Circulating Renalase as Predictor of Renal and Cardiovascular Outcomes in Pre-Dialysis CKD Patients: A 5-Year Prospective Cohort Study
Abstract
1. Introduction
2. Methods
Statistical Analysis
3. Ethics
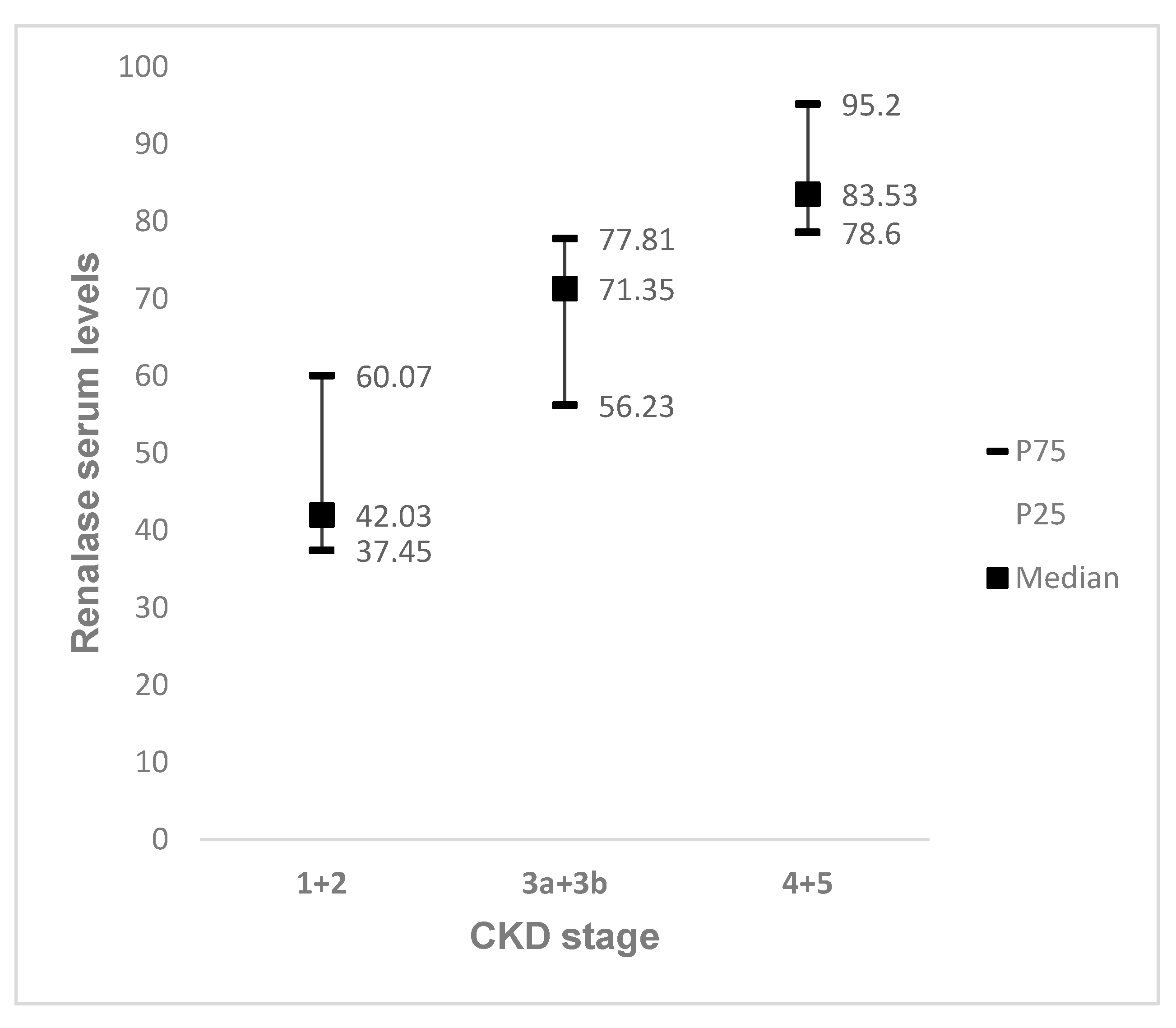
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barsoum, R.S. Chronic kidney disease in the developing world. N. Engl. J. Med. 2006, 354, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.A.; Fathima, S.F.; Feigl, A.B.; Chyung, D. The burden of Chronic Kidney Disease on developing nations: A 21st century challenge in global health. Nephron Clin. Pract. 2011, 118, c269–c277. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, G.; Tighiouart, H.; Ibrahim, H.; MacLeod, B.; Salem, D.N.; Griffith, J.L.; Coresh, J.; Levey, A.S.; Sarnak, M.J. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. J. Am. Coll. Cardiol. 2003, 63, 1121–1129. [Google Scholar] [CrossRef]
- Anavekar, N.S.; Pfeffer, M.A. Cardiovascular risk in chronic kidney disease. Kidney Int. 2004, 66, S11–S15. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Michael, E.K.; Klag, J.; et al. Wilson Kidney Disease as a Risk Factor for Development of Cardiovascular Disease. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef]
- Irie, F.; Iso, H.; Sairenchi, T.; Fukasawa, N.; Yamagishi, K.; Ikehara, S.; Kanashiki, M.; Saito, Y.; Ota, H.; Nose, T. The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int. 2006, 69, 1264–1271. [Google Scholar] [CrossRef]
- Tripepi, G.; Raso, F.M.; Sijbrands, E.; Seck, M.S.; Maas, R.; Boger, R.; Witteman, J.; Rapisarda, F.; Malatino, L.; Mallamaci, F.; et al. Inflammation and asymmetric dimethylarginine for predicting death and cardiovascular events in ESRD patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- D’Elia, E.; Iacovoni, A.; Vaduganathan, M.; Lorini, F.L.; Perlini, S.; Senni, M. Neprilysin inhibition in heart failure: Mechanisms and substrates beyond modulating natriuretic peptides. Eur. J. Heart Fail. 2017, 19, 710–717. [Google Scholar] [CrossRef]
- Unger, T.; Paulis, L.; Sica, D.A. Therapeutic perspectives in hypertension: Novel means for renin-angiotensin-aldosterone system modulation and emerging device-based approaches. Eur. Heart J. 2011, 32, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, G.; Wang, P.; Velazquez, H.; Yao, X.; Li, Y.; Wu, Y.; Peixoto, A.; Crowley, S.; Desir, G.V. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J. Clin. Investig. 2005, 115, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Hennebry, S.C.; Eikelis, N.; Socratous, F.; Desir, G.; Lambert, G.; Schlaich, M. Renalase, a novel soluble FAD-dependent protein, is synthesized in the brain and peripheral nerves. Mol. Psychiatry 2010, 15, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Maciorkowska, D.; Zbroch, E.; Malyszko, J. Circulating renalase, catecholamines, and vascular adhesion protein 1 in hypertensive patients. J. Am. Soc. Hypertens. 2015, 9, 855–864. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Xing, T.; Xie, Y.; Wang, N. Serum renalase is related to catecholamine levels and renal function. Clin. Exp. Nephrol. 2015, 19, 92–98. [Google Scholar] [CrossRef]
- Wybraniec, M.T.; Wieczorek, J.; Woźniak-Skowerska, I.; Hoffmann, A.; Nowak, S.; Cichoń, M.; Szydło, K.; Wnuk-Wojnar, A.; Chudek, J.; Więcek, A.; et al. Renalase is associated with adverse left atrial remodelling and disease burden in patients with atrial fibrillation undergoing pulmonary vein isolation. Kardiol. Pol. 2018, 76, 1232–1241. [Google Scholar] [CrossRef]
- Lee, I.-T.; Sheu, W.H.-H. Serum Renalase Levels Are Predicted by Brain-Derived Neurotrophic Factor and Associated with Cardiovascular Events and Mortality after Percutaneous Coronary Intervention. J. Clin. Med. 2018, 7, 437. [Google Scholar] [CrossRef]
- Baek, S.H.; Cha, R.H.; Kang, S.W.; Park, C.W.; Cha, D.R.; Kim, S.G.; Yoon, S.A.; Kim, S.; Han, S.Y.; Park, J.H.; et al. Circulating renalase predicts all-cause mortality and renal outcomes in patients with advanced chronic kidney disease. Korean J. Intern. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Zbroch, E.; Malyszko, J.S.; Koc-Zorawska, E.; Mysliwiec, M. Renalase, a novel regulator of blood pressure, is predicted by kidney function in renal transplant recipients. Transplant. Proc. 2011, 43, 3004–3007. [Google Scholar] [CrossRef] [PubMed]
- Quelhas-Santos, J.; Soares-Silva, I.; Fernandes-Cerqueira, C.; Simoes-Silva, L.; Ferreira, I.; Carvalho, C.; Coentrao, L.; Vaz, R.; Sampaio-Maia, B.; Pestana, M. Plasma and urine renalase levels and activity during the recovery of renal function in kidney transplant recipients. Exp. Biol. Med. 2014, 239, 502–508. [Google Scholar] [CrossRef]
- Zbroch, E.; Malyszko, J.; Malyszko, J.; Koc-Zorawska, E.; Mysliwiec, M. Renalase in peritoneal dialysis patients is not related to blood pressure, but to dialysis vintage. Perit. Dial. Int. 2012, 32, 348–351. [Google Scholar] [CrossRef]
- Zbroch, E.; Malyszko, J.; Malyszko, J.S.; Koc-Zorawska, E.; Mysliwiec, M. Renalase, a Novel Enzyme Involved in Blood Pressure Regulation, Is Related to Kidney Function but Not to Blood Pressure in Hemodialysis Patients. Kidney Blood Press. Res. 2012, 35, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Gluba-Brzozka, A.; Michalska-Kasiczak, M.; Franczyk-Skora, B.; Nocun, M.; Banach, M.; Rysz, J. Markers of increased cardiovascular risk in patients with chronic kidney disease. Lipids Health Dis. 2014, 13, 135. [Google Scholar] [CrossRef]
- Skrzypczyk, P.; Okarska-Napierala, M.; Stelmaszczyk-Emmel, A.; Gorska, E.; Panczyk-Tomaszewska, M. Renalase in children with chronic kidney disease. Biomarkers 2019, 24, 638–644. [Google Scholar] [CrossRef]
- Wisniewska, M.; Serwin, N.; Dziedziejko, V.; Marchelek-Mysliwiec, M.; Dolegowska, B.; Domanski, L.; Ciechanowski, K.; Safranow, K.; Pawlik, A. Chronic kidney disease is associated with increased levels of renalase in serum and decreased in erythrocytes. Pol. Arch. Intern. Med. 2019, 129, 790–797. [Google Scholar] [CrossRef]
- Serwin, N.M.; Wisniewska, M.; Cecerska-Heryc, E.; Safranow, K.; Skwirczynska, E.; Dolegowska, B. Serum-to-urine renalase ratio and renalase fractional excretion in healthy adults and chronic kidney disease patients. BMC Nephrol. 2020, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Koc-Zorawska, E.; Malyszko, J.S.; Kozminski, P.; Zbroch, E.; Mysliwiec, M. Renalase, stroke, and hypertension in hemodialyzed patients. Ren. Fail. 2012, 34, 727–731. [Google Scholar] [CrossRef]
- Koc-Zorawska, E.; Malyszko, J.; Zbroch, E.; Malyszko, J.; Mysliwiec, M. Vascular adhesion protein-1 and renalase in regard to diabetes in hemodialysis patients. Arch. Med. Sci. 2012, 8, 1048–1052. [Google Scholar] [CrossRef]
- Zbroch, E.; Koc-Zorawska, E.; Malyszko, J.; Malyszko, J.; Mysliwiec, M. Circulating levels of renalase, norepinephrine, and dopamine in dialysis patients. Ren. Fail. 2013, 35, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, M.; Petkowicz, B.; Bednarek-Skublewska, A.; Solski, J.; Buczaj, A.; Choina, P. Relationship between renalase and N-terminal pro-B-type natriuretic peptide (NT pro-BNP) in haemodialysis patients. Ann. Agric. Environ. Med. 2014, 21, 132–135. [Google Scholar] [PubMed]
- Oguz, E.G.; Gursoy, G.K.; Yayar, O.; Yildirim, T.; Cimen, T.; Bulut, C.; Eser, B.; Canbakan, B.; Yeter, E.; Ayli, M.D. Increased serum renalase in hemodialysis patients: Is it related to left ventricular hypertrophy? Ren. Fail. 2016, 38, 1180–1186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gok Oguz, E.; Akoglu, H.; Ulusal Okyay, G.; Karaveli Gursoy, G.; Yildirim, T.; Merhametsiz, O.; Cimen, T.; Canbakan, B.; Yeter, E.; Ayli, M.D. Increased serum renalase in peritoneal dialysis patients: Is it related to cardiovascular disease risk? Nefrologia 2017, 37, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, M.; Serwin, N.; Dziedziejko, V.; Marchelek-Mysliwiec, M.; Dolegowska, B.; Domanski, L.; Ciechanowski, K.; Safranow, K.; Pawlik, A. Renalase in Haemodialysis Patients with Chronic Kidney Disease. J. Clin. Med. 2021, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Zbroch, E.; Malyszko, J.; Malyszko, J.; Koc-Zorawska, E.; Mysliwiec, M. Renalase, kidney function, and markers of endothelial dysfunction in renal transplant recipients. Pol. Arch. Med. Wewn 2012, 122, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, D.; Cvetkovic, T.; Stojanovic, M.; Bojanic, V.; Stefanovic, N.; Stojanovic, I. The assessment of renalase: Searching for the best predictor of early renal dysfunction by multivariate modeling in stable renal transplant recipients. Ann. Transplant. 2015, 20, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, D.; Cvetkovic, T.; Stojanovic, M.; Stefanovic, N.; Velickovic-Radovanovic, R.; Zivkovic, N. Renalase Assessment With Regard to Kidney Function, Lipid Disturbances, and Endothelial Dysfunction Parameters in Stable Renal Transplant Recipients. Prog. Transplant. 2017, 27, 125–130. [Google Scholar] [CrossRef] [PubMed]
| Variable | ||
|---|---|---|
| Follow-Up (months), median (P25–P75) | 65.4 | (57.4–68.6) |
| Demographic data | ||
| Gender (male), n (%) | 21 | (52.5) |
| Age (years), median (P25–P75) | 61 | (45–66) |
| Charlson Index, median (P25–P75) | 4.5 | (2–6) |
| Clinical data | ||
| Systolic blood pressure (mmHg), (P25–P75) | 137 | (117–150) |
| Diastolic blood pressure (mmHg), (P25–P75) | 76 | (66–83) |
| Body mass index (kg/m2), median (P25–P75) | 27.5 | (25–30) |
| ACEI/ARBs, n (%) | 38 | (95.0) |
| Renalase levels (ug/mL), median (P25–P75) | 63.53 | (48.42–82.69) |
| CKD related parameters | ||
| Diabetic Nephropathy n (%) | 6 | (15.0) |
| eGFR CKD–EPI (mL/min/1.3 m2), median (min–max) | 47 | (13–119) |
| Creatinine (mg/dL), median (P25–P75) | 1.44 | (1.02–2.46) |
| Urea (mg/dL), median (P25–P75) | 73.5 | (44.5–109.0) |
| Calcium (mg/dL), median (P25–P75) | 4.75 | (4.60–4.90) |
| Phosphate (mg/dL), median (P25–P75) | 3.25 | (2.90–4.00) |
| Parathormone (pg/mL), median (P25–P75) | 97.0 | (45.0–120.0) |
| 25-OH-Vitamin D (ng/mL), median (P25–P75) | 20 | (11–31) |
| Protein/creatinine ratio (mg/g), median (P25–P75) | 455.5 | (154.2–966.8) |
| Cardiovascular related parameters | ||
| Hemoglobin (g/dL), median (P25–P75) | 12.7 | (11.60–13.75) |
| Albumin (g/dL), median (P25–P75) | 41.05 | (39.20–43.55) |
| Total Cholesterol (mg/dL), median (P25–P75) | 184 | (155–207) |
| HDL Cholesterol (mg/dL), median (P25–P75) | 49 | (41–59) |
| Triglycerides (mg/dL), median (P25–P75) | 121 | (95–180) |
| Uric Acid (mg/dL), median (P25–P75) | 6.4 | (5.3–8.7) |
| C reactive protein (mg/L), median (P25–P75) | 2.2 | (1.2–5.7) |
| BNP (pg/mL), median (P25–P75) | 66.0 | (24.0–177.0) |
| Renalase | ||
|---|---|---|
| Spearman Correlation | p-Value | |
| Demographic data | ||
| Age (years) | 0.407 | 0.009 |
| Charlson Index | 0.704 | <0.001 |
| CKD related parameters | ||
| eGFR CKD-EPI (mL/min/1.73 m2) | −0.883 | <0.001 |
| Creatinine (mg/dL) | 0.877 | <0.001 |
| Urea (mg/dL) | 0.818 | <0.001 |
| Protein/creatinine ratio (mg/g) | 0.133 | 0.426 |
| Phosphate (mg/dL) | 0.590 | <0.001 |
| Parathormone (pg/mL) | 0.694 | <0.001 |
| 25-OH-Vitamin D (ng/mL) | 0.163 | 0.357 |
| Cardiovascular related parameters | ||
| Hemoglobin (g/dL) | −0.360 | 0.023 |
| Albumin (g/dL) | −0.095 | 0.559 |
| Total Cholesterol (mg/dL) | −0.236 | 0.480 |
| HDL Cholesterol (mg/dL) | −0.455 | 0.004 |
| Triglycerides (mg/dL) | 0.383 | 0.016 |
| Uric Acid (mg/dL) | 0.565 | <0.001 |
| C reactive protein (mg/L) | 0.153 | 0.372 |
| BNP (pg/mL) | 0.546 | 0.003 |
| Renalase | |||||
|---|---|---|---|---|---|
| P25 | Median | P75 | n | p-Value | |
| Hypertension | |||||
| No | 33.94 | 45.51 | 83.88 | 4 | 0.279 |
| Yes | 49.18 | 68.17 | 82.69 | 36 | |
| Diabetes | |||||
| No | 42.03 | 61.42 | 78.60 | 29 | 0.209 |
| Yes | 54.06 | 65.63 | 99.54 | 11 | |
| Dyslipidemia | |||||
| No | 41.20 | 51.69 | 77.62 | 16 | 0.104 |
| Yes | 56.23 | 71.35 | 83.74 | 23 | |
| Cardiovascular disease | |||||
| No | 40.95 | 59.44 | 78.60 | 29 | 0.028 |
| Yes | 70.71 | 76.64 | 86.61 | 11 | |
| Cerebrovascular disease | |||||
| No | 47.98 | 60.82 | 83.31 | 38 | 0.420 |
| Yes | 75.64 | 78.54 | 81.43 | 2 | |
| Outcome | n (%) |
|---|---|
| MACCEs | 2 (5.0) |
| Acute myocardial infarction | 1 (2.5) |
| Stroke | 1 (2.5) |
| Hospital admission for medical causes | 17 (42.5) |
| Death | 3 (7.5) |
| Death by MACCEs | 2 (5) |
| CKD progression | 16 (40.0) |
| RRT | 12 (30.0) |
| Renalase | ||
|---|---|---|
| Median (P25–P75) | p-Value 1 | |
| CKD progression | 0.001 | |
| No | 51.79 (40.6–73.5) | |
| Yes | 80.4 (62.93–88.07) | |
| Mortality | 0.022 | |
| No | 60.22 (48–78.6) | |
| Yes | 95.20 (81.4–113.9) | |
| Hospitalization | 0.001 | |
| No | 53.86 (40.4–71.4) | |
| Yes | 81.43(65.6–95.1) | |
| MACCEs | 0.094 | |
| No | 60.82 (48–82.1) | |
| Yes | 95.12 (81.4–108.1) | |
| CKD Progression | Hospitalizations | |||||
|---|---|---|---|---|---|---|
| OR | IC 95% | p | OR | IC 95% | p | |
| Model 1 | ||||||
| Renalase | 1.055 | 1.015–1.096 | 0.007 | 1.071 | 1.025–1.119 | 0.002 |
| Hosmer Lemeshow p-value | 0.565 | 0.797 | ||||
| Model 2 | ||||||
| Renalase | 1.064 | 1.019–1.112 | 0.005 | 1.074 | 1.025–1.126 | 0.003 |
| Age | 0.971 | 0.921–1.023 | 0.265 | 0.988 | 0.937–1.041 | 0.645 |
| Hosmer Lemeshow p–value | 0.095 | 0.673 | ||||
| Model 3 | ||||||
| Renalase | 1.050 | 1.001–1.101 | 0.044 | 1.062 | 1.008–1.119 | 0.023 |
| Charlson comorbidity index | 1.060 | 0.715–1.570 | 0.773 | 1.117 | 0.740–1.684 | 0.599 |
| Hosmer Lemeshow p-value | 0.840 | 0.521 | ||||
| Model 4 | ||||||
| Renalase | 1.068 | 1.021–1.117 | 0.004 | 1.067 | 1.020–1.116 | 0.005 |
| Cardiovascular disease | 0.269 | 0.045–1.607 | 0.150 | 1.518 | 0.287–8.028 | 0.623 |
| Hosmer Lemeshow p-value | 0.646 | 0.848 | ||||
| Model 5 | ||||||
| Renalase | 1.054 | 1.015–1.095 | 0.007 | 1.070 | 1.024–1.117 | 0.002 |
| Hypertension | 2.261 | 0.084–60.550 | 0.627 | 2.954 | 0.057–153.369 | 0.591 |
| Hosmer Lemeshow p-value | 0.477 | 0.789 | ||||
| Model 6 | ||||||
| Renalase | 1.055 | 1.012–1.101 | 0.012 | 1.074 | 1.023–1.127 | 0.004 |
| Diabetes | 3.333 | 0.635–17.502 | 0.155 | 3.050 | 0.517–17.984 | 0.218 |
| Hosmer Lemeshow p-value | 0.775 | 0.775 | ||||
| Model 7 | ||||||
| Renalase | 1.052 | 1.012–1.095 | 0.011 | 1.072 | 1.026–1.121 | 0.002 |
| Dyslipidemia | 2.413 | 0.510–11.423 | 0.267 | 0.797 | 0.157–4.058 | 0.785 |
| Hosmer Lemeshow p-value | 0.561 | 0.461 | ||||
| Levels of Circulating Renalase by Elisa Kit | Correlation with Renal and CV Outcomes | |
|---|---|---|
| CKD Patients Stages 1–4 | ||
| A. Gluba-Brzózka et al. 2014 [23] 139 CKD patients 45 healthy volunteers | Increased concentrations of renalase control vs. CKD group 251.0 ± 157 vs. 316.1 ± 155.3 ng/mL, p = 0.026 USCN Life Science, E92845Hu | Increased concentration of osteocalcin, renalase, MMP-2 and TIMP-2 suggest that these factors may be involved in the pathogenesis of CAD in patients with CKD. |
| J. Quelhas-Santos et al. 2014 [20] 26 ESRD patients | Plasma renalase levels (ug/mL) 4.7 ± 0.5 LKD 29.4 ± 4.0 LKR before TX (p < 0.05) USCN Life Science, E92845Hu | Plasma renalase levels closely depend on renal function and sympathetic nervous system activity. |
| F. Wang et al. 2015 [15] 87 CKD patients stages I to IV | Renalase levels not different between groups CKD1–2 (162.1 ± 40.1 ng/L) vs. healthy control group (167.8 ± 69.4 ng/L) group CKD3–5 (217.4 ± 103.8 ng/L) were significantly increased compared with group CKD1–2 (p < 0.05) ELISA Yaji Biological Corp | Serum renalase levels were positively correlated with CKD stage (p < 0.05), while negatively correlated with eGFR (p < 0.05) |
| S. H. Baek et al. 2019 [18] 383 patients with CKD | Mean level of serum renalase was 75.8 ± 34.8 μg/mL ELISA Cloud Clone Corp | Higher serum creatinine levels were significantly associated with a higher renalase levels. Increase in serum renalase was associated with all-cause mortality and adverse renal outcomes, but not associated with the rate of MACCEs. |
| P. Skrzypczyk et al. 2019 [24] 38 children with CKD (stage G2-5) 38 healthy children | Renalase level was higher in the study group compared to control group values (p < 0.001) ELISA CloudClone Corp | In multivariate analysis GFR (β = −0.63, p < 0.001), was determinant of renalase In children with CKD there is a strong correlation between renalase level and CKD stage. |
| M. Wiśniewska et al. 2019 [25] 155 white patients with CKD 30 healthy controls | Serum renalase levels were higher in patients with CKD than in controls: median (Q1-Q3), 103 ng/mL (55.6–166 ng/mL) vs. 17.7 ng/mL (16.3–21.8 ng/mL); p < 0.001 ELISA kit EIAab | No association between serum renalase and eGFR. No associations were found between renalase concentrations and other causes of CKD. |
| N. M. Serwin et al. 2020 [26] 62 CKD patients stages I to IV 28 healthy controls | The concentration of renalase in the serum of CKD patients was much higher in comparison to material from healthy individuals 36.1 (18.3–109.1) vs. 11.1 (2.5–26.5) ng/mL ELISA kit EIAab | Renalase levels in serum are not related to the glomerular filtration rate. |
| HD and DP Patients | ||
| E. Zbroch et al. 2012 [22] 104 HD patients | Mean serum renalase in the study cohort was significantly higher than in the control group (27.53 ± 7.18 vs. 3.86 ± 0.73 µg/mL, p < 0.001) USCN Life Science, E92845Hu | Significant inverse correlation between the serum renalase and residual renal function (r = −0.327, p = 0.001). Renalase was not related to blood pressure, heart rate or hemodialysis vintage. |
| J. Malyszko et al. 2012 [27] 34 HD patients | Mean serum renalase concentration in the study cohort was 17.51 6.73 μg/mL and it was significantly higher when compared with the healthy volunteers—3.99 1.73 μg/mL (p < 0.001) USCN Life Science, E92845Hu | Serum renalase correlated with creatinine (r = 0.43, p < 0.05), residual renal function (r = 0.39, p < 0.05). The only predictor of renalase in multiple regression analysis was the presence of hypertension explaining 90% of the renalase variations. |
| E.-Zorawska et al. 2012 [28] 60 HD patients | Mean level of renalase was significantly higher in HD patients when compared to the control group (27.53 ± 9.39 µg/mL vs. 4.00 ± 1.37 µg/mL, p < 0.001 USCN Life Science, E92845Hu | Renalase appeared to be unrelated to Vascular adhesion protein-1. |
| E. Zbroch et al. 2012 [21] 26 PD patients | Serum concentration of renalase was significantly higher in patients dialyzed for more than 6 months than in those dialyzed for fewer than 6 months (21.15 ± 4.58 μg/mL vs. 16.63 ± 2.86 μg/mL, p = 0.008) USCN Life Science, E92845Hu | Renalase was not related to BP control, BP level, sex, dialysis adequacy, or residual renal function. |
| E. Zbroch et al. 2013 [29] 75 HD patients 26 PD patients | HD patients had higher renalase levels (27.49 ± 6.9 ug/mL) Renalase were higher in dialyzed groups (19.24 ± 4.5 ug/mL) comparing to healthy volunteers (3.86 ± 0.74 ug/mL) USCN Life Science, E92845Hu | Renalase correlated with dialysis vintage and inversely with residual diuresis. HD population with CAD had higher renalase level than their PD counterparts. |
| M. Dziedzic et al. 2014 [30] 49 HD patients | The mean concentration of renalase in the entire study population was 126.59 ± 32.63 ng/mL USCN Life Science, E92845Hu | Inverse correlation between NT-proBNP and renalase plasma levels in HD patients were due to impaired kidney function, accompanied by increased sympathetic nerve activity, which have an impact on the development of hypertension and cardiovascular complications. |
| E. G. Oguz et al. 2016 [31] 50 HD patients 35 healthy controls | Serum renalase levels were significantly higher in HD patients (212 ± 127 ng/mL) compared to controls (116 ± 67 ng/mL) (p < 0.001). USCN Life Science, E92845Hu | Renalase was positively correlated with serum creatinine and dialysis vintage (r = 0.677, p <0.001 and r = 0.625, p < 0.001, respectively). There was no significant association of renalase with LVMI in the HD patients (r = 0.263, p = 0.065). |
| E. G. Oguz et al. 2017 [32] 40 PD patients 40 healthy controls | Serum renalase level was significantly higher in the PD patients than in the control group [176.5 (100–278.3) vs. 122 (53.3–170.0) ng/mL] (p = 0.001) USCN Life Science, E92845Hu | Renalase was negatively correlated with RRF (r = −0.511, p = 0.021). Renalase is associated with residual renal function but not with CVD risk factors in PD patients. |
| M. Wisniewska et al. 2021 [33] 77 HD patients 30 healthy controls | Renalase serum concentrations in CKD patients were significantly increased when compared with control subjects (185.5 ± 64.3 vs. 19.6 ± 5.0 ng/mL; p < 0.00001 ELISA kit EIAab | The decreased plasma concentrations of catecholamines may be due to their increased degradation by plasma renalase. |
| Renal Transplant | ||
| J. Malyszko et al. 2011 [19] 89 kidney allograft recipients 27 healthy volunteers | The mean serum renalase among recipients was significantly higher compared with the control group (6.72 ± 4.50 µg/mL vs. 3.86 ± 0.73 µg/mL; p < 0.001) USCN Life Science, E92845Hu | In kidney transplant recipients, renalase correlated serum creatinine (r = 0.49; p < 0.001) and estimated glomerular filtration rate r = −0.44; p < 0.0001 |
| E. Zbroch et al. 2012 [34] 62 kidney allograft recipients 27 healthy volunteers | The mean serum renalase level in kidney allograft recipients was significantly higher compared with the control group (6.72 ± 2.86 µg/mL vs. 3.86 ± 0.73 µg/mL, p < 0.001 USCN Life Science, E92845Hu | In hypertensive allograft recipients, renalase was significantly higher than in normotensives. A multiple regression analysis showed that renalase was predicted in 58% by serum creatinine. |
| D. Stojanovic et al. 2015 [35] 73 renal TX recipientes | Renalase ng/mL Renal transplant recipients (141.82 ± 36.47) Control group (16.36 ± 4.13) USCN Life Science, E92845Hu | Significant risk of reduced glomerular filtration rate in transplant recipients with increased renalase concentration (p = 0.026). Renalase was shown to be strong predictor of decreased glomerular filtration rate. |
| D. Stojanovic et al. 2017 [36] 73 renal TX recipients | Plasma renalase level was increased compared to controls, 141.82 ng/mL vs. 16.36 ng/mL, p < 0.0001 USCN Life Science, E92845Hu | Significant inverse correlation between renalase and estimated glomerular filtration rate (r = −0.552, p < 0.001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerqueira, A.; Quelhas-Santos, J.; Ferreira, I.; Sampaio, S.; Relvas, M.; Marques, N.; Dias, C.C.; Pestana, M. Circulating Renalase as Predictor of Renal and Cardiovascular Outcomes in Pre-Dialysis CKD Patients: A 5-Year Prospective Cohort Study. Life 2021, 11, 210. https://doi.org/10.3390/life11030210
Cerqueira A, Quelhas-Santos J, Ferreira I, Sampaio S, Relvas M, Marques N, Dias CC, Pestana M. Circulating Renalase as Predictor of Renal and Cardiovascular Outcomes in Pre-Dialysis CKD Patients: A 5-Year Prospective Cohort Study. Life. 2021; 11(3):210. https://doi.org/10.3390/life11030210
Chicago/Turabian StyleCerqueira, Ana, Janete Quelhas-Santos, Inês Ferreira, Susana Sampaio, Miguel Relvas, Nídia Marques, Cláudia Camila Dias, and Manuel Pestana. 2021. "Circulating Renalase as Predictor of Renal and Cardiovascular Outcomes in Pre-Dialysis CKD Patients: A 5-Year Prospective Cohort Study" Life 11, no. 3: 210. https://doi.org/10.3390/life11030210
APA StyleCerqueira, A., Quelhas-Santos, J., Ferreira, I., Sampaio, S., Relvas, M., Marques, N., Dias, C. C., & Pestana, M. (2021). Circulating Renalase as Predictor of Renal and Cardiovascular Outcomes in Pre-Dialysis CKD Patients: A 5-Year Prospective Cohort Study. Life, 11(3), 210. https://doi.org/10.3390/life11030210







