work-Up in Case of Suspected Metabolic Disease
3.12. Scenario 11—Hospital Pulse Oximetry Monitoring
3.13. Scenario 12—Home Cardiorespiratory Monitoring
3.14. Protocol
3.14.1. Primary Prevention
3.14.2. Emergency Management of BRUE and ALTE
3.14.3. Hospital Admission
3.14.4. Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institutes of Health Consensus Development Conference on Infantile Apnea and Home Monitoring, 29 Sept to 1 Oct 1986. Pediatrics 1987, 79, 292–299.
- Tieder, J.S.; Bonkowsky, J.L.; Etzel, R.A.; Franklin, W.H.; Gremse, D.A.; Herman, B.; Katz, E.S.; Krilov, L.R.; Merritt, J.L.; Norlin, C.; et al. Brief resolved unexplained events (formerly apparent life-threatening events) and evaluation of lower-risk infants. Pediatrics 2016, 137, e20160591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piumelli, R.; Davanzo, R.; Nassi, N.; Salvatore, S.; Arzilli, C.; Peruzzi, M.; Agosti, M.; Palmieri, A.; Paglietti, M.G.; Nosetti, L.; et al. Apparent Life-Threatening Events (ALTE): Italian guidelines. Ital. J. Pediatr. 2017, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.Y.; Moon, R.Y. Apparent life-threatening events: An update. Pediatr. Rev. 2012, 33, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Hicks, N.R. Some observations on attempts to measure appropriateness of care. BMJ 1994, 309, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitch, K.; Bernstein, S.J.; Aguilar, M.D.; Burnand, B.; LaCalle, J.R.; Lazaro, P.; van het Loo, M.; McDonnell, J.; Vader, J.; Kahan, J.P. The RAND/UCLA Appropriateness Method User’s Manual Approved for Public Release; RAND Corporation: Santa Monica, CA, USA, 2001. [Google Scholar]
- Humphrey-Murto, S.; Varpio, L.; Gonsalves, C.; Wood, T.J. Using consensus group methods such as Delphi and Nominal Group in medical education research. Med. Teach. 2017, 39, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lerner, S.F.; Singh, K.; Susanna, R.; Wilson, M.R.; Lee, B.L.; Maul, E. RAND-like appropriateness methodology consensus for primary open-angle glaucoma in Latin America. Am. J. Ophthalmol. 2012, 154, 460–465.e7. [Google Scholar] [CrossRef]
- Meddings, J.; Skolarus, T.A.; Fowler, K.E.; Bernstein, S.J.; Dimick, J.B.; Mann, J.D.; Saint, S. Michigan Appropriate Perioperative (MAP) criteria for urinary catheter use in common general and orthopedic surgeries: Results obtained using the RAND/UCLA Appropriateness Method. BMJ Qual. Saf. 2019, 28, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Mainiero, M.B.; Moy, L.; Baron, P.; Didwania, A.D.; diFlorio, R.M.; Green, E.D.; Heller, S.L.; Holbrook, A.I.; Lee, S.J.; Lewin, A.A.; et al. ACR Appropriateness Criteria® Breast Cancer Screening. J. Am. Coll. Radiol. 2017, 14, S383–S390. [Google Scholar] [CrossRef]
- Whitehead, M.T.; Cardenas, A.M.; Corey, A.S.; Policeni, B.; Burns, J.; Chakraborty, S.; Crowley, R.W.; Jabbour, P.; Ledbetter, L.N.; Lee, R.K.; et al. ACR Appropriateness Criteria® Headache. J. Am. Coll. Radiol. 2019, 16, S364–S377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.D.; Broderick, D.F.; Burns, J.; Deshmukh, T.K.; Fries, I.B.; Harvey, H.B.; Holly, L.; Hunt, C.H.; Jagadeesan, B.D.; Kennedy, T.A.; et al. ACR Appropriateness Criteria Low Back Pain. J. Am. Coll. Radiol. 2016, 13, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Vandenplas, Y.; Singendonk, M.; Cabana, M.; Dilorenzo, C.; Gottrand, F.; Gupta, S.; Langendam, M.; Staiano, A.; Thapar, N.; et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 516–554. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, C.D. Physical Abuse of Children. N. Engl. J. Med. 2017, 376, 1659–1666. [Google Scholar] [CrossRef]
- Brand, D.A.; Altman, R.L.; Purtill, K.; Edwards, K.S. Yield of diagnostic testing in infants who have had an apparent life-threatening event. Pediatrics 2005, 115, 885–893. [Google Scholar] [CrossRef] [PubMed]
- De Piero, A.D.; Teach, S.J.; Chamberlain, J.M. ED Evaluation of Infants after an apparent Life-Threatening Event. Am. J. Emerg. Med. 2004, 22, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Stefanelli, P.; Fry, N.K.; Fedele, G.; He, Q.; Paterson, P.; Tan, T.; Knuf, M.; Rodrigo, C.; Weil Olivier, C.; et al. Pertussis Prevention: Reasons for Resurgence, and Differences in the Current Acellular Pertussis Vaccines. Front. Immunol. 2019, 10, 1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, A. Recommended clinical evaluation of infants with an apparent life-threatening event. Consensus document of the European Society for the Study and Prevention of Infant Death, 2003. Eur. J. Pediatr. 2004, 163, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Hirtz, D.; Ashwal, S.; Berg, A.; Bettis, D.; Camfield, C.; Camfield, P.; Crumrine, P.; Elterman, R.; Schneider, S.; Shinnar, S. Practice parameter: Evaluating a first nonfebrile seizure in children: Report of the Quality Standards Subcommittee of the American Academy of Neurology, the Child Neurology Society, and the American Epilepsy Society. Neurology 2000, 55, 616–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arens, R.; Gozal, D.; Williams, J.C.; Davidson Ward, S.L.; Keens, T.G. Recurrent apparent life-threatening events during infancy: A manifestation of inborn errors of metabolism. J. Pediatr. 1993, 123, 415–418. [Google Scholar] [CrossRef]
- Hoki, R.; Bonkowsky, J.L.; Minich, L.L.A.; Srivastava, R.; Pinto, N.M. Cardiac testing and outcomes in infants after an apparent life-threatening event. Arch. Dis. Child 2012, 97, 1034–1038. [Google Scholar] [CrossRef] [Green Version]
- Ueda, R.; Nomura, O.; Maekawa, T.; Sakai, H.; Nakagawa, S.; Ishiguro, A. Independent risk factors for recurrence of apparent life-threatening events in infants. Eur. J. Pediatr. 2017, 176, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.Y.; Darnall, R.A.; Goodstein, M.H.; Hauck, F.R.; Willinger, M.; Shapiro-Mendoza, C.K.; Couto, J. SIDS and other sleep-related infant deaths: Expansion of recommendations for a safe infant sleeping environment. Pediatrics 2011, 128, e1341–e1367. [Google Scholar] [PubMed] [Green Version]
- Hauck, F.R.; Omojokun, O.O.; Siadaty, M.S. Do pacifiers reduce the risk of sudden infant death syndrome? A meta-analysis. Pediatrics 2005, 116, e716–e723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeWolfe, C.C. Apparent life-threatening event: A review. Pediatr. Clin. N. Am. 2005, 52, 1127–1146. [Google Scholar] [CrossRef] [PubMed]
- Côté, A. Home and hospital monitoring for ALTE. Pediatr. Respir. Rev. 2006, 7 (Suppl. 1), S199–S201. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; Corwin, M.J.; Hunt, C.E.; Lister, G.; Tinsley, L.R.; Baird, T.; Silvestri, J.M.; Crowell, D.H.; Hufford, D.; Martin, R.J.; et al. Cardiorespiratory events recorded on home monitors: Comparison of healthy infants with those at increased risk for SIDS. J. Am. Med. Assoc. 2001, 285, 2199–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halbower, A.C. Pediatric home apnea monitors: Coding, billing, and updated prescribing information for practice management. Chest 2008, 134, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, J.M. Indications for Home Apnea Monitoring (or Not). Clin. Perinatol. 2009, 36, 7–99. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.S.; Parker, M.G.; Colvin, B.N.; Forbes, E.S.; Brown, K.; Colson, E.R. Understanding the barriers and facilitators to safe infant sleep for mothers of preterm infants. J. Perinatol. 2020. [Google Scholar] [CrossRef] [PubMed]
| Definition | Definition |
|---|---|
| SIDS | Sudden, unexpected death before 12 months of age occurring in a previously healthy infant, in which the cause remains unknown despite thorough investigations (including an appropriate autopsy, death scene investigation, and analysis of the clinical history). |
| BRUE | Sudden, brief (<1 min) episode without other explainable cause occurring in an infant younger than 12 months of age characterized by ≥1 among cyanosis or pallor, absent/ decreased/ or irregular breathing, marked change in tone, altered level of responsiveness. |
| ALTE | Episode that is frightening to the observer, characterized by ≥1 among apnoea (central or occasionally obstructive), color change, marked change in muscle tone, choking or gagging. |
| History | Information |
|---|---|
| Family history | Episodes of SIDS or sudden death in family members before age 35 years Episodes of ALTE/BRUE Cardiac diseases (i.e., long QT syndrome, arrhythmia) Inborn error of metabolism or genetic disease Developmental delay Allergies Epilepsy (i.e., infantile spasms) Malformations |
| Past history | Pre-/perinatal history Prematurity and neonatal history Past medical history Feeding problems, reflux Growth Neurocognitive development, behavior Previous episodes/BRUE Respiratory problems (in sleep and wakefulness) Past injuries Previous hospitalization Immunization Medications |
| Recent history | General conditions in the previous 48 h (i.e., illness, signs and symptoms, feeding problems, vaccinations) Injuries, falls, unexplained bruising Drugs New food introduction Sleep pattern alterations, sleep deprivation |
| History of the event | Full description Duration Person that reported the event Witnesses of the event (and level of reliability) |
| Circumstances of the event | Location Position of the baby (i.e., prone, supine, sitting) If awake: ask for sounds, breathing abnormalities, vomit, regurgitation If asleep: ask for cough, vomit, rigidity or cry before the episode During feeding (or time since last feeding) During the bath Ambiental risk factors: smoke, CO, temperature, clothing, blankets, close objects, accidental events |
| Appearance of the baby during the event | Skin color and lips color Muscle tone Consciousness Limb movements, other movements Skin temperature Sweating Respiratory distress Apnea Bleeding |
| End of the event and action | Duration (</≥1 min) Baby aspect before resolution Spontaneous resolution or after intervention Gradual or rapid resolution Time from beginning to first intervention Time from first intervention to return to normality Need for vigorous interventions or resuscitation maneuvers Intervention provided by caregiver or healthcare personnel |
| Socio-environmental history | Family structure Home condition Recent changes, stressful conditions, or conflicts Drugs/toxic substances exposure Social services assistance Psychiatric problems Adults with history of substance abuse |
| Considerations for possible child abuse | Social services assistance Incongruences between the descriptions and child’s developmental stage Infant blamed for bad behavior Changing versions of the history Unexplained bruises |
| General conditions and vital signs | Auxological parameters General aspect (color, perfusion/ capillar refill, crying, hydration) Respiratory function Cardiac function, pulse, pressure Vital signs monitoring |
| Physical examination | Eyes (i.e., extrinsic ocular motility, pupillary response, conjunctiva, fundoscopy) Ears and pharynx (i.e., nasal congestion and secretions, blood in nostrils or oral cavity, evidence of injuries or obstructions) Chest (i.e., ribs fractures, asymmetry) Abdomen (i.e., organomegaly, masses, abdominal distension) Genitals Skin (i.e., bruises or other lesions) Dysmorphisms (i.e., especially craniofacial) Skeletal muscle (i.e., neck mobility, other joints, lesions, deformities, fractures, muscle tone, and strength) Nervous system (i.e., alertness, responsiveness, response to visual and sound stimuli, tendon reflexes, meningeal symptoms, asymmetries of the movements, anterior fontanelle) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prezioso, G.; Perrone, S.; Biasucci, G.; Pisi, G.; Fainardi, V.; Strisciuglio, C.; Marzano, F.N.; Moretti, S.; Pisani, F.; Tchana, B.; et al. Management of Infants with Brief Resolved Unexplained Events (BRUE) and Apparent Life-Threatening Events (ALTE): A RAND/UCLA Appropriateness Approach. Life 2021, 11, 171. https://doi.org/10.3390/life11020171
Prezioso G, Perrone S, Biasucci G, Pisi G, Fainardi V, Strisciuglio C, Marzano FN, Moretti S, Pisani F, Tchana B, et al. Management of Infants with Brief Resolved Unexplained Events (BRUE) and Apparent Life-Threatening Events (ALTE): A RAND/UCLA Appropriateness Approach. Life. 2021; 11(2):171. https://doi.org/10.3390/life11020171
Chicago/Turabian StylePrezioso, Giovanni, Serafina Perrone, Giacomo Biasucci, Giovanna Pisi, Valentina Fainardi, Caterina Strisciuglio, Francesco Nonnis Marzano, Sabrina Moretti, Francesco Pisani, Bertrand Tchana, and et al. 2021. "Management of Infants with Brief Resolved Unexplained Events (BRUE) and Apparent Life-Threatening Events (ALTE): A RAND/UCLA Appropriateness Approach" Life 11, no. 2: 171. https://doi.org/10.3390/life11020171
APA StylePrezioso, G., Perrone, S., Biasucci, G., Pisi, G., Fainardi, V., Strisciuglio, C., Marzano, F. N., Moretti, S., Pisani, F., Tchana, B., Argentiero, A., Neglia, C., Caffarelli, C., Bertolini, P., Bersini, M. T., Canali, A., Voccia, E., Squarcia, A., Ghi, T., ... Esposito, S. (2021). Management of Infants with Brief Resolved Unexplained Events (BRUE) and Apparent Life-Threatening Events (ALTE): A RAND/UCLA Appropriateness Approach. Life, 11(2), 171. https://doi.org/10.3390/life11020171











