Treadmill Exercise after Controlled Abnormal Joint Movement Inhibits Cartilage Degeneration and Synovitis
Abstract
1. Introduction
2. Materials and Methods
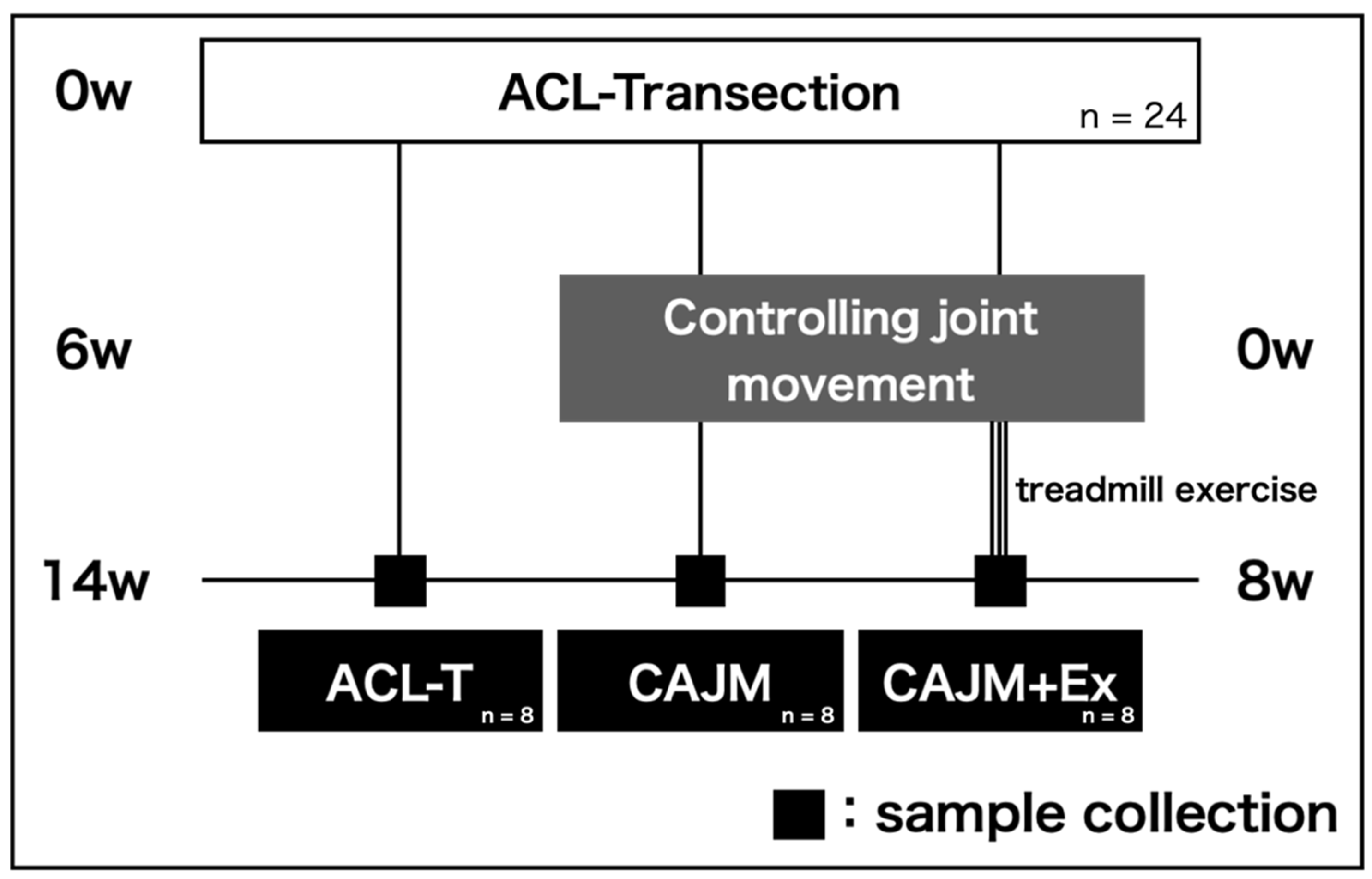
2.1. Research Design
2.2. Surgical Procedure
2.3. Exercise Intervention
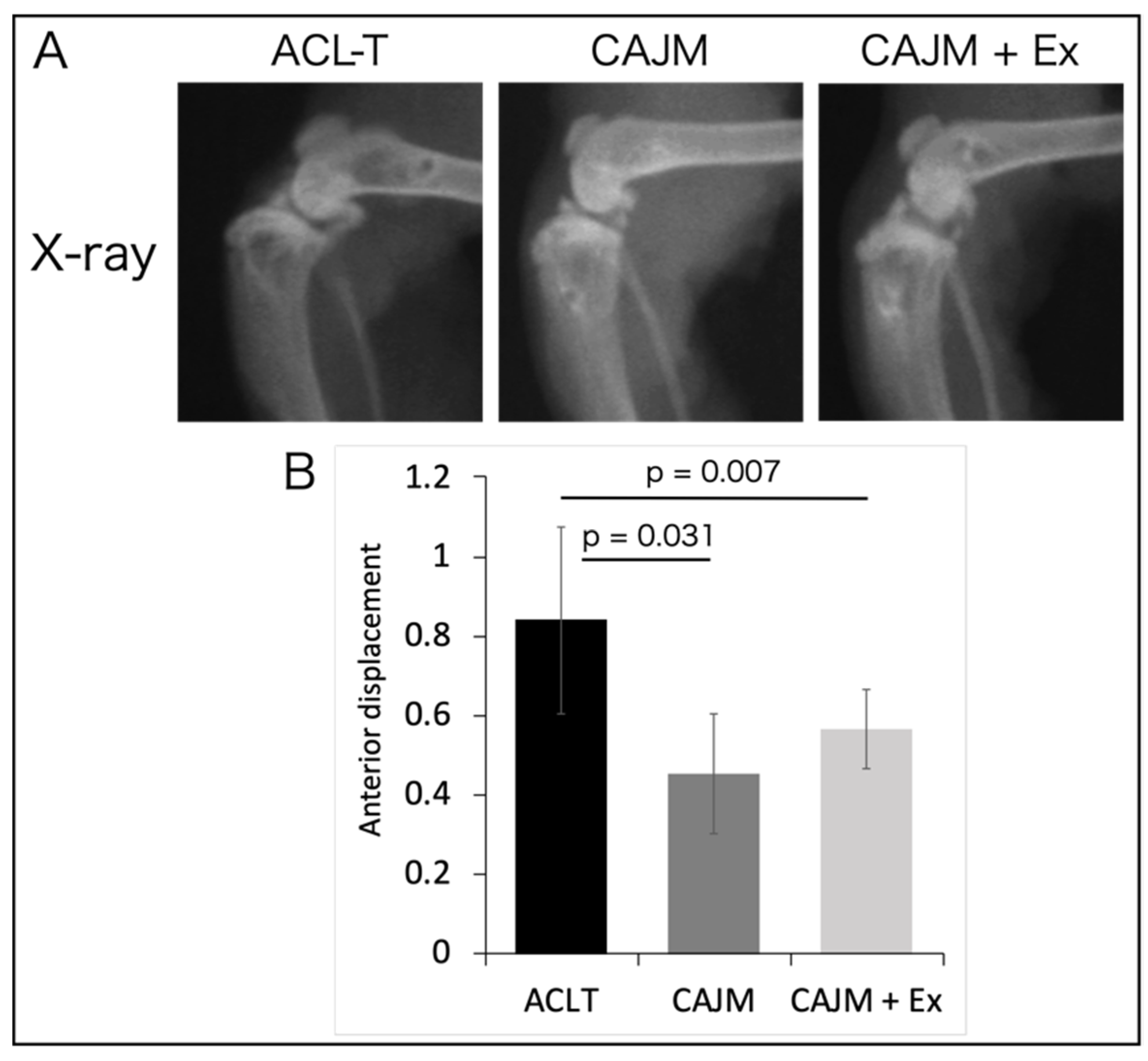
2.4. Evaluation of Tibial Malposition
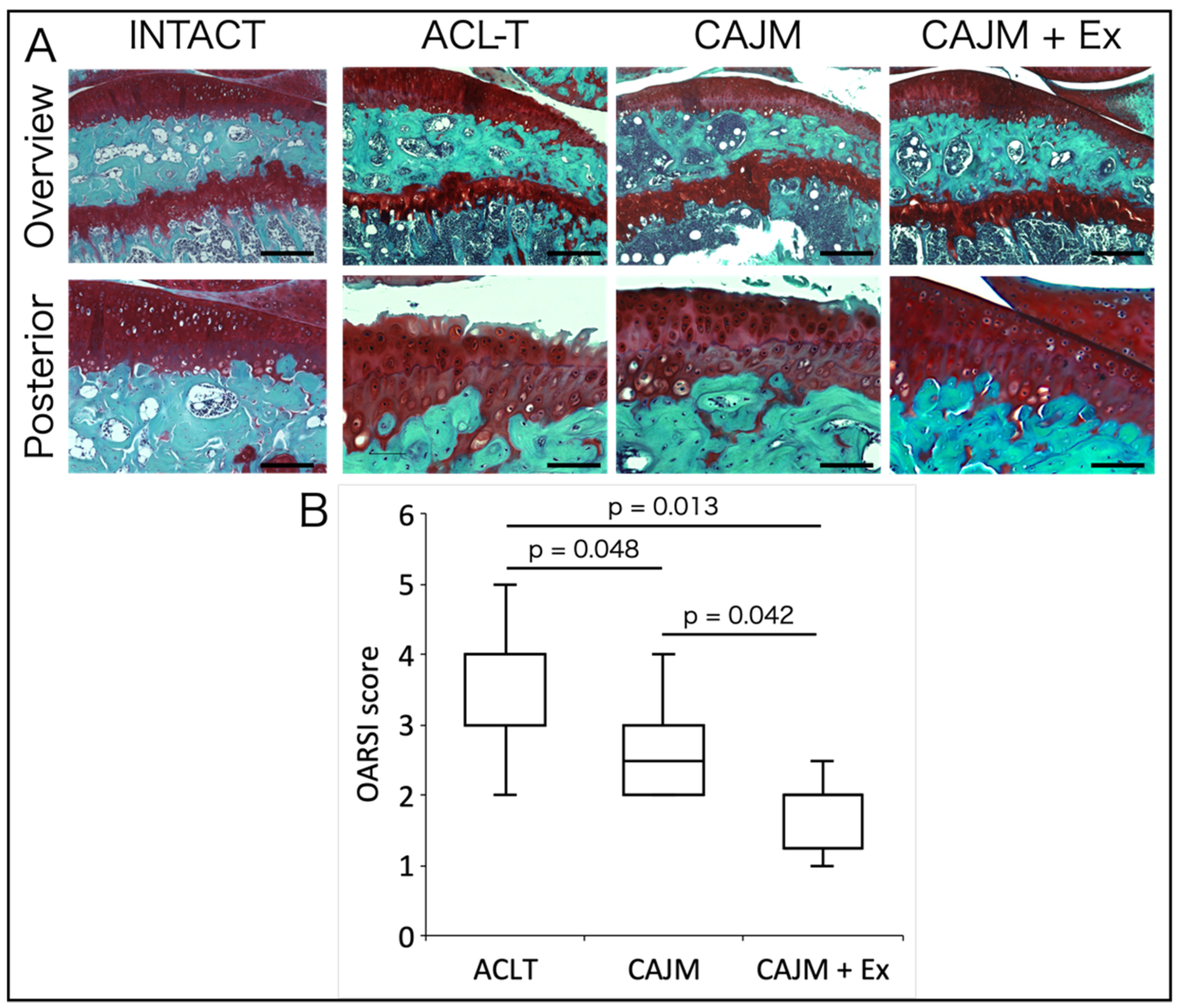
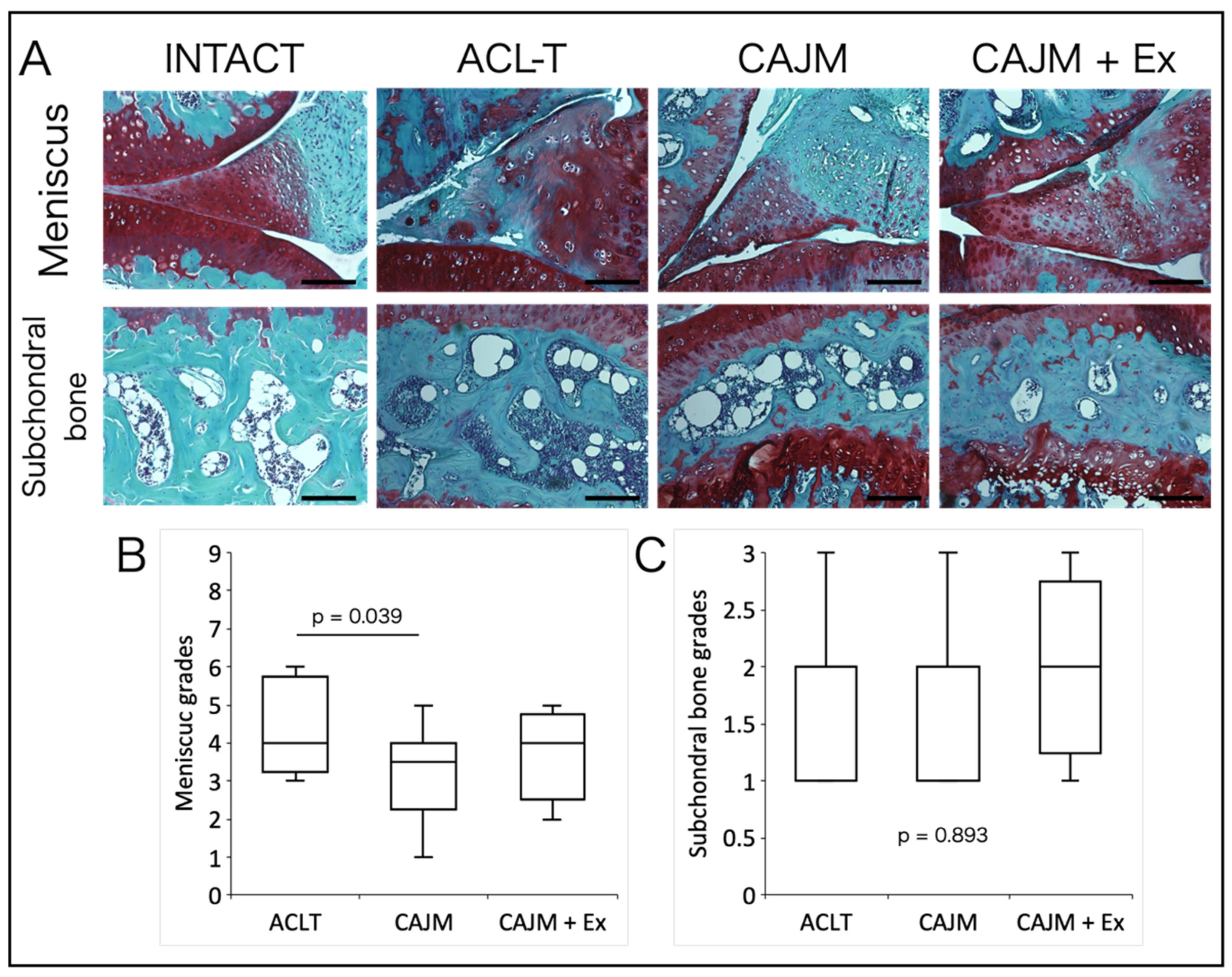
2.5. Histological Analysis
2.6. Immunohistochemistry (IHC) Analysis
2.7. Statistical Analysis
3. Results
3.1. CAJM Suppresses Anterior Displacement of the Tibia
3.2. Treadmill Exercise After CAJM Inhibits Cartilage Degeneration
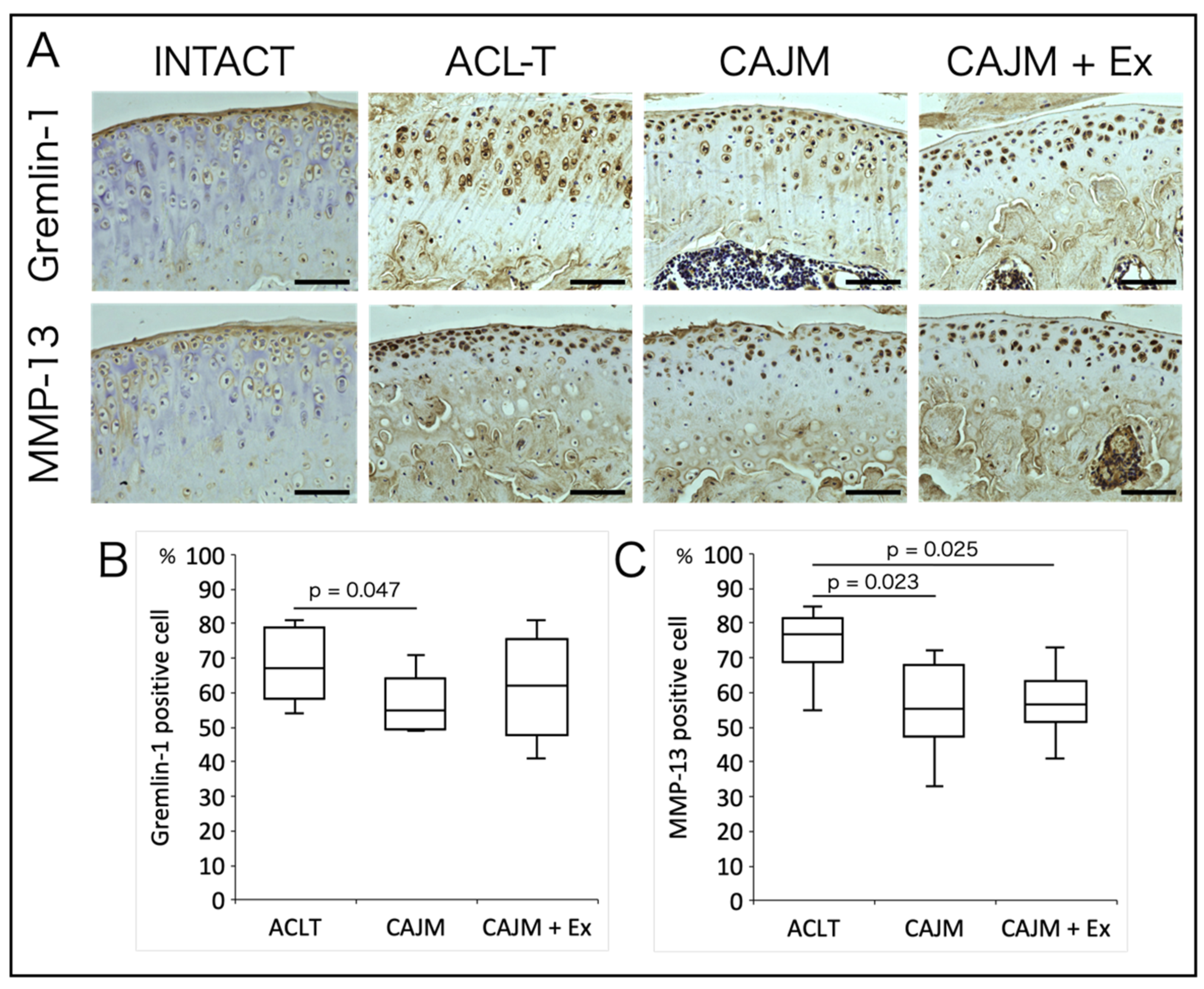
3.3. CAJM Inhibits the Expression of Gremlin-1
3.4. Treadmill Exercise Suppresses Synovitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef]
- Collins, K.H.; Reimer, R.A.; Seerattan, R.A.; Leonard, T.R.; Herzog, W. Using diet-induced obesity to understand a metabolic subtype of osteoarthritis in rats. Osteoarthr. Cartil. 2015, 23, 957–965. [Google Scholar] [CrossRef]
- Gabay, O.; Hall, D.J.; Berenbaum, F.; Henrotin, Y.; Sanchez, C. Osteoarthritis and obesity: Experimental models. Jt. Bone Spine 2008, 75, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Nelson, F.; Billinghurst, R.C.; Pidoux, R.T.; Reiner, A.; Langworthy, M.; McDermott, M.; Malogne, T.; Sitler, D.F.; Kilambi, N.R.; Lenczner, E.; et al. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthr. Cartil. 2006, 14, 114–119. [Google Scholar] [CrossRef]
- Felson, D.T. Osteoarthritis as a disease of mechanics. Osteoarthr. Cartil. 2013, 21, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Tomazoni, S.S.; Leal-Junior, E.C.P.; Frigo, L.; Pallotta, R.C.; Teixeira, S.; de Almeida, P.; Bjordal, J.M.; Lopes-Martins, R.Á.B. Isolated and combined effects of photobiomodulation therapy, topical nonsteroidal anti-inflammatory drugs, and physical activity in the treatment of osteoarthritis induced by papain. J. Biomed. Opt. 2016, 21, 108001. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Leal-Junior, E.C.P.; Pallotta, R.C.; Teixeira, S.; de Almeida, P.; Lopes-Martins, R.Á.B. Effects of photobiomodulation therapy, pharmacological therapy, and physical exercise as single and/or combined treatment on the inflammatory response induced by experimental osteoarthritis. Lasers Med. Sci. 2017, 32, 101–108. [Google Scholar] [CrossRef]
- Assis, L.; Milares, L.P.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.R.; Medalha, C.; Renno, A.C.M. Aerobic exercise training and low-level laser therapy modulate in fl ammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cartil. 2016, 24, 169–177. [Google Scholar] [CrossRef]
- Pallotta, R.C.; Bjordal, J.M.; Frigo, L.; Leal Junior, E.C.P.; Teixeira, S.; Marcos, R.L.; Ramos, L.; De Moura Messias, F.; Lopes-Martins, R.Á.B. Infrared (810-nm) low-level laser therapy on rat experimental knee inflammation. Lasers Med. Sci. 2012, 27, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.A.; Oliveira, P.; Fernandes, K.R.; Rhon, L.; Tim, C.R.; Vasilceac, F.A.; Pinto, K.N.Z.; Mattiello, S.M.; Parizotto, N.A.; Renno, A.C.M. Effects of low-level laser therapy on cartilage repair in an experimental model of osteoarthritis. Photonics Lasers Med. 2014, 3, 255–264. [Google Scholar] [CrossRef]
- Lim, B.W.; Hinman, R.S.; Wrigley, T.V.; Sharma, L.; Bennell, K.L. Does knee malalignment mediate the effects of quadriceps strengthening on knee adduction moment, pain, and function in medial knee osteoarthritis? A randomized controlled trial. Arthritis Care Res. 2008, 59, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Blazek, A.D.; Nam, J.; Gupta, R.; Pradhan, M.; Perera, P.; Weisleder, N.L.; Hewett, T.E.; Chaudhari, A.M.; Lee, B.S.; Leblebicioglu, B.; et al. Exercise-driven metabolic pathways in healthy cartilage. Osteoarthr. Cartil. 2016, 24, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Perera, P.; Liu, J.; Wu, L.C.; Rath, B.; Butterfield, T.A.; Agarwal, S. Transcriptome-wide gene regulation by gentle treadmill walking during the progression of monoiodoacetate-induced arthritis. Arthritis Rheum. 2011, 63, 1613–1625. [Google Scholar] [CrossRef]
- Galois, L.; Etienne, S.; Grossin, L.; Watrin-Pinzano, A.; Cournil-Henrionnet, C.; Loeuille, D.; Netter, P.; Mainard, D.; Gillet, P. Dose-response relationship for exercise on severity of experimental osteoarthritis in rats: A pilot study. Osteoarthr. Cartil. 2004, 12, 779–786. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Aoyama, T.; Ito, A.; Nagai, M.; Iijima, H.; Zhang, X.; Tajino, J.; Kuroki, H. Effects of exercise level on biomarkers in a rat knee model of osteoarthritis. J. Orthop. Res. 2013, 31, 1026–1031. [Google Scholar] [CrossRef]
- Iijima, H.; Aoyama, T.; Ito, A.; Yamaguchi, S.; Nagai, M.; Tajino, J.; Zhang, X.; Kuroki, H. Effects of short-term gentle treadmill walking on subchondral bone in a rat model of instability-induced osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1563–1574. [Google Scholar] [CrossRef]
- Iijima, H.; Aoyama, T.; Ito, A.; Tajino, J.; Yamaguchi, S.; Nagai, M.; Kiyan, W.; Zhang, X.; Kuroki, H. Exercise intervention increases expression of bone morphogenetic proteins and prevents the progression of cartilage-subchondral bone lesions in a post-traumatic rat knee model. Osteoarthr. Cartil. 2016, 24, 1092–1102. [Google Scholar] [CrossRef]
- Iijima, H.; Ito, A.; Nagai, M.; Tajino, J.; Yamaguchi, S.; Kiyan, W.; Nakahata, A.; Zhang, J.; Wang, T.; Aoyama, T.; et al. Physiological exercise loading suppresses post-traumatic osteoarthritis progression via an increase in bone morphogenetic proteins expression in an experimental rat knee model. Osteoarthr. Cartil. 2017, 25, 964–975. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Li, X.; Zhang, H.; Gang, Y.; Bai, L. Alterations of autophagy in knee cartilage by treatment with treadmill exercise in a rat osteoarthritis model. Int. J. Mol. Med. 2019, 43, 336–344. [Google Scholar] [CrossRef]
- Chen, L.; Lou, Y.; Pan, Z.; Cao, X.; Zhang, L.; Zhu, C.; Liang, J. Treadmill and wheel exercise protect against JNK/NF-κB induced inflammation in experimental models of knee osteoarthritis. Biochem. Biophys. Res. Commun. 2020, 523, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Kanemura, N.; Kokubun, T.; Fujino, T.; Morishita, Y.; Onitsuka, K.; Fujiwara, S.; Nakajima, A.; Shimizu, D.; Takayanagi, K. Controlling joint instability delays the degeneration of articular cartilage in a rat model. Osteoarthr. Cartil. 2017, 25, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Kokubun, T.; Morishita, Y.; Onitsuka, K.; Fujiwara, S.; Nakajima, A.; Fujino, T.; Takayanagi, K.; Kanemura, N. Controlling Abnormal Joint Movement Inhibits Response of Osteophyte Formation. Cartilage 2017, 194760351770095. [Google Scholar] [CrossRef] [PubMed]
- Onitsuka, K.; Murata, K.; Kokubun, T.; Fujiwara, S.; Nakajima, A.; Morishita, Y.; Kanemura, N. Effects of Controlling Abnormal Joint Movement on Expression of MMP13 and TIMP-1 in Osteoarthritis. Cartilage 2018. [Google Scholar] [CrossRef]
- Oka, Y.; Murata, K.; Kano, T.; Ozone, K.; Arakawa, K.; Kokubun, T.; Kanemura, N. Impact of Controlling Abnormal Joint Movement on the Effectiveness of Subsequent Exercise Intervention in Mouse Models of Early Knee Osteoarthritis. Cartilage 2019. [Google Scholar] [CrossRef]
- McNulty, M.A.; Loeser, R.F.; Davey, C.; Callahan, M.F.; Ferguson, C.M.; Carlson, C.S. Histopathology of naturally occurring and surgically induced osteoarthritis in mice. Osteoarthr. Cartil. 2012, 20, 949–956. [Google Scholar] [CrossRef]
- Kwok, J.; Onuma, H.; Olmer, M.; Lotz, M.K.; Grogan, S.P.; D’Lima, D.D. Histopathological analyses of murine menisci: Implications for joint aging and osteoarthritis. Osteoarthr. Cartil. 2016, 24, 709–718. [Google Scholar] [CrossRef]
- Aho, O.M.; Finnilä, M.; Thevenot, J.; Saarakkala, S.; Lehenkari, P. Subchondral bone histology and grading in osteoarthritis. PLoS ONE 2017, 12, 1–16. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef]
- Chang, S.H.; Mori, D.; Kobayashi, H.; Mori, Y.; Nakamoto, H.; Okada, K.; Taniguchi, Y.; Sugita, S.; Yano, F.; Chung, U.; et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1–NF-κB pathway. Nat. Commun. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Kamekura, S.; Hoshi, K.; Shimoaka, T.; Chung, U.; Chikuda, H.; Yamada, T.; Uchida, M.; Ogata, N.; Seichi, A.; Nakamura, K.; et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr. Cartil. 2005, 13, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, T.; Kanemura, N.; Murata, K.; Moriyama, H.; Morita, S.; Jinno, T.; Ihara, H.; Takayanagi, K. Effect of Changing the Joint Kinematics of Knees with a Ruptured Anterior Cruciate Ligament on the Molecular Biological Responses and Spontaneous Healing in a Rat Model. Am. J. Sports Med. 2016, 44, 2900–2910. [Google Scholar] [CrossRef]
- Murata, K.; Kokubun, T.; Onitsuka, K.; Oka, Y.; Kano, T.; Morishita, Y.; Ozone, K.; Kuwabara, N.; Nishimoto, J.; Isho, T.; et al. Controlling joint instability after anterior cruciate ligament transection inhibits transforming growth factor-beta-mediated osteophyte formation. Osteoarthr. Cartil. 2019, 27, 1185–1196. [Google Scholar] [CrossRef]
- Proctor, C.S.; Schmidt, M.B.; Whipple, R.R.; Kelly, M.A.; Mow, V.C. Material properties of the normal medial bovine meniscus. J. Orthop. Res. 1989, 7, 771–782. [Google Scholar] [CrossRef]
- Radin, E.L.; Parker, H.G.; Pugh, J.W.; Steinberg, R.S.; Paul, I.L.; Rose, R.M. Response of joints to impact loading—III. Relationship between trabecular microfractures and cartilage degeneration. J. Biomech. 1973, 6. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Plaas, A.; Crow, M.K. Innate immune system activation in osteoarthritis: Is osteoarthritis a chronic wound? Curr. Opin. Rheumatol. 2008, 20, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Lopa, S.; Leijs, M.J.C.; Moretti, M.; Lubberts, E.; van Osch, G.J.V.M.; Bastiaansen-Jenniskens, Y.M. Arthritic and non-arthritic synovial fluids modulate IL10 and IL1RA gene expression in differentially activated primary human monocytes. Osteoarthr. Cartil. 2015, 23, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Tsuji, K.; Onuma, H.; Udo, M.; Ueki, H.; Akiyama, M.; Abula, K.; Katagiri, H.; Miyatake, K.; Watanabe, T.; et al. Persistent synovial inflammation plays important roles in persistent pain development in the rat knee before cartilage degradation reaches the subchondral bone. BMC Musculoskelet. Disord. 2018, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Yano, H.; Yokogawa, Y.; Suzuki, K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 2010, 16, 105–118. [Google Scholar]
- Mawatari, T.; Lindsey, D.P.; Harris, A.H.S.; Goodman, S.B.; Maloney, W.J.; Smith, R.L. Effects of tensile strain and fluid flow on osteoarthritic human chondrocyte metabolism in vitro. J. Orthop. Res. 2010, 28, 907–913. [Google Scholar] [CrossRef]
- Chu, F.; Feng, Q.; Hu, Z.; Shen, G. Appropriate cyclic tensile strain promotes biological changes of cranial base synchondrosis chondrocytes. Orthod. Craniofacial Res. 2017, 20, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Brinkley, T.E.; Nicklas, B.J. Effect of exercise training on chronic inflammation. Clin. Chim. Acta 2010, 411, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.K.; Dykes, R.; Douglas, J.E.; Krishnaswamy, G.; Berk, S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. J. Am. Med. Assoc. 1999, 281, 1722–1727. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oka, Y.; Murata, K.; Ozone, K.; Kano, T.; Minegishi, Y.; Kuro-Nakajima, A.; Arakawa, K.; Kokubun, T.; Kanemura, N. Treadmill Exercise after Controlled Abnormal Joint Movement Inhibits Cartilage Degeneration and Synovitis. Life 2021, 11, 303. https://doi.org/10.3390/life11040303
Oka Y, Murata K, Ozone K, Kano T, Minegishi Y, Kuro-Nakajima A, Arakawa K, Kokubun T, Kanemura N. Treadmill Exercise after Controlled Abnormal Joint Movement Inhibits Cartilage Degeneration and Synovitis. Life. 2021; 11(4):303. https://doi.org/10.3390/life11040303
Chicago/Turabian StyleOka, Yuichiro, Kenij Murata, Kaichi Ozone, Takuma Kano, Yuki Minegishi, Aya Kuro-Nakajima, Kohei Arakawa, Takanori Kokubun, and Naohiko Kanemura. 2021. "Treadmill Exercise after Controlled Abnormal Joint Movement Inhibits Cartilage Degeneration and Synovitis" Life 11, no. 4: 303. https://doi.org/10.3390/life11040303
APA StyleOka, Y., Murata, K., Ozone, K., Kano, T., Minegishi, Y., Kuro-Nakajima, A., Arakawa, K., Kokubun, T., & Kanemura, N. (2021). Treadmill Exercise after Controlled Abnormal Joint Movement Inhibits Cartilage Degeneration and Synovitis. Life, 11(4), 303. https://doi.org/10.3390/life11040303





