Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy
Abstract
1. Introduction
2. Andrographis paniculata—“The King of Bitter”
2.1. Botanical Data of A. paniculata
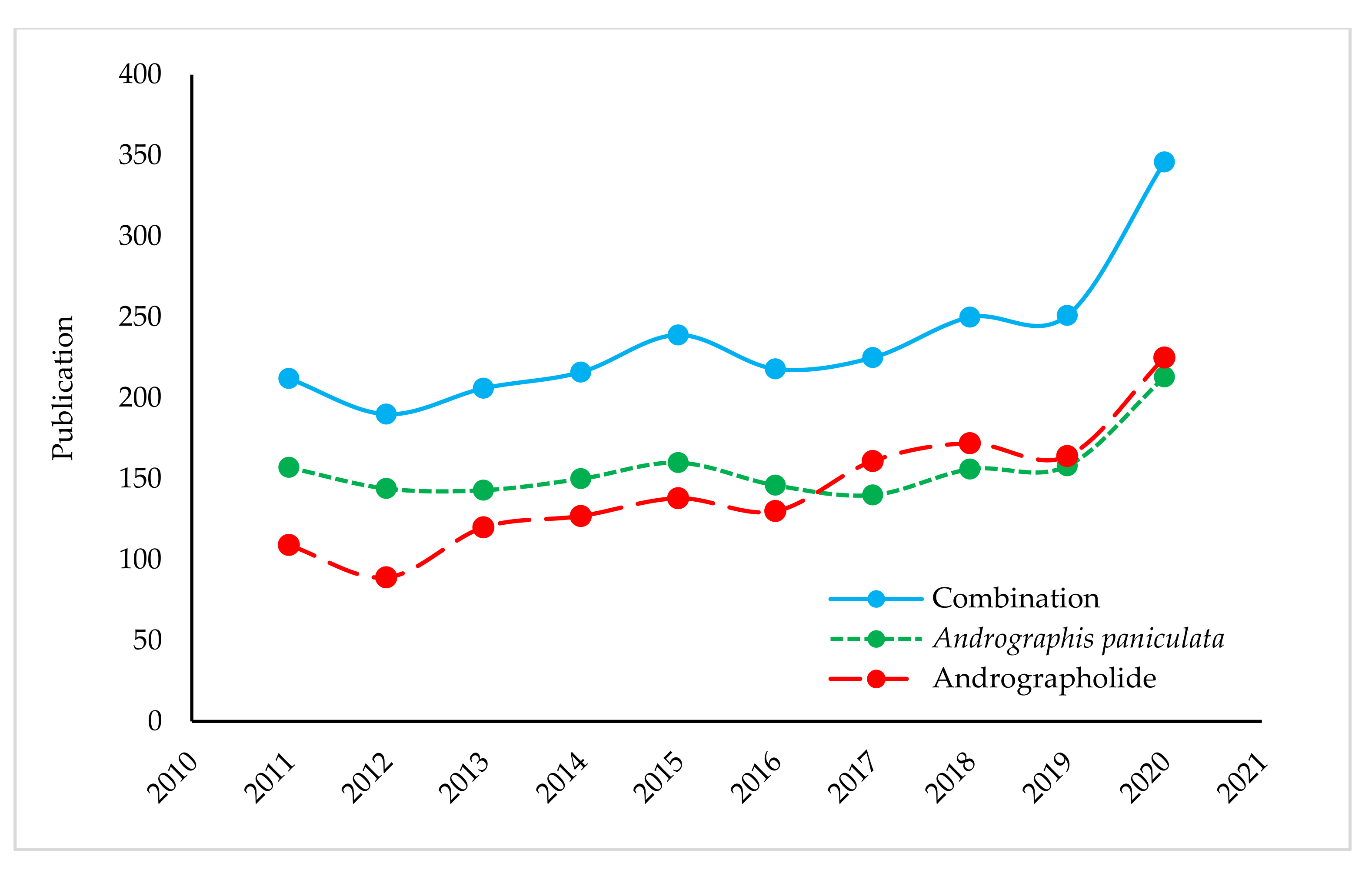
2.2. Recent Progress in Publication of A. paniculata
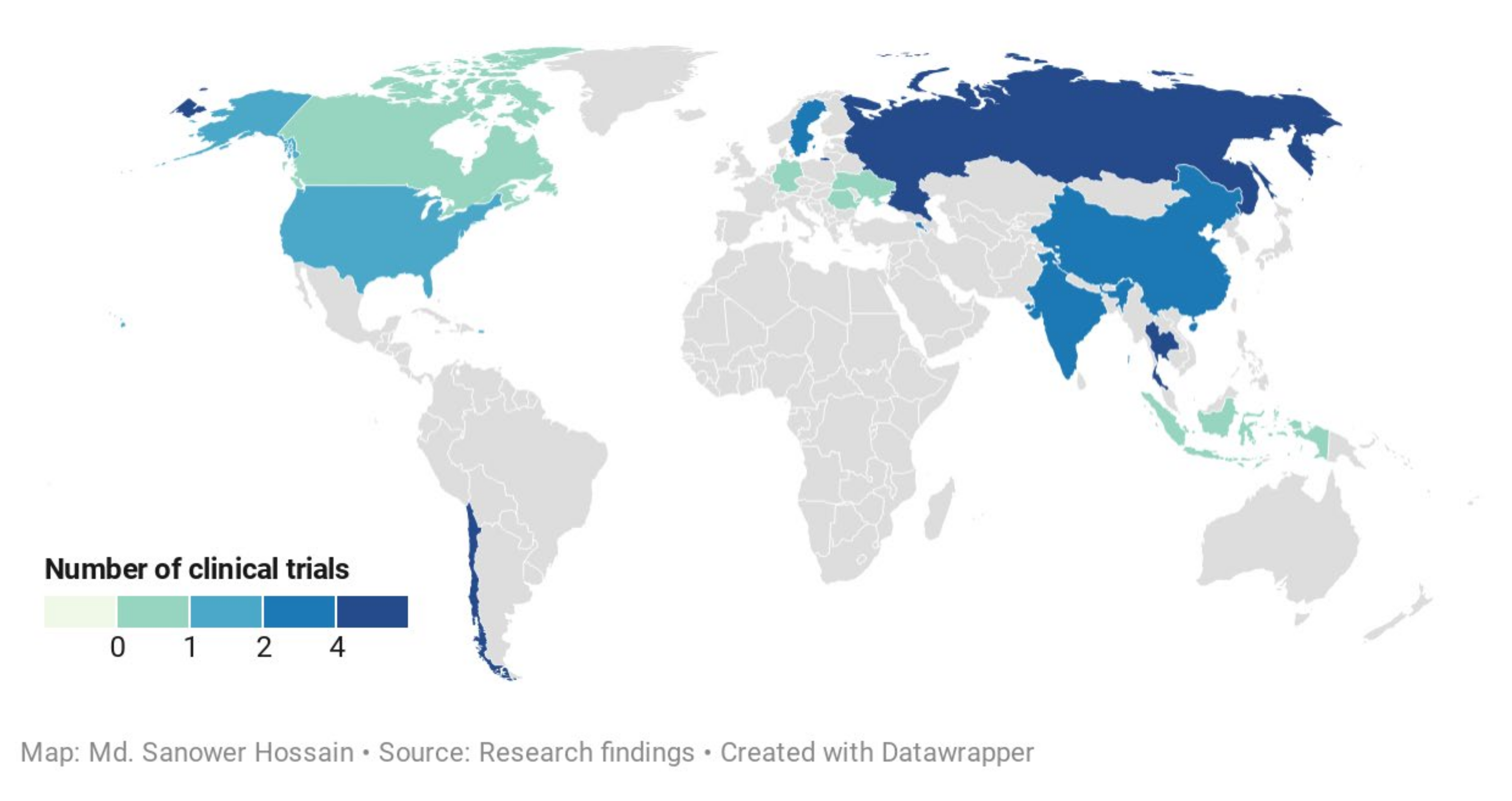
3. Invasive Microbes Used in the Antimicrobial Study of A. paniculata
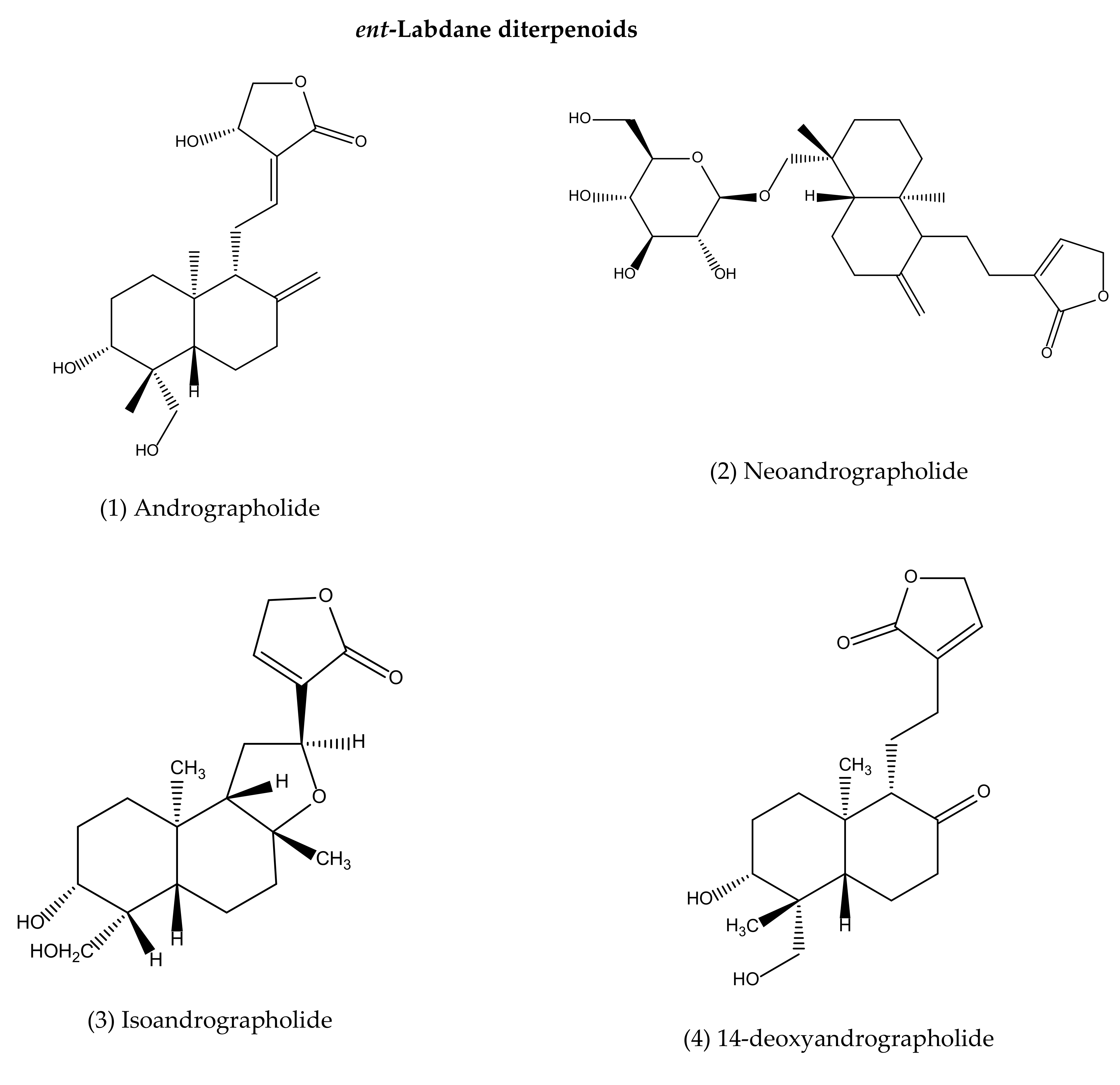
4. Antimicrobial Secondary Metabolites of A. paniculata
| No. | Name | Sources | Extraction Solvent | Analytical Technique | Antimicrobial Potentiality |
|---|---|---|---|---|---|
| ent-Labdane diterpenoids | |||||
| 1 | Andrographolide | AeP, L, R, WP | AW, E, H, M | HPLC, HPLC-MS, MECC, FIS, UPLC, HPLC-DAD | Antibacterial [139,170,171,172,173], anti-biofilm [88,139], anti-CHIKV [91], Anti-HIV [177,178,179], anti-influenza [99,180], anti-HSV-1 [83,99,181], anti-DVS-1 [92,93,94,182], anti-EBV [97], anti-HPV-16 [96], anti-HBV [186], anti-HCV [194], antimalarial [95,184,185], anti-Leishmaniasis [98], pestivirus and flavivirus [90] |
| 2 | Neoandrographolide | AeP, L, R, WP | AW, E, M | TLC | Anti-HSV-1 [99], antimalarial [184] |
| 3 | Isoandrographolide | AeP, L, R, WP | AW, E, M | HPLC, TLC | Antibacterial [109,174,175], anti-fungal [183] |
| 4 | 14-deoxyandrographolide | AeP, L, WP | AW, E, H, M | HPLC, TLC | Antibacterial [174,175], anti-fungal [183], antimalarial [109,184] |
| 5 | 14-deoxy-11, 12-didehydroxiandrographolide | AeP, L, WP | E, H, M, DCM | HPLC, TLC | Antibacterial [109], anti-biofilm [88], Antifungal [109,183], anti-HIV [177], Anti-HSV [99] |
| 6 | 14-deoxy—11-oxo- andrographolide | AeP, L | AW, M | SGC | Anti-Leishmaniasis [89,167] |
| 7 | 3-O-β-D-glucosyl-14-deoxy- andrographolide | AeP, WP | E, M | HPLC, TLC | Antibacterial [174,175], anti-fungal [183] |
| 8 | 14-Deoxy-12-hydroxy- andrographolide | AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 9 | 3-O-β-D-glucopyranosyl- 14,19-dideoxyandrographolide | AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 10 | 3-O-β-D-glucopyranosyl- andrographolide | AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 11 | 8,17-Epoxy-14-deoxy-andrographolide | AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 12 | 14-Deoxy-17-β-hydroxy- andrographolide | AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 13 | 19-O-[β-D-apiofuranosyl-β-D-glucopyranoyl]-3,14-dideoxy- andrographolide | AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 14 | Echiodinin | Callus | AW, M | TLC | Antibacterial [80] |
| 15 | Andrograpanin | AeP, L | E, H | HPLC, TLC, SGC | Antibacterial [109], anti-fungal [109], Antibiofilm [168,176] |
| 16 | Andrographiside | WP | n-butanol | TLC | Antimalarial [184] |
| Xanthones | |||||
| 17 | 1,2-Dihydroxy-6,8-dimethoxyxanthone | R | CF, M, PE, W | TLC | Antimalarial [190,191] |
| 18 | 1,8-Dihydroxy-3,7-dimethoxyxanthone | R | CF, M, PE, W | TLC | Antimalarial [190,191] |
| 19 | 3,7,8-Trimethoxy-1-hydroxyxanthone | R | CF, M, PE, W | TLC | Antimalarial [190,191] |
| 20 | 4,8-Dihydroxy-2,7-dimethoxyxanthone | R | CF, M, PE, W | TLC | Antimalarial [190,191] |
5. Antimicrobial Pharmacology
5.1. Antibacterial Effects
5.1.1. A. paniculata Extracts as Antibacterial Agents
| Plant Part | Extraction Methods | Assay | Number of Test MOs | Most Inhibited Mos | MEIC | ZOI (mm or %) | Remarks | Reference |
|---|---|---|---|---|---|---|---|---|
| AeP | Chloroform | AWDM | 9 | Enterobacter faecalis | 250 μg/mL | 35 | Seven out of 9 pathogens were inhibited that were comparable with antibiotic, amikacin | [203] |
| L | Water | DDM | 5 | P. aeruginosa S. aureus MRSA | 2 µg/disc 1000 µg/disc 250 µg/disc | 8 ± 0.1 6 ± 0.1 8 ± 0.1 | No activity against K. pneumoniae and E. coli. | [200] |
| L | 70% Methanol | Two-fold broth MDM | 10 | Edwardsiella tarda E. coli Flavobacterium sp. P. aeruginosa Vibrio cholerae | 31.5 mg/L | - | All the test MOs were inhibited | [204] |
| WP | DCM | DDM | 12 | E. faecalis S. aureus S. saprophyticus | 1000 µg/disc | 21.33 ± 1.53 20.00 ± 1.50 19.33 ± 1.15 | Gram-negative bacteria were more resistant. | [205] |
| WP | Methanol | E. faecalis S. aureus S. saprophyticus | 1000 µg/disc | 24.00 ± 0.00 22.00 ± 0.00 22.00 ± 1.53 | No activity observed against S. saprophyticus at 250 µg/disc | |||
| WP | Aqueous | M. luteus S. pyogenes E. faecalis | 1000 µg/disc | 23.17 ± 0.76 22.67 ± 0.58 22.00 ± 1.00 | No activity was observed against M. luteus, S. pyogenes, E. faecalis and K. pneumoniae at 250 µg/disc | |||
| R | Hexane | Broth MDM | 4 | B. pumilus B. subtilis E. coli Proteus vulgaris | 100 mg/mL 100 mg/mL 200 mg/mL 200 mg/mL | 12 12 13 12 | Hexane and methanolic extracts were more efficient against all tested MOs | [206] |
| R | Methanol | Broth MDM | 4 | E. coli B. subtilis Proteus vulgaris | 100 mg/mL 200 mg/mL 200 mg/mL | 12 12 13 | ||
| WP | Methanol | CPADM | 5 | S. aureus | 1000 μg/mL | 19.67 ± 0.76 | Gram-negative bacteria were more resistant to methanol extracts | [174] |
| AeP | Ethanol | AWDM | 11 | S. typhi V. cholerae | 200 μg/mL | 14 13 | The ethanol extract was efficient | [173] |
| L | Methanol | AWDM | 6 | S. aureus | 50 mg/mL | 24 ± 0.2 | Inhibit both Gram-positive and negative bacteria, but gram-negative bacteria are less susceptible | [201] |
| WP | DCM | DDM | 10 | S. aureus | 1000 μg/disc | 20 ± 1.50 | Aqueous extracts were more effective compared to the DCM and methanol extracts | [207] |
| Methanol | S. aureus S.saprophyticus | 1000 μg/disc | 22 ± 0.00 22 ± 1.53 | |||||
| Aqueous | M. luteus | 1000 μg/disc | 23.17 ± 0.76 | |||||
| WP | Methanol | CPADM | 5 | S. aureus M. luteus | 1000 μg/mL | 19.67 ± 0.76 18.50 ± 0.58 | Effective against all test MOs | [175] |
| L | Chloroform | AWDM | 6 | B. subtilis | 22 ± 0.071 | Chloroform extract of leaves was more efficient to inhibit all tested MOs than other extracts | [208] | |
| Aqueous | 6 | K. pneumoniae S. aureus B. subtilis | 12 ± 0.344 12 ± 0.447 12 ± 0.084 | |||||
| Acetone | 6 | S. aureus | 13 ± 0.416 | |||||
| Ethyl acetate | 6 | B. subtilis | 15 ± 0.152 | |||||
| Petroleum ether | - | - | - | No inhibitory activity | ||||
| R | Chloroform | AWDM | 6 | B. subtilis | 18 ± 0.055 | Chloroform extract of roots was more efficient to inhibit all tested MOs than other extracts | [208] | |
| Aqueous | K. pneumoniae | 14 ± 0.297 | ||||||
| Acetone | S. aureus | 15 ± 0.055 | ||||||
| Ethyl acetate | B. subtilis | 10 ± 0.626 | ||||||
| DMSO | S. aureus | 14 ± 0.187 | ||||||
| Petroleum ether | - | - | - | No inhibitory activity | ||||
| S | Ethyl acetate | AWDM | 6 | S. aureus B. subtilis | 8 ± 0.303 8 ± 0.327 | Chloroform extract of stems was more efficient to inhibit all tested MOs than other extracts | [208] | |
| DMSO | S. aureus | 16 ± 0.332 | ||||||
| Acetone | S. aureus | 16 ± 0.374 | ||||||
| Chloroform | B. subtilis | 24 ± 0.219 | ||||||
| Aqueous | B. subtilis | 13 ± 0.373 | ||||||
| Petroleum ether | - | - | - | No inhibitory activity |
5.1.2. Isolated Compound as Antibacterial Agent: Mechanisms of Action
5.1.3. Mechanisms of Action Influence on Biofilm Production by Pure Compounds
5.2. Antiviral Effects
5.3. Antifungal Effects
5.4. Anti-Parasitic Effects
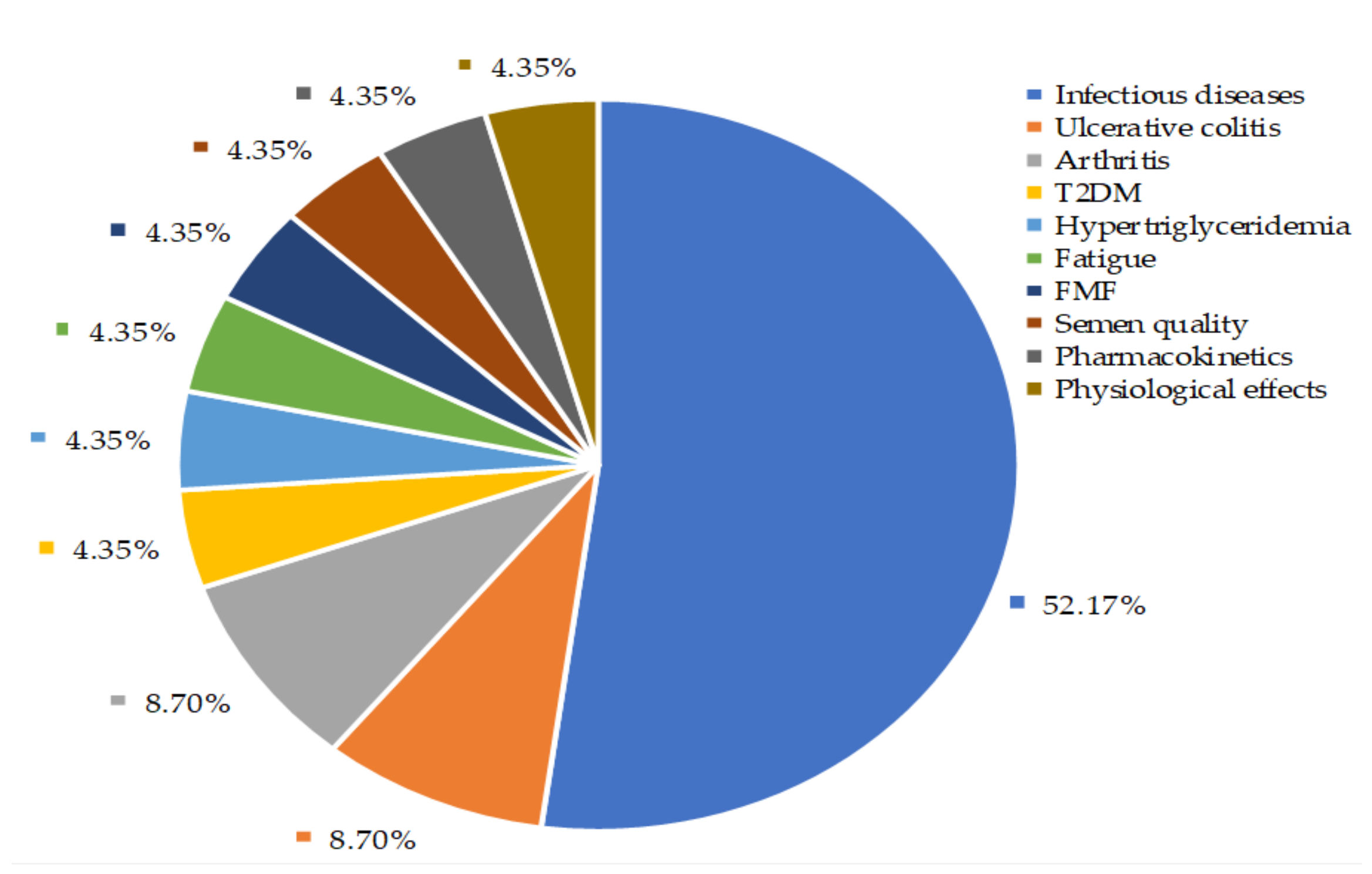
6. Controlled Clinical Trials of A. paniculata Treatment: A Systematic Evaluation
6.1. Evaluation of A. paniculata Efficacy Against Infections
6.2. Evaluation of Safety of A. paniculata Treatment
7. Methodology
8. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloom, D.E.; Cadarette, D. Infectious Disease Threats in the Twenty-First Century: Strengthening the Global Response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.F.; Alto, N.M. Cooperative Immune Suppression by Escherichia coli and Shigella Effector Proteins. Infect. Immun. 2018, 86, e00517–e00560. [Google Scholar] [CrossRef]
- Bizzell, E. Microbial Ninja Warriors: Bacterial Immune Evasion. Available online: https://asm.org/Articles/2018/December/Microbial-Ninja-Warriors-Bacterial-Immune-Evasion (accessed on 15 February 2021).
- WHO. Antimicrobial Resistance. Available online: http://www.who.int/mediacentre/factsheets/fs194/en/ (accessed on 20 October 2020).
- Namita, P.; Mukesh, R. Medicinal plants used as antimicrobial agents: A review. Int. Res. J. Pharm. 2012, 3, 31–40. [Google Scholar]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Bomar, P.A. Upper Respiratory Tract Infection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Aabenhus, R.; Hansen, M.P.; Saust, L.T.; Bjerrum, L. Characterisation of antibiotic prescriptions for acute respiratory tract infections in Danish general practice: A retrospective registry based cohort study. NPJ Prim. Care Respir Med. 2017, 27, 37. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.; O’Doherty, J.; O’Regan, A.; Dunne, C. Antibiotic use for acute respiratory tract infections (ARTI) in primary care; what factors affect prescribing and why is it important? A narrative review. Ir. J. Med. Sci. 2018, 187, 969–986. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.; Leader, L.F.W.; O’Regan, A.; Dunne, C.; Puthoopparambil, S.J.; O’Connor, R. Over prescribing of antibiotics for acute respiratory tract infections; a qualitative study to explore Irish general practitioners’ perspectives. BMC Fam. Pract. 2019, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Suriyo, T.; Pholphana, N.; Ungtrakul, T.; Rangkadilok, N.; Panomvana, D.; Thiantanawat, A.; Pongpun, W.; Satayavivad, J. Clinical Parameters following Multiple Oral Dose Administration of a Standardized Andrographis paniculata Capsule in Healthy Thai Subjects. Planta Med. 2017, 83, 778–789. [Google Scholar] [CrossRef]
- Poolsup, N.; Suthisisang, C.; Prathanturarug, S.; Asawamekin, A.; Chanchareon, U. Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: Systematic review of randomized controlled trials. J. Clin. Pharm. Ther. 2004, 29, 37–45. [Google Scholar] [CrossRef]
- Little, A. Review: Antibiotics are not effective for upper respiratory tract infection in children. Evid. Based Nurs. 1999, 2, 77. [Google Scholar] [CrossRef]
- DeCorte, B.L. Underexplored Opportunities for Natural Products in Drug Discovery. J. Med. Chem. 2016, 59, 9295–9304. [Google Scholar] [CrossRef]
- Fang, J.; Cai, C.; Wang, Q.; Lin, P.; Zhao, Z.; Cheng, F. Systems Pharmacology-Based Discovery of Natural Products for Precision Oncology Through Targeting Cancer Mutated Genes. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 177–187. [Google Scholar] [CrossRef]
- Hazrati, S.; Govahi, M.; Sedaghat, M.; Kashkooli, A.B. A comparative study of essential oil profile, antibacterial and antioxidant activities of two cultivated Ziziphora species (Z. clinopodioides and Z. tenuior). Ind. Crop. Prod. 2020, 157, 7. [Google Scholar] [CrossRef]
- De Veras, B.O.; de Oliveira, J.R.S.; de Menezes Lima, V.L.; do Amaral Ferraz Navarro, D.M.; de Oliveira Farias de Aguiar, J.C.R.; de Medeiros Moura, G.M.; da Silva, J.W.; de Assis, C.R.D.; Gorlach-Lira, K.; de Assis, P.A.C.; et al. The essential oil of the leaves of Verbesina macrophylla (Cass.) S.F.Blake has antimicrobial, anti-inflammatory and antipyretic activities and is toxicologically safe. J. Ethnopharmacol. 2021, 265, 113248. [Google Scholar] [CrossRef]
- de Araujo, M.R.C.; Maciel, P.P.; Castellano, L.R.C.; Bonan, P.R.F.; Alves, D.D.N.; de Medeiros, A.C.D.; de Castro, R.D. Efficacy of essential oil of cinnamon for the treatment of oral candidiasis: A randomized trial. Spec. Care Dent. 2021, 9. [Google Scholar] [CrossRef]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, A.; Hosseini, S.; Martinez-Chapa, S.O.; Cordell, G.A. Multi-target Activities of Selected Alkaloids and Terpenoids. Mini Rev. Org. Chem. 2017, 14, 272–279. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting new molecular targets for known drugs. Nature 2009, 462, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xie, L.; Kinnings, S.L.; Bourne, P.E. Novel computational approaches to polypharmacology as a means to define responses to individual drugs. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 361–379. [Google Scholar] [CrossRef]
- Yildirim, M.A.; Goh, K.I.; Cusick, M.E.; Barabasi, A.L.; Vidal, M. Drug-target network. Nat. Biotechnol. 2007, 25, 1119–1126. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Tan, Z.; Zhang, S. Curation and analysis of multitargeting agents for polypharmacological modeling. J. Chem. Inf. Model 2014, 54, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Zhang, S. Polypharmacology: Drug discovery for the future. Expert Rev. Clin. Pharmacol. 2013, 6, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.F.; Yanez, M.; Moghadam, S.E.; Moridi Farimani, M.; Soroury, S.; Ebrahimi, S.N.; Tabefam, M.; Jabbarzadeh, E. 7-epi-Clusianone, a Multi-Targeting Natural Product with Potential Chemotherapeutic, Immune-Modulating, and Anti-Angiogenic Properties. Molecules 2019, 24, 4415. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Ismail, N.A.; Hossain, M.S.; Mustafa, N.H.M.; Phang, I.C. Morpho-physiological characterizatics, selected macronutrient uptak, and oxidative stress level of Andrographis paniculata under salinity condition. J. Teknol. 2015, 77, 135–140. [Google Scholar] [CrossRef][Green Version]
- Hossain, M.S.; Urbi, Z.; Evamoni, F.Z.; Zohora, F.T.; Rahman, K.M.H. A secondary research on medicinal plants mentioned in the Holy Qur’an. J. Med. Plants 2016, 15, 81–97. [Google Scholar]
- Urbi, Z.; Hossain, M.S.; Rahman, K.M.H.; Zayed, T.M. Grape: A Medicinal Fruit Species in the Holy Qur’an and its Ethnomedinical Importance Department of Basic Medical Sciences, Faculty of Pharmacy. World Appl. Sci. J. 2014, 30, 253–265. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Kumar, A.; Dora, J.; Singh, A.; Tripathi, R. A review on King of Bitter (Kalmegh). Int. J. Res. Pharm. Chem. 2012, 2, 116–124. [Google Scholar]
- Akbar, S. Andrographis paniculata: A review of pharmacological activities and clinical effects. Altern. Med. Rev. 2011, 16, 66–77. [Google Scholar]
- Benoy, G.K.; Animesh, D.K.; Aninda, M.; Priyanka, D.K.; Sandip, H. An overview on Andrographis paniculata (Burm. F.) Nees. Int. J. Res. Ayur. Pharm. 2012, 3, 752–760. [Google Scholar]
- Hossain, M.S.; Urbi, Z. Effect of Naphthalene Acetic Acid on the Adventitious Rooting in Shoot Cuttings of Andrographis paniculata (Burm.f.) Wall. ex Nees: An Important Therapeutical Herb. Int. J. Agron. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Hossain, M.S.; Urbi, Z.; Sule, A.; Hafizur Rahman, K.M. Andrographis paniculata (Burm. f.) Wall. ex Nees: A review of ethnobotany, phytochemistry, and pharmacology. Sci. World J. 2014, 2014, 274905. [Google Scholar] [CrossRef] [PubMed]
- Anju, D.; Jugnu, G.; Kavita, S.; Arun, N.; Sandeep, D. A review on medicinal prospectives of Andrographis paniculata Nees. J. Pharm. Sci. Innov. 2012, 1, 1–4. [Google Scholar]
- Mussard, E.; Jousselin, S.; Cesaro, A.; Legrain, B.; Lespessailles, E.; Esteve, E.; Berteina-Raboin, S.; Toumi, H. Andrographis paniculata and Its Bioactive Diterpenoids Against Inflammation and Oxidative Stress in Keratinocytes. Antioxidants 2020, 9, 530. [Google Scholar] [CrossRef]
- Lee, D.; Baek, C.Y.; Hwang, J.H.; Kim, M.Y. Andrographis paniculata Extract Relieves Pain and Inflammation in Monosodium Iodoacetate-Induced Osteoarthritis and Acetic Acid-Induced Writhing in Animal Models. Processes 2020, 8, 873. [Google Scholar] [CrossRef]
- Li, X.; Yuan, K.; Zhu, Q.; Lu, Q.; Jiang, H.; Zhu, M.; Huang, G.; Xu, A. Andrographolide Ameliorates Rheumatoid Arthritis by Regulating the Apoptosis-NETosis Balance of Neutrophils. Int. J. Mol. Sci. 2019, 20, 5035. [Google Scholar] [CrossRef]
- Gu, L.; Yu, Q.; Li, Q.; Zhang, L.; Lu, H.; Zhang, X. Andrographolide Protects PC12 Cells against β-Amyloid-Induced Autophagy-Associated Cell Death Through Activation of the Nrf2-Mediated p62 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2844. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, a Natural Antioxidant: An Update. Antioxidants 2019, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.T.; Bin Mohd Sarib, M.S.; Ismail, I.S.; Abas, F.; Ismail, A.; Lajis, N.H.; Shaari, K. Anti-Diabetic Activity and Metabolic Changes Induced by Andrographis paniculata Plant Extract in Obese Diabetic Rats. Molecules 2016, 21, 1026. [Google Scholar] [CrossRef]
- Qader, S.W.; Abdulla, M.A.; Chua, L.S.; Najim, N.; Zain, M.M.; Hamdan, S. Antioxidant, total phenolic content and cytotoxicity evaluation of selected Malaysian plants. Molecules 2011, 16, 3433–3443. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Chen, H.W.; Lii, C.K.; Jhuang, J.H.; Huang, C.S.; Li, M.L.; Yao, H.T. A Diterpenoid, 14-Deoxy-11, 12-Didehydroandrographolide, in Andrographis paniculata Reduces Steatohepatitis and Liver Injury in Mice Fed a High-Fat and High-Cholesterol Diet. Nutrients 2020, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Hanapi, N.A.; Ab Halim, M.R.; Uchaipichat, V.; Mackenzie, P.I. Effects of Andrographis paniculata and Orthosiphon stamineus extracts on the glucuronidation of 4-methylumbelliferone in human UGT isoforms. Molecules 2010, 15, 3578–3592. [Google Scholar] [CrossRef]
- Loh, S.H.; Tsai, Y.T.; Huang, S.F.; Yu, T.C.; Kuo, P.C.; Chao, S.C.; Chou, M.F.; Tsai, C.S.; Lee, S.P. Effects of Andrographolide on Intracellular pH Regulation, Cellular Migration, and Apoptosis in Human Cervical Cancer Cells dagger. Cancers 2020, 12, 387. [Google Scholar] [CrossRef]
- Panossian, A.; Brendler, T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals 2020, 13, 236. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, J.; Yang, Y.; Yang, M.; Zheng, Q. Screening and Identification for Immunological Active Components from Andrographis Herba Using Macrophage Biospecific Extraction Coupled with UPLC/Q-TOF-MS. Molecules 2018, 23, 1047. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, P.; Gupta, G.K.; Ntie-Kang, F.; Kumar, D. Structure-Activity-Relationship and Mechanistic Insights for Anti-HIV Natural Products. Molecules 2020, 25, 2070. [Google Scholar] [CrossRef]
- Calabrese, C.; Berman, S.H.; Babish, J.G.; Ma, X.; Shinto, L.; Dorr, M.; Wells, K.; Wenner, C.A.; Standish, L.J. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 2000, 14, 333–338. [Google Scholar] [CrossRef]
- Chuthaputti, A.; Pornpatkul, V.; Suwankiri, U. The Efficacy of Andrographis paniculata (Burm. f.) Wall. ex Nees for the Relief of the Symptoms of Influenza. J. Thai Tradit. Altern. Med. 2007, 5, 1–10. [Google Scholar]
- Hancke, J.; Burgos, R.; Caceres, D.; Wikman, G. A double-blind study with a new monodrug Kan Jang: Decrease of symptoms and improvement in the recovery from common colds. Phytother. Res. 1995, 9, 559–562. [Google Scholar] [CrossRef]
- Kulichenko, L.L.; Kireyeva, L.V.; Malyshkina, E.N.; Wikman, G. A randomized, controlled study of Kan Jang versus amantadine in the treatment of influenza in Volgograd. J. Herb. Pharmacother. 2003, 3, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Melchior, J.; Palm, S.; Wikman, G. Controlled clinical study of standardized Andrographis paniculata extract in common cold—A pilot trial. Phytomedicine 1997, 3, 315–318. [Google Scholar] [CrossRef]
- Saxena, R.C.; Singh, R.; Kumar, P.; Yadav, S.C.; Negi, M.P.; Saxena, V.S.; Joshua, A.J.; Vijayabalaji, V.; Goudar, K.S.; Venkateshwarlu, K.; et al. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection. Phytomedicine 2010, 17, 178–185. [Google Scholar] [CrossRef]
- Thamlikitkul, V.; Dechatiwongse, T.; Theerapong, S.; Chantrakul, C.; Boonroj, P.; Punkrut, W.; Ekpalakorn, W.; Boontaeng, N.; Taechaiya, S.; Petcharoen, S.; et al. Efficacy of Andrographis paniculata, Nees for pharyngotonsillitis in adults. J. Med. Assoc. Thai 1991, 74, 437–442. [Google Scholar] [PubMed]
- Widjajakusuma, E.C.; Jonosewojo, A.; Hendriati, L.; Wijaya, S.; Surjadhana, A.; Sastrowardoyo, W.; Monita, N.; Muna, N.M.; Fajarwati, R.P.; Ervina, M.; et al. Phytochemical screening and preliminary clinical trials of the aqueous extract mixture of Andrographis paniculata (Burm. f.) Wall. ex Nees and Syzygium polyanthum (Wight.) Walp leaves in metformin treated patients with type 2 diabetes. Phytomedicine 2019, 55, 137–147. [Google Scholar] [CrossRef]
- Tang, T.; Targan, S.R.; Li, Z.S.; Xu, C.; Byers, V.S.; Sandborn, W.J. Randomised clinical trial: Herbal extract HMPL-004 in active ulcerative colitis-a double-blind comparison with sustained release mesalazine. Aliment. Pharmacol. Ther. 2011, 33, 194–202. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Targan, S.R.; Byers, V.S.; Rutty, D.A.; Mu, H.; Zhang, X.; Tang, T. Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am. J. Gastroenterol. 2013, 108, 90–98. [Google Scholar] [CrossRef]
- Phunikhom, K.; Khampitak, K.; Aromdee, C.; Arkaravichien, T.; Sattayasai, J. Effect of Andrographis paniculata Extract on Triglyceride Levels of the Patients with Hypertriglyceridemia: A Randomized Controlled Trial. J. Med. Assoc. Thai 2015, 98 (Suppl. 6), S41–S47. [Google Scholar]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Islam, M.A.; Shaw, S.; Khan, I.N.; Saravi, S.S.S.; Ahmad, S.; Rehman, S.; Gupta, V.K.; et al. Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer. Cancer Lett. 2018, 420, 129–145. [Google Scholar] [CrossRef]
- Hancke, J.L.; Srivastav, S.; Caceres, D.D.; Burgos, R.A. A double-blind, randomized, placebo-controlled study to assess the efficacy of Andrographis paniculata standardized extract (ParActin(R)) on pain reduction in subjects with knee osteoarthritis. Phytother. Res. 2019, 33, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, J.C.; Baumgartner, M.; Palma, R.; Ciampi, E.; Carcamo, C.; Caceres, D.D.; Acosta-Jamett, G.; Hancke, J.L.; Burgos, R.A. Andrographis paniculata decreases fatigue in patients with relapsing-remitting multiple sclerosis: A 12-month double-blind placebo-controlled pilot study. BMC Neurol. 2016, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.; Hovhannisyan, A.; Jamalyan, K.; Narimanyan, M. Antitussive effect of a fixed combination of Justicia adhatoda, Echinacea purpurea and Eleutherococcus senticosus extracts in patients with acute upper respiratory tract infection: A comparative, randomized, double-blind, placebo-controlled study. Phytomedicine 2015, 22, 1195–1200. [Google Scholar] [CrossRef]
- Sgorlon, S.; Colitti, M.; Asquini, E.; Ferrarini, A.; Pallavicini, A.; Stefanon, B. Administration of botanicals with the diet regulates gene expression in peripheral blood cells of Sarda sheep during ACTH challenge. Domest Anim. Endocrinol. 2012, 43, 213–226. [Google Scholar] [CrossRef]
- Raghavan, R.; Cheriyamundath, S.; Madassery, J. Andrographolide, a new potential NF-kappa B inhibitor: Docking simulation and evaluation of drug-likeness. Mol. Simulat. 2012, 38, 582–588. [Google Scholar] [CrossRef]
- Novianto, F.; Zulkarnain, Z.; Mana, T.A. A Clinical Observation to Understand the Safety of Herbs Used for Diabetes mellitus. Media Penelit Pengem 2018, 28, 9–14. [Google Scholar] [CrossRef]
- He, Y.; Yang, J.; Zeng, G.; Shen, T.; Fontaine, R.E.; Zhang, L.; Shi, G.; Wang, Y.; Li, Q.; Long, J. Risk factors for critical disease and death from hand, foot and mouth disease. Pediatr. Infect. Dis. J. 2014, 33, 966–970. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ciampi, E.; Uribe-San-Martin, R.; Carcamo, C.; Cruz, J.P.; Reyes, A.; Reyes, D.; Pinto, C.; Vasquez, M.; Burgos, R.A.; Hancke, J. Efficacy of andrographolide in not active progressive multiple sclerosis: A prospective exploratory double-blind, parallel-group, randomized, placebo-controlled trial. BMC Neurol. 2020, 20, 173. [Google Scholar] [CrossRef]
- Panossian, A.; Hovhannisyan, A.; Mamikonyan, G.; Abrahamian, H.; Hambardzumyan, E.; Gabrielian, E.; Goukasova, G.; Wikman, G.; Wagner, H. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine 2000, 7, 351–364. [Google Scholar] [CrossRef]
- Li, L.; Wijaya, H.; Samanta, S.; Lam, Y.; Yao, S.Q. In situ imaging and proteome profiling indicate andrographolide is a highly promiscuous compound. Sci. Rep. 2015, 5, 11522. [Google Scholar] [CrossRef] [PubMed]
- Bisson, J.; McAlpine, J.B.; Friesen, J.B.; Chen, S.N.; Graham, J.; Pauli, G.F. Can Invalid Bioactives Undermine Natural Product-Based Drug Discovery? J. Med. Chem. 2016, 59, 1671–1690. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S. The Effect of Salinity Stress on the Morpho-physiology and Protein Profile of Andrographis Paniculata. Master’s Thesis, Department of Biotechnology, Kulliyyah of Science, International Islamic University Malaysia, Kuantan, Pahang, Malaysia, 2016. [Google Scholar]
- Hossain, M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar]
- Hossain, M.S. Proteomic Studies: Contribution to Understanding Plant Salinity Stress Response. Glob. J. Bot. Sci. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Arifullah, M.; Namsa, N.D.; Mandal, M.; Chiruvella, K.K.; Vikrama, P.; Gopal, G.R. Evaluation of anti-bacterial and anti-oxidant potential of andrographolide and echiodinin isolated from callus culture of Andrographis paniculata Nees. Asian Pac. J. Trop. Biomed. 2013, 3, 604–610. [Google Scholar] [CrossRef]
- Editorial. Microbiology by numbers. Nat. Rev. Microbiol. 2011, 9, 628. [Google Scholar] [CrossRef]
- Sharma, A.; Lal, K.; Handa, S.S. Standardization of the Indian Crude Drug Kalmegh by High-Pressure Liquid-Chromatographic Determination of Andrographolide. Phytochem. Anal. 1992, 3, 129–131. [Google Scholar] [CrossRef]
- Seubsasana, S.; Pientong, C.; Ekalaksananan, T.; Thongchai, S.; Aromdee, C. A potential andrographolide analogue against the replication of herpes simplex virus type 1 in vero cells. Med. Chem. 2011, 7, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Morita, H.; Tezuka, Y. Preferentially Cytotoxic Constituents of Andrographis paniculata and their Preferential Cytotoxicity against Human Pancreatic Cancer Cell Lines. Nat. Prod. Commun. 2015, 10, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Jin, X.; Lu, Y.; Chen, D.F. Anticomplement ent-labdane diterpenoids from the aerial parts of Andrographis paniculata. Fitoterapia 2020, 142, 104528. [Google Scholar] [CrossRef] [PubMed]
- Koteswara Rao, Y.; Vimalamma, G.; Rao, C.V.; Tzeng, Y.M. Flavonoids and andrographolides from Andrographis paniculata. Phytochemistry 2004, 65, 2317–2321. [Google Scholar] [CrossRef]
- Reddy, M.K.; Reddy, M.V.; Gunasekar, D.; Murthy, M.M.; Caux, C.; Bodo, B. A flavone and an unusual 23-carbon terpenoid from Andrographis paniculata. Phytochemistry 2003, 62, 1271–1275. [Google Scholar] [CrossRef]
- Majumdar, M.; Misra, T.K.; Roy, D.N. In vitro anti-biofilm activity of 14-deoxy-11,12-didehydroandrographolide from Andrographis paniculata against Pseudomonas aeruginosa. Braz. J. Microbiol. 2020, 51, 15–27. [Google Scholar] [CrossRef]
- Lala, S.; Nandy, A.K.; Mahato, S.B.; Basu, M.K. Delivery in vivo of 14-deoxy-11-oxoandrographolide, an antileishmanial agent, by different drug carriers. Indian J. Biochem. Biophys. 2003, 40, 169–174. [Google Scholar] [PubMed]
- Liu, R.H.; Jacob, J.R.; Tennant, B. Andrographolide Derivatives to Treat Viral Infections Patent No. US8445533B2, 21 May 2013.
- Wintachai, P.; Kaur, P.; Lee, R.C.; Ramphan, S.; Kuadkitkan, A.; Wikan, N.; Ubol, S.; Roytrakul, S.; Chu, J.J.; Smith, D.R. Activity of andrographolide against chikungunya virus infection. Sci. Rep. 2015, 5, 14179. [Google Scholar] [CrossRef]
- Paemanee, A.; Hitakarun, A.; Wintachai, P.; Roytrakul, S.; Smith, D.R. A proteomic analysis of the anti-dengue virus activity of andrographolide. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 322–332. [Google Scholar] [CrossRef]
- Panraksa, P.; Ramphan, S.; Khongwichit, S.; Smith, D.R. Activity of andrographolide against dengue virus. Antiviral. Res. 2017, 139, 69–78. [Google Scholar] [CrossRef]
- Edwin, E.S.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Thanigaivel, A.; Ponsankar, A.; Pradeepa, V.; Selin-Rani, S.; Kalaivani, K.; Hunter, W.B.; Abdel-Megeed, A.; et al. Anti-dengue efficacy of bioactive andrographolide from Andrographis paniculata (Lamiales: Acanthaceae) against the primary dengue vector Aedes aegypti (Diptera: Culicidae). Acta Trop. 2016, 163, 167–178. [Google Scholar] [CrossRef]
- Prakoso, N.I.; Zakiyah, Z.N.; Liyanita, A.; Rubiyanto, D.; Fitriastuti, D.; Ramadani, A.P.; Kamari, A.; Mow, S.K. Antimalarial Activity of Andrographis Paniculata Ness‘s N-hexane Extract and Its Major Compounds. Open Chem. 2019, 17, 788–797. [Google Scholar] [CrossRef]
- Fangkham, S.; Ekalaksananan, T.; Aromdee, C.; Seubsasana, S.; Kongyingyoes, B.; Patarapadungkit, N.; Pientong, C. The effect of andrographolide on Human papillomavirus type 16 (HPV16) positive cervical cancer cells (SiHa). Int. J. Infect. Dis. 2012, 16, E80. [Google Scholar] [CrossRef][Green Version]
- Lin, T.P.; Chen, S.Y.; Duh, P.D.; Chang, L.K.; Liu, Y.N. Inhibition of the epstein-barr virus lytic cycle by andrographolide. Biol. Pharm. Bull 2008, 31, 2018–2023. [Google Scholar] [CrossRef] [PubMed]
- Sinha, J.; Mukhopadhyay, S.; Das, N.; Basu, M.K. Targeting of liposomal andrographolide to L. donovani-infected macrophages in vivo. Drug Deliv. 2000, 7, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Wiart, C.; Kumar, K.; Yusof, M.Y.; Hamimah, H.; Fauzi, Z.M.; Sulaiman, M. Antiviral properties of ent-labdene diterpenes of Andrographis paniculata nees, inhibitors of herpes simplex virus type 1. Phytother. Res. 2005, 19, 1069–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.L.; Chen, L.X.; Zhuang, Y.L.; Wang, N.L.; Yao, X.S.; Qiu, F. Two new ent-labdane diterpenoid glycosides from the aerial parts of Andrographis paniculata. J. Asian Nat. Prod. Res. 2008, 10, 939–943. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Yu, J.; Zeng, H.; Jiang, S.; Chen, X. Separation of five compounds from leaves of Andrographis paniculata (Burm. f.) Nees by off-line two-dimensional high-speed counter-current chromatography combined with gradient and recycling elution. J. Sep. Sci. 2015, 38, 1476–1483. [Google Scholar] [CrossRef]
- Wu, T.S.; Chern, H.J.; Damu, A.G.; Kuo, P.C.; Su, C.R.; Lee, E.J.; Teng, C.M. Flavonoids and ent-labdane diterpenoids from Andrographis paniculata and their antiplatelet aggregatory and vasorelaxing effects. J. Asian Nat. Prod. Res. 2008, 10, 17–24. [Google Scholar] [CrossRef]
- Wang, C.H.; Li, W.; Qiu, R.X.; Jiang, M.M.; Li, G.Q. A new diterpenoid from the aerial parts of Andrographis paniculata. Nat. Prod. Commun. 2014, 9, 13–14. [Google Scholar] [CrossRef]
- Suriyo, T.; Pholphana, N.; Rangkadilok, N.; Thiantanawat, A.; Watcharasit, P.; Satayavivad, J. Andrographis paniculata extracts and major constituent diterpenoids inhibit growth of intrahepatic cholangiocarcinoma cells by inducing cell cycle arrest and apoptosis. Planta Med. 2014, 80, 533–543. [Google Scholar] [CrossRef]
- Tran, Q.T.N.; Tan, W.S.D.; Wong, W.S.F.; Chai, C.L.L. Polypharmacology of andrographolide: Beyond one molecule one target. Nat. Prod. Rep. 2020. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmad, S.H.; Mohamed, M.T.; Ab Rahman, M.Z. Antimicrobial compounds from leaf extracts of Jatropha curcas, Psidium guajava, and Andrographis paniculata. Sci. World J. 2014, 2014, 635240. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.J.; Zheng, X.W.; Feng, T.; Huang, J.; Chen, J.; Wu, Y.Y.; Zhou, L.M.; Tu, W.W.; Li, H. Andrographolide exerted its antimicrobial effects by upregulation of human beta-defensin-2 induced through p38 MAPK and NF-kappaB pathway in human lung epithelial cells. Can. J. Physiol. Pharmacol. 2012, 90, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kaushik, S.; Pandit, P.; Dhull, D.; Yadav, J.P.; Kaushik, S. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl. Microbiol. Biotechnol. 2019, 103, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; Li, R.T.; Xiao, W.L.; Xu, G.; Lin, Z.W.; Zhao, Q.S.; Sun, H.D. ent-Labdane diterpenoids from Andrographis paniculata. J. Nat. Prod. 2006, 69, 319–322. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Use of asiatic pennywort Centella asiatica aqueous extract as a bath treatment to control columnaris in Nile tilapia. J. Aquat. Anim. Health 2010, 22, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Yasmin, S.; Ghosh, S.; Bhattacharya, S.; Banerjee, D. Anti-Infective Metabolites of a Newly Isolated Bacillus thuringiensis KL1 Associated with Kalmegh (Andrographis paniculata Nees.), a Traditional Medicinal Herb. Microbiol. Insights 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Pfisterer, P.H.; Rollinger, J.M.; Schyschka, L.; Rudy, A.; Vollmar, A.M.; Stuppner, H. Neoandrographolide from Andrographis paniculata as a potential natural chemosensitizer. Planta Med. 2010, 76, 1698–1700. [Google Scholar] [CrossRef]
- Radhika, P.; Prasad, Y.R.; Sowjanya, K. A new diterpene from the leaves of Andrographis paniculata Nees. Nat. Prod. Commun. 2012, 7, 485–486. [Google Scholar] [CrossRef]
- Pholphana, N.; Rangkadilok, N.; Saehun, J.; Ritruechai, S.; Satayavivad, J. Changes in the contents of four active diterpenoids at different growth stages in Andrographis paniculata (Burm.f.) Nees (Chuanxinlian). Chin. Med. 2013, 8, 2. [Google Scholar] [CrossRef]
- Radhika, P.; Prasad, Y.R.; Lakshmi, K.R. Flavones from the stem of Andrographis paniculata Nees. Nat. Prod. Commun. 2010, 5, 59–60. [Google Scholar] [CrossRef]
- Pramanick, S.; Banerjee, S.; Achari, B.; Das, B.; Sen, A.K.; Mukhopadhyay, S.; Neuman, A.; Prange, T. Andropanolide and isoandrographolide, minor diterpenoids from Andrographis paniculata: Structure and X-ray crystallographic analysis. J. Nat. Prod. 2006, 69, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Kuroyanagi, M.; Sugiyama, S.; Umehara, K.; Ueno, A.; Nishi, K. Cell differentiation-inducing diterpenes from Andrographis paniculata Nees. Chem. Pharm. Bull. 1994, 42, 1216–1225. [Google Scholar] [CrossRef]
- Hossain, M.M.; Polash, S.A.; Takikawa, M.; Shubhra, R.D.; Saha, T.; Islam, Z.; Hossain, S.; Hasan, M.A.; Takeoka, S.; Sarker, S.R. Investigation of the Antibacterial Activity and in vivo Cytotoxicity of Biogenic Silver Nanoparticles as Potent Therapeutics. Front. Bioeng. Biotechnol. 2019, 7, 239. [Google Scholar] [CrossRef]
- Malahubban, M.; Alimon, A.R.; Sazili, A.Q.; Fakurazi, S.; Zakry, F.A. Phytochemical analysis of Andrographis paniculata and Orthosiphon stamineus leaf extracts for their antibacterial and antioxidant potential. Trop. Biomed. 2013, 30, 467–480. [Google Scholar] [PubMed]
- Li, W.; Xu, X.; Zhang, H.; Ma, C.; Fong, H.; van Breemen, R.; Fitzloff, J. Secondary metabolites from Andrographis paniculata. Chem. Pharm. Bull. 2007, 55, 455–458. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, G.; Jeong, M.; Lee, M.J.; Kang, K.; Cho, J. Hierarchical surface atomic structure of a manganese-based spinel cathode for lithium-ion batteries. Angew. Chem. Int. Ed. Engl. 2015, 54, 1153–1158. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Sato, M.; Ueno, A.; Nishi, K. Flavonoids from Andrographis-Paniculata. Chem. Pharm. Bull. 1987, 35, 4429–4435. [Google Scholar] [CrossRef]
- Hu, X.Y.; Wu, R.H.; Logue, M.; Blondel, C.; Lai, L.Y.W.; Stuart, B.; Flower, A.; Fei, Y.T.; Moore, M.; Shepherd, J.; et al. Andrographis paniculata (Chuan Xin Lian) for symptomatic relief of acute respiratory tract infections in adults and children: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0181780. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.A.F.; Overton, K.H.; Rycroft, D.S. Formation of three new flavones by differentiating callus cultures of andrographis paniculata. Phytochemistry 1979, 18, 149–151. [Google Scholar] [CrossRef]
- Geethangili, M.; Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Cytotoxic constituents from Andrographis paniculata induce cell cycle arrest in jurkat cells. Phytother. Res. 2008, 22, 1336–1341. [Google Scholar] [CrossRef]
- Das, B.; Khan, M.I.; Jayabalan, R.; Behera, S.K.; Yun, S.I.; Tripathy, S.K.; Mishra, A. Understanding the Antifungal Mechanism of Ag@ZnO Core-shell Nanocomposites against Candida krusei. Sci. Rep. 2016, 6, 36403. [Google Scholar] [CrossRef]
- Hapuarachchi, S.D.; Ali, Z.; Abe, N.; Sugandhika, S.T.; Sandun, S.T.; Khan, I.A. Andrographidine G, a new flavone glucoside from Andrographis paniculata. Nat. Prod. Commun. 2013, 8, 333–334. [Google Scholar] [CrossRef]
- Hanh, T.T.H.; My, N.T.T.; Cham, P.T.; Quang, T.H.; Cuong, N.X.; Huong, T.T.; Nam, N.H.; Minh, C.V. Diterpenoids and Flavonoids from Andrographis paniculata. Chem. Pharm. Bull. 2020, 68, 96–99. [Google Scholar] [CrossRef]
- Feng, J.; Leone, J.; Schweig, S.; Zhang, Y. Evaluation of Natural and Botanical Medicines for Activity against Growing and Non-growing Forms of B. burgdorferi. Front. Med. 2020, 7, 6. [Google Scholar] [CrossRef]
- Gupta, K.K.; Taneja, S.C.; Dhar, K.L.; Atal, C.K. Flavonoids of Andrographis-Paniculata. Phytochemistry 1983, 22, 314–315. [Google Scholar] [CrossRef]
- Dedhia, J.; Mukharjee, E.; Luke, A.M.; Mathew, S.; Pawar, A.M. Efficacy of Andrographis paniculata compared to Azadirachta indica, Curcuma longa, and sodium hypochlorite when used as root canal irrigants against Candida albicans and Staphylococcus aureus: An in vitro antimicrobial study. J. Conserv. Dent. JCD 2018, 21, 642–645. [Google Scholar] [CrossRef]
- Fujita, T.; Fujitani, R.; Takeda, Y.; Takaishi, Y.; Yamada, T.; Kido, M.; Miura, I. On the Diterpenoids of Andrographis-Paniculata-X-Ray Crystallographic Analysis of Andrographolide and Structure Determination of New Minor Diterpenoids. Chem. Pharm. Bull. 1984, 32, 2117–2125. [Google Scholar] [CrossRef]
- Chen, L.X.; Qiu, F.; Wei, H.; Qu, G.X.; Yao, X.S. Nine new ent-labdane diterpenoids from the aerial parts of Andrographis paniculata. Helvetica Chim. Acta 2006, 89, 2654–2664. [Google Scholar] [CrossRef]
- Chen, L.X.; He, H.; Xia, G.Y.; Zhou, K.L.; Qiu, F. A new flavonoid from the aerial parts of Andrographis paniculata. Nat. Prod. Res. 2014, 28, 138–143. [Google Scholar] [CrossRef]
- Awang, K.; Abdullah, N.H.; Hadi, A.H.; Fong, Y.S. Cardiovascular activity of labdane diterpenes from Andrographis paniculata in isolated rat hearts. J. Biomed. Biotechnol. 2012, 2012, 876458. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Kuo, Y.H.; Lin, B.F. Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-kappaB transactivation inhibition. J. Agric. Food Chem. 2010, 58, 2505–2512. [Google Scholar] [CrossRef]
- Burgos, R.A.; Alarcon, P.; Quiroga, J.; Manosalva, C.; Hancke, J. Andrographolide, an Anti-Inflammatory Multitarget Drug: All Roads Lead to Cellular Metabolism. Molecules 2020, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, H.; Wang, R.; Zhou, K.; Jing, Y.; Qiu, F. ent-Labdane diterpenoid lactone stereoisomers from Andrographis paniculata. J. Nat. Prod. 2008, 71, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Parai, D.; Chattopadhyay, S.; Mukherjee, S.K. Andrographolide: Antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiol. 2017, 62, 237–244. [Google Scholar] [CrossRef]
- Zou, Q.Y.; Li, N.; Dan, C.; Deng, W.L.; Peng, S.L.; Ding, L.S. A new ent-labdane diterpenoid from Andrographis paniculata. Chin. Chem. Lett. 2010, 21, 1091–1093. [Google Scholar] [CrossRef]
- Xu, C.; Chou, G.X.; Wang, C.H.; Wang, Z.T. Rare noriridoids from the roots of Andrographis paniculata. Phytochemistry 2012, 77, 275–279. [Google Scholar] [CrossRef]
- Xu, C.; Chou, G.X.; Wang, Z.T. A new diterpene from the leaves of Andrographis paniculata Nees. Fitoterapia 2010, 81, 610–613. [Google Scholar] [CrossRef]
- Wang, G.Y.; Wen, T.; Liu, F.F.; Tian, H.Y.; Chun-Lin, F.; Huang, X.J.; Ye, W.C.; Wang, Y. Two new diterpenoid lactones isolated from Andrographis paniculata. Chin. J. Nat. Med. 2017, 15, 458–462. [Google Scholar] [CrossRef]
- Weiming, C.; Xiaotian, L. Deoxyandrographolide-19β-D-Glucoside from the Leaves of Andrographis paniculata. Planta Med. 1982, 45, 245–246. [Google Scholar] [CrossRef]
- Tanwar, A.; Chawla, R.; Chakotiya, A.S.; Thakur, P.; Goel, R.; Basu, M.; Arora, R.; Khan, H.A. Effect of Holarrhena antidysentrica (Ha) and Andrographis paniculata (Ap) on the biofilm formation and cell membrane integrity of opportunistic pathogen Salmonella typhimurium. Microb. Pathog. 2016, 101, 76–82. [Google Scholar] [CrossRef]
- Maity, G.N.; Maity, P.; Choudhuri, I.; Sahoo, G.C.; Maity, N.; Ghosh, K.; Bhattacharyya, N.; Dalai, S.; Mondal, S. Green synthesis, characterization, antimicrobial and cytotoxic effect of silver nanoparticles using arabinoxylan isolated from Kalmegh. Int. J. Biol. Macromol. 2020, 162, 1025–1034. [Google Scholar] [CrossRef]
- Xu, F.-F.; Fu, S.-J.; Gu, S.-P.; Wang, Z.-M.; Wang, Z.-Z.; He, X.; Xiao, W. Simultaneous determination of andrographolide, dehydroandrographolide and neoandrographolide in dog plasma by LC–MS/MS and its application to a dog pharmacokinetic study of Andrographis paniculata tablet. J. Chromatogr. B 2015, 990, 125–131. [Google Scholar] [CrossRef]
- Pholphana, N.; Panomvana, D.; Rangkadilok, N.; Suriyo, T.; Ungtrakul, T.; Pongpun, W.; Thaeopattha, S.; Satayavivad, J. A Simple and Sensitive LC-MS/MS Method for Determination of Four Major Active Diterpenoids from Andrographis paniculata in Human Plasma and Its Application to a Pilot Study. Planta Med. 2016, 82, 113–120. [Google Scholar] [CrossRef]
- Bera, R.; Ahmed, S.K.; Sarkar, L.; Sen, T.; Karmakar, S. Pharmacokinetic analysis and tissue distribution of andrographolide in rat by a validated LC-MS/MS method. Pharm. Biol. 2014, 52, 321–329. [Google Scholar] [CrossRef]
- Tu, Y.S.; Sun, D.M.; Zhang, J.J.; Jiang, Z.Q.; Chen, Y.X.; Zeng, X.H.; Huang, D.E.; Yao, N. Preparation and characterisation of andrographolide niosomes and its anti-hepatocellular carcinoma activity. J. Microencapsul. 2014, 31, 307–316. [Google Scholar] [CrossRef]
- Chen, L.; Yu, A.; Zhuang, X.; Zhang, K.; Wang, X.; Ding, L.; Zhang, H. Determination of andrographolide and dehydroandrographolide in rabbit plasma by on-line solid phase extraction of high-performance liquid chromatography. Talanta 2007, 74, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.B.; Zhang, H.; Wang, Y.Q. HPLC determination of andrographolide in rat whole blood: Study on the pharmacokinetics of andrographolide incorporated in liposomes and tablets. Biomed. Chromatogr. 2007, 21, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ma, J.; Liu, Y.; Chen, B.; Yao, S. Determination of andrographolide in human plasma by high-performance liquid chromatography/mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 854, 328–331. [Google Scholar] [CrossRef]
- Ruengsitagoon, W.; Anuntakarun, K.; Aromdee, C. Flow injection spectrophotometric determination of andrographolide from Andrographis paniculata. Talanta 2006, 69, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, G.A.; Zhari, I.; Norhayati, I.; Mariam, A. HPLC and HPTLC densitometric determination of andrographolides and antioxidant potential of Andrographis paniculata. J. Food Compos. Anal. 2006, 19, 118–126. [Google Scholar] [CrossRef]
- Srivastava, A.; Misra, H.; Verma, R.K.; Gupta, M.M. Chemical fingerprinting of Andrographis paniculata using HPLC, HPTLC and densitometry. Phytochem. Anal. 2004, 15, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Pholphana, N.; Rangkadilok, N.; Thongnest, S.; Ruchirawat, S.; Ruchirawat, M.; Satayavivad, J. Determination and variation of three active diterpenoids in Andrographis paniculata (Burm.f.) Nees. Phytochem. Anal. 2004, 15, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, G.; Liu, H.; Wang, D.; Song, X.; Chen, Y. Determination of andrographolide, deoxyandrographolide and neoandrographolide in the Chinese herb Andrographis paniculata by micellar electrokinetic capillary chromatography. Phytochem. Anal. 2002, 13, 222–227. [Google Scholar] [CrossRef]
- Cheung, H.Y.; Cheung, C.S.; Kong, C.K. Determination of bioactive diterpenoids from Andrographis paniculata by micellar electrokinetic chromatography. J. Chromatogr. A 2001, 930, 171–176. [Google Scholar] [CrossRef]
- Jain, D.C.; Gupta, M.M.; Saxena, S.; Kumar, S. LC analysis of hepatoprotective diterpenoids from Andrographis paniculata. J. Pharm. Biomed. Anal. 2000, 22, 705–709. [Google Scholar] [CrossRef]
- Kumaran, K.S.; Thirugnanasambantham, P.; Viswanathan, S.; Murthy, M.S.R. An HPLC method for the estimation of andrographolide in rabbit serum. Indian J. Pharmacol. 2003, 35, 109–112. [Google Scholar]
- Li, W.; Fitzloff, J.F. HPLC–PDA determination of bioactive diterpenoids from plant materials and commercial products of Andrographis paniculata. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 2407–2420. [Google Scholar] [CrossRef]
- Wongkittipong, R.; Prat, L.; Damronglerd, S.; Gourdon, C. Solid–liquid extraction of andrographolide from plants—experimental study, kinetic reaction and model. Sep. Purif. Technol. 2004, 40, 147–154. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Ouyang, X.; Han, Y.; Zhu, H.; Chen, Q. Fingerprint profile of active components for Andrographis paniculata Nees by HPLC-DAD. Sens. Instrum. Food Qual. Saf. 2009, 3, 165–179. [Google Scholar] [CrossRef]
- Kavuri, S.R.; Mukkamala, S.B.; Ramesh, P. Quantitative determination of two bioactive compounds in Andrographis paniculata (Burm. f) nees by ultra performance liquid chromatography. J. Pharm. Res. 2010, 3, 1682–1684. [Google Scholar]
- Du, Q.Z.; Jerz, G.; Winterhalter, P. Separation of andrographolide and neoandrographolide from the leaves of Andrographis paniculata using high-speed counter-current chromatography. J. Chromatogr. A 2003, 984, 147–151. [Google Scholar] [CrossRef]
- Kulyal, P.; Tiwari, U.K.; Shukla, A.; Gaur, A.K. Chemical constituents isolated from Andrographis paniculata. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 2010, 49, 356–359. [Google Scholar]
- Liu, J.; Wang, Z.T.; Ge, B.X. Andrograpanin, isolated from Andrographis paniculata, exhibits anti-inflammatory property in lipopolysaccharide-induced macrophage cells through down-regulating the p38 MAPKs signaling pathways. Int. Immunopharmacol. 2008, 8, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Aminah, N.S.; Tun, K.N.W.; Kristanti, A.N.; Aung, H.T.; Takaya, Y.; Choudhary, M.I. Chemical constituents and their biological activities from Taunggyi (Shan state) medicinal plants. Heliyon 2021, 7, e06173. [Google Scholar] [CrossRef]
- Xu, Y.; Marshall, R.L.; Mukkur, T.K. An Investigation on the Antimicrobial Activity of Andrographis paniculata Extracts and Andrographolide in vitro. Asian J. Plant Sci. 2006, 5, 527–530. [Google Scholar]
- Banerjee, M.; Moulick, S.; Bhattacharya, K.K.; Parai, D.; Chattopadhyay, S.; Mukherjee, S.K. Attenuation of Pseudomonas aeruginosa quorum sensing, virulence and biofilm formation by extracts of Andrographis paniculata. Microb. Pathog. 2017, 113, 85–93. [Google Scholar] [CrossRef]
- Voravuthikunchai, S.P.; Limsuwan, S. Medicinal Plant Extracts as AntiEscherichia coli O157: H7 Agents and Their Effects on Bacterial Cell Aggregation. J. Food Prot. 2006, 69, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.S.; Mishra, A.; Kumari, R.; Murthy, P.N.; Naik, B.S. Antibacterial Activity of Ethanol Extract of Andrographis paniculata. Indian J. Pharm. Sci. 2009, 71, 436–438. [Google Scholar] [CrossRef]
- Ahmed, Q.U.; Samah, O.A.; Sule, A. Andrographis paniculata (Burm.f) Wall. ex Ness: A Potent Antibacterial Plant. In Antimicrobial Agents; Bobbarala, V., Ed.; IntechOpen: London, UK, 2012; pp. 345–360. [Google Scholar] [CrossRef]
- Sule, A.; Ahmed, Q.U.; Samah, O.A.; Omar, M.N.; Hassan, N.M.; Kamal, L.Z.M.; Yarmo, M.A. Bioassay guided isolation of antibacterial compounds from Andrographis paniculata (Burm.f.) Wall. ex Nees (Hempedeu bumi). Am. J. Appl. Sci. 2011, 8, 525–534. [Google Scholar] [CrossRef]
- Majumdar, M.; Dubey, A.; Goswami, R.; Misra, T.K.; Roy, D.N. In vitro and in silico studies on the structural and biochemical insight of anti-biofilm activity of andrograpanin from Andrographis paniculata against Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2020, 36, 143. [Google Scholar] [CrossRef]
- Reddy, V.L.; Reddy, S.M.; Ravikanth, V.; Krishnaiah, P.; Goud, T.V.; Rao, T.P.; Ram, T.S.; Gonnade, R.G.; Bhadbhade, M.; Venkateswarlu, Y. A new bis-andrographolide ether from Andrographis paniculata nees and evaluation of anti-HIV activity. Nat. Prod. Res. 2005, 19, 223–230. [Google Scholar] [CrossRef]
- Chang, R.S.; Ding, L.; Chen, G.Q.; Pan, Q.C.; Zhao, Z.L.; Smith, K.M. Dehydroandrographolide succinic acid monoester as an inhibitor against the human immunodeficiency virus. Proc. Soc. Exp. Biol. Med. 1991, 197, 59–66. [Google Scholar] [CrossRef]
- Xu, H.X.; Wan, M.; Loh, B.N.; Kon, O.L.; Chow, P.W.; Sim, K.Y. Screening of traditional medicines for their inhibitory activity against HIV-1 protease. Phytother. Res. 1996, 10, 207–210. [Google Scholar] [CrossRef]
- Chen, J.X.; Xue, H.J.; Ye, W.C.; Fang, B.H.; Liu, Y.H.; Yuan, S.H.; Yu, P.; Wang, Y.Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009, 32, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Aromdee, C.; Suebsasana, S.; Ekalaksananan, T.; Pientong, C.; Thongchai, S. Stage of action of naturally occurring andrographolides and their semisynthetic analogues against herpes simplex virus type 1 in vitro. Planta Med. 2011, 77, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.I.; Ling, A.P.; Koh, R.Y.; Chye, S.M.; Voon, K.G. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement Altern. Med. 2012, 12, 3. [Google Scholar] [CrossRef]
- Sule, A.; Ahmed, Q.U.; Latip, J.; Samah, O.A.; Omar, M.N.; Umar, A.; Dogarai, B.B. Antifungal activity of Andrographis paniculata extracts and active principles against skin pathogenic fungal strains in vitro. Pharm. Biol. 2012, 50, 850–856. [Google Scholar] [CrossRef]
- Misra, P.; Pal, N.; Guru, P.; Katiyar, J.; Srivastava, V.; Tandon, J. Antimalarial activity of Andrographis paniculata (Kalmegh) against Plasmodium berghei NK 65 in Mastomys natalensis. Pharm. Biol. 1992, 30, 263–274. [Google Scholar] [CrossRef]
- Dua, V.K.; Ojha, V.P.; Biswas, S.; Valecha, N.; Singh, N.; Sharma, V.P. Antimalarial activity of different fractions isolated from the leaves of Andrographis paniculata. J. Med. Aromat. Plant Sci. 1999, 21, 1069–1073. [Google Scholar]
- Chen, H.; Ma, Y.B.; Huang, X.Y.; Geng, C.A.; Zhao, Y.; Wang, L.J.; Guo, R.H.; Liang, W.J.; Zhang, X.M.; Chen, J.J. Synthesis, structure-activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorganic Med. Chem. Lett. 2014, 24, 2353–2359. [Google Scholar] [CrossRef]
- Gan, L.; Zheng, Y.; Deng, L.; Sun, P.; Ye, J.; Wei, X.; Liu, F.; Yu, L.; Ye, W.; Fan, C.; et al. Diterpenoid Lactones with Anti-Inflammatory Effects from the Aerial Parts of Andrographis paniculata. Molecules 2019, 24, 2726. [Google Scholar] [CrossRef]
- Sharma, S.N.; Sahu, S.; Jha, Z.; Sharma, D.K. Evaluation of seasonal variation in relation to secondary metabolite and biomass production of Andrographis paniculata. J. Nat. Remedies 2012, 12, 39–46. [Google Scholar]
- Sharma, M.; Sharma, R. Identification, purification and quantification of andrographolide from Andrographis paniculata (burm. F.) Nees by HPTLC at different stages of life cycle of crop. J. Curr. Chem. Pharm. Sci. 2013, 3, 23–32. [Google Scholar]
- Dua, V.K.; Ojha, V.P.; Roy, R.; Joshi, B.C.; Valecha, N.; Devi, C.U.; Bhatnagar, M.C.; Sharma, V.P.; Subbarao, S.K. Anti-malarial activity of some xanthones isolated from the roots of Andrographis paniculata. J. Ethnopharmacol. 2004, 95, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Dua, V.K.; Verma, G.; Dash, A.P. In vitro antiprotozoal activity of some xanthones isolated from the roots of Andrographis paniculata. Phytother. Res. 2009, 23, 126–128. [Google Scholar] [CrossRef] [PubMed]
- ChemFinder. Structure Search. Available online: https://www.sigmaaldrich.com/catalog/search/substructure/SubstructureSearchPage (accessed on 25 November 2020).
- ChemSpider. Structure Search. Available online: https://www.chemspider.com/StructureSearch.aspx (accessed on 25 November 2020).
- Lee, J.C.; Tseng, C.K.; Young, K.C.; Sun, H.Y.; Wang, S.W.; Chen, W.C.; Lin, C.K.; Wu, Y.H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br. J. Pharmacol. 2014, 171, 237–252. [Google Scholar] [CrossRef]
- Singha, P.K.; Roy, S.; Dey, S. Antimicrobial activity of Andrographis paniculata. Fitoterapia 2003, 74, 692–694. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, N.R.; Akerele, O.; Bingel, A.S.; Soejarto, D.D.; Guo, Z. Medicinal plants in therapy. Bull. World Health Organ. 1985, 63, 965–981. [Google Scholar] [CrossRef]
- Deng, W.L. Outline of current clinical and pharmacological research on Andrographis paniculata in China. Newsl. Chin. Herb. Med. 1978, 10, 27–31. [Google Scholar]
- Parveen, R.; Parveen, B.; Parveen, A.; Ahmad, S. Andrographis paniculata: From traditional to nano drug for cancer therapy. In Nanomaterials and Plant Potential; Husen, A., Iqbal, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 317–345. [Google Scholar]
- Zaidan, M.R.; Noor Rain, A.; Badrul, A.R.; Adlin, A.; Norazah, A.; Zakiah, I. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop. Biomed. 2005, 22, 165–170. [Google Scholar] [PubMed]
- Sahalan, A.Z.; Sulaiman, N.; Mohammed, N.; Ambia, K.M.; Lian, H.H. Antibacterial activity of Andrographis paniculata and Euphorbia hirta methanol extracts. J. Sains Kesihat. Malays. 2007, 5, 1–8. [Google Scholar]
- Gupta, S.; Yadava, J.N.S.; Tandon, J.S. Antisecretory (Antidiarrhoeal) Activity of Indian Medicinal Plants Against Escherichia Coli Enterotoxin-Induced Secretion in Rabbit and Guinea Pig Ileal Loop Models. Int. J. Pharmacogn. 2008, 31, 198–204. [Google Scholar] [CrossRef]
- Roy, S.; Rao, K.; Bhuvaneswari, C.; Giri, A.; Mangamoori, L.N. Phytochemical analysis of Andrographis paniculata extract and its antimicrobial activity. World J. Microbiol. Biotechnol. 2010, 26, 85–91. [Google Scholar] [CrossRef]
- Wei, L.S.; Wee, W.; Siong, J.Y.F.; Syamsumir, D.F. Characterizaion of antimicrobial, antioxidant, anticancer properties and chemical composition of Malaysian Andrographis paniculata leaf extract. Pharmacologyonline 2011, 2, 996–1002. [Google Scholar]
- Sule, A.; Ahmed, Q.U.; Samah, O.A.; Omar, M.N. Screening for Antibacterial Activity of Andrographis paniculata Used in Malaysian Folkloric Medicine: A Possible Alternative for the Treatment of Skin Infections. Ethnobot. Leafl. 2010, 14, 445–456. [Google Scholar]
- Radhika, P.; Sastry, B.S.; Madhu, H.B. Antimicrobial screening of Andrographis paniculata (Acanthaceae) root extracts. Res. J. Biotechnol. 2008, 3, 62–63. [Google Scholar]
- Sule, A.; Ahmed, Q.U.; Samah, O.A.; Omar, M.N. Bacteriostatic and bactericidal activities of Andrographis paniculata extracts on skin disease causing pathogenic bacteria. J. Med. Plants Res. 2011, 5, 7–14. [Google Scholar]
- Kataky, A.; Handique, P.J. Antimicrobial activity and phytochemical estimation of micropropagated Andrographis paniculata (Burm.f) Nees. Asian J. Sci. Technol. 2010, 5, 091–094. [Google Scholar]
- Monegro, A.F.; Muppidi, V.; Regunath, H. Hospital Acquired Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Taylor, T.A.; Unakal, C.G. Staphylococcus aureus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Wang, W.; Wang, J.; Dong, S.F.; Liu, C.H.; Italiani, P.; Sun, S.H.; Xu, J.; Boraschi, D.; Ma, S.P.; Qu, D. Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacol. Sin. 2010, 31, 191–201. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, L.; Li, M.; Hu, Y.; Zhang, E.; Jiang, Q.; Han, G.; Jin, Y. Inhalable Andrographolide-beta-cyclodextrin Inclusion Complexes for Treatment of Staphylococcus aureus Pneumonia by Regulating Immune Responses. Mol. Pharm. 2017, 14, 1718–1725. [Google Scholar] [CrossRef]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, Y.; Kolter, R. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 5383–5386. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, X.; Liang, H.; Che, Y.; Chen, C.; Dai, H.; Yu, K.; Liu, M.; Ma, L.; Yang, C.H.; et al. Effects of 14-alpha-lipoyl andrographolide on quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2012, 56, 6088–6094. [Google Scholar] [CrossRef]
- Wu, C.M.; Cao, J.L.; Zheng, M.H.; Ou, Y.; Zhang, L.; Zhu, X.Q.; Song, J.X. Effect and mechanism of andrographolide on the recovery of Pseudomonas aeruginosa susceptibility to several antibiotics. J. Int. Med. Res. 2008, 36, 178–186. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.Y.; Wu, S.C.; Xia, F.; Fu, Y.X.; Wu, Y.L.; Leng, C.Q.; Yi, P.F.; Shen, H.Q.; Wei, X.B.; et al. Andrographolide interferes quorum sensing to reduce cell damage caused by avian pathogenic Escherichia coli. Vet. Microbiol. 2014, 174, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013, 1–51. [Google Scholar] [CrossRef]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrob. Resist Infect. Control 2020, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Jiao, Y.; Cody, G.D.; Harding, A.K.; Wilmes, P.; Schrenk, M.; Wheeler, K.E.; Banfield, J.F.; Thelen, M.P. Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl. Environ. Microbiol. 2010, 76, 2916–2922. [Google Scholar] [CrossRef]
- Otake, T.; Mori, H.; Morimoto, M.; Ueba, N.; Sutardjo, S.; Kusumoto, I.T.; Hattori, M.; Namba, T. Screening of Indonesian plant extracts for anti-human immunodeficiency virus—type 1 (HIV-1) activity. Phytother. Res. 1995, 9, 6–10. [Google Scholar] [CrossRef]
- Yao, X.J.; Wainberg, M.A.; Parniak, M.A. Mechanism of inhibition of HIV-1 infection in vitro by purified extract of Prunella vulgaris. Virology 1992, 187, 56–62. [Google Scholar] [CrossRef]
- St-Pierre, C.; Ouellet, M.; Tremblay, M.J.; Sato, S. Galectin-1 and HIV-1 Infection. In Glycobiology; Fukuda, M., Ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 480, pp. 267–294. [Google Scholar]
- Basak, A.; Cooper, S.; Roberge, A.G.; Banik, U.K.; Chretien, M.; Seidah, N.G. Inhibition of proprotein convertases-1, -7 and furin by diterpines of Andrographis paniculata and their succinoyl esters. Biochem. J. 1999, 338, 107–113. [Google Scholar] [CrossRef]
- Ekalaksananan, T.; Sookmai, W.; Fangkham, S.; Pientong, C.; Aromdee, C.; Seubsasana, S.; Kongyingyoes, B. Activity of Andrographolide and Its Derivatives on HPV16 Pseudovirus Infection and Viral Oncogene Expression in Cervical Carcinoma Cells. Nutr. Cancer 2015, 67, 687–696. [Google Scholar] [CrossRef]
- Gopalasatheeskumar, K.; Karthikeyen, L.; Anguraj, M.; Jerad, S.; Kalaichelvan, V.K.; Kumudhaveni, B. Screening of Kabasura Kudineer Chooranam against COVID-19 through Targeting of Main Protease and RNA-Dependent RNA Polymerase of SARS-Cov-2 by Molecular Docking Studies; SSRN: Rochester, NY, USA, 2020; 38p. [Google Scholar] [CrossRef]
- Suritra, B.; Omobolanle Abimbola, A.; Blessing Chinweotito, O.; Adeola Tawakalitu, K.-M.; Emmanuel Ifeanyi, A.; Lawrence, E.; Ankita, K.; Ravindran, J.; Niyi Samuel, A. Polypharmacology of Some Medicinal Plant Metabolites Against SARS-CoV-2 and Host Targets: Molecular Dynamics Evaluation of NSP9 RNA Binding Protein. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Sukardiman, M.E.; Pratama, M.R.; Poerwono, H.; Siswodihardjo, S. The coronavirus disease 2019 main protease inhibitor from Andrographis paniculata (Burm. f) Ness. J. Adv. Pharm. Technol. Res. 2020, 11, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Enmozhi, S.K.; Raja, K.; Sebastine, I.; Joseph, J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: An in silico approach. J. Biomol. Struct. Dyn. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Dey, Y.N.; Patil, R.; Chikhale, R.; Wanjari, M.M.; Gurav, S.S.; Patil, B.M.; Srivastava, B.; Gaidhani, S.N. Combination of system biology to probe the anti-viral activity of andrographolide and its derivative against COVID-19. RSC Adv. 2021, 11, 5065–5079. [Google Scholar] [CrossRef]
- Wanchaitanawong, P.; Chaungwanit, P.; Poovarodom, N.; Nitisinprasert, S. In vitro antifungal activity of Thai herb and spice extracts against food spoilage fungi. Kasetsart J. Nat. Sci. 2005, 39, 400–405. [Google Scholar]
- Rehman, A.N.N.; Furuta, T.; Kojima, S.; Takane, K.; Ali Mohd, M. Antimalarial activity of extracts of Malaysian medicinal plants. J. Ethnopharmacol. 1999, 64, 249–254. [Google Scholar] [CrossRef]
- Mishra, K.; Dash, A.P.; Dey, N. Andrographolide: A Novel Antimalarial Diterpene Lactone Compound from Andrographis paniculata and Its Interaction with Curcumin and Artesunate. J. Trop. Med. 2011, 2011, 579518. [Google Scholar] [CrossRef]
- Sachdeva, M. Analysis of in-vitro antimalarial activity of Andrographolide and 5-hydroxy-7,8-dimethoxyflavone Isolated from andrographis paniculata against plasmodium Berghei parasite. Pharma. Sci. Monit. 2011, 2, 104–116. [Google Scholar]
- Kaleysa, R.R. Screening of indigenous plants for anthelmintic action against human Ascaris lumbricoides. Indian J. Physiol. Pharmacol. 1975, 19, 47–49. [Google Scholar]
- Dutta, A.; Sukul, N.C. Filaricidal properties of a wild herb, Andrographis paniculata. J. Helminthol. 1982, 56, 81–84. [Google Scholar] [CrossRef]
- Spasov, A.; Ostrovskij, O.; Chernikov, M.; Wikman, G. Comparative controlled study of Andrographis paniculata fixed combination, Kan Jang® and an echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytother. Res. 2004, 18, 47–53. [Google Scholar] [CrossRef]
- Gabrielian, E.S.; Shukarian, A.K.; Goukasova, G.I.; Chandanian, G.L.; Panossian, A.G.; Wikman, G.; Wagner, H. A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine 2002, 9, 589–597. [Google Scholar] [CrossRef]
- Melchior, J.; Spasov, A.A.; Ostrovskij, O.V.; Bulanov, A.E.; Wikman, G. Double-blind, placebo-controlled pilot and phase III study of activity of standardized Andrographis paniculata Herba Nees extract fixed combination (Kan jang) in the treatment of uncomplicated upper-respiratory tract infection. Phytomedicine 2000, 7, 341–350. [Google Scholar] [CrossRef]
- Caceres, D.D.; Hancke, J.L.; Burgos, R.A.; Wikman, G.K. Prevention of common colds with Andrographis paniculata dried extract. A Pilot double blind trial. Phytomedicine 1997, 4, 101–104. [Google Scholar] [CrossRef]
- Caceres, D.D.; Hancke, J.L.; Burgos, R.A.; Sandberg, F.; Wikman, G.K. Use of visual analogue scale measurements (VAS) to asses the effectiveness of standardized Andrographis paniculata extract SHA-10 in reducing the symptoms of common cold. A randomized double blind-placebo study. Phytomedicine 1999, 6, 217–223. [Google Scholar] [CrossRef]
- Mkrtchyan, A.; Panosyan, V.; Panossian, A.; Wikman, G.; Wagner, H. A phase I clinical study of Andrographis paniculata fixed combination Kan Jang versus ginseng and valerian on the semen quality of healthy male subjects. Phytomedicine 2005, 12, 403–409. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Liu, Y.M.; Liu, G.Y.; Zhang, M.Q.; Jia, J.Y.; Lu, C.; Yu, C. Pharmacokinetics and tolerance of dehydroandrographolide succinate injection after intravenous administration in healthy Chinese volunteers. Acta Pharmacol. Sin. 2012, 33, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.A.; Hancke, J.L.; Bertoglio, J.C.; Aguirre, V.; Arriagada, S.; Calvo, M.; Caceres, D.D. Efficacy of an Andrographis paniculata composition for the relief of rheumatoid arthritis symptoms: A prospective randomized placebo-controlled trial. Clin. Rheumatol. 2009, 28, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Amaryan, G.; Astvatsatryan, V.; Gabrielyan, E.; Panossian, A.; Panosyan, V.; Wikman, G. Double-blind, placebo-controlled, randomized, pilot clinical trial of ImmunoGuard—A standardized fixed combination of Andrographis paniculata Nees, with Eleutherococcus senticosus Maxim, Schizandra chinensis Bail. and Glycyrrhiza glabra L. extracts in patients with Familial Mediterranean Fever. Phytomedicine 2003, 10, 271–285. [Google Scholar] [CrossRef]
- Ang, L.P.; Ng, P.W.; Lean, Y.L.; Kotra, V.; Kifli, N.; Goh, H.P.; Lee, K.S.; Sarker, M.M.R.; Al-Worafi, Y.M.; Ming, L.C. Herbal Products Containing Aristolochic Acids: A Call to Revisit the Context of Safety. J. Herb. Med. 2021, 100447. [Google Scholar] [CrossRef]

 ), the Black arrow (
), the Black arrow ( ): regular process/pathway, Black dotted rectangle area: AG Induction of Salmonella-specific cell-mediated immune response in Salmonella typhimurum, Blue dotted rectangle area: AG inhibits cellular inflammatory factor in Propionibacterium acnes, COX-2: Cyclooxygenase-2, ENB: Enterotoxin B, FBP: Fibronectin-binding protein, Green dotted arrow (
): regular process/pathway, Black dotted rectangle area: AG Induction of Salmonella-specific cell-mediated immune response in Salmonella typhimurum, Blue dotted rectangle area: AG inhibits cellular inflammatory factor in Propionibacterium acnes, COX-2: Cyclooxygenase-2, ENB: Enterotoxin B, FBP: Fibronectin-binding protein, Green dotted arrow ( ): expected regulation process, Green dotted arrow with a flat bottom (
): expected regulation process, Green dotted arrow with a flat bottom ( ): attachment of AG to the target site, Green down arrow (
): attachment of AG to the target site, Green down arrow ( ): downregulation and expected outcomes, Green up arrow (
): downregulation and expected outcomes, Green up arrow ( ): upregulation and expected outcomes, HaCaT: Human Epidermal Keratinocyte line, HLN: Hemolysin, IFN-γ: Interferon-gamma, IgG: Immunoglobulin G, IL-6: Interleukin 6, IL-8: Interleukin 8, NF-κB: Nuclear factor-kappa-light-chain-enhancer of activated B cell, Orange dotted rectangle area: AG analogue influences the quorum sensing system and inhibits exopolysaccharides generation in Pseudomonas aeruginosa. psl production is also significantly inhibited in this biofilm-forming bacteria, (
): upregulation and expected outcomes, HaCaT: Human Epidermal Keratinocyte line, HLN: Hemolysin, IFN-γ: Interferon-gamma, IgG: Immunoglobulin G, IL-6: Interleukin 6, IL-8: Interleukin 8, NF-κB: Nuclear factor-kappa-light-chain-enhancer of activated B cell, Orange dotted rectangle area: AG analogue influences the quorum sensing system and inhibits exopolysaccharides generation in Pseudomonas aeruginosa. psl production is also significantly inhibited in this biofilm-forming bacteria, ( ): Phosphorylation, Purple dotted rectangle area: AG influences quorum sensing system and reduces the expression of F1 pili, P pili and Tsh by downregulating fimA, papC and tsh in Escherichia coli. All these virulence genes help bacteria to the adherence cell surface. The red arrow (
): Phosphorylation, Purple dotted rectangle area: AG influences quorum sensing system and reduces the expression of F1 pili, P pili and Tsh by downregulating fimA, papC and tsh in Escherichia coli. All these virulence genes help bacteria to the adherence cell surface. The red arrow ( ): Inhibition/downregulation of the process, Red up arrow (
): Inhibition/downregulation of the process, Red up arrow ( ): upregulation (at disease or infection stage), TNF-α: Tumor Necrosis Factor- alpha, TSST-1: Toxic Shock Syndrome Toxin-1.
): upregulation (at disease or infection stage), TNF-α: Tumor Necrosis Factor- alpha, TSST-1: Toxic Shock Syndrome Toxin-1.
 ), the Black arrow (
), the Black arrow ( ): regular process/pathway, Black dotted rectangle area: AG Induction of Salmonella-specific cell-mediated immune response in Salmonella typhimurum, Blue dotted rectangle area: AG inhibits cellular inflammatory factor in Propionibacterium acnes, COX-2: Cyclooxygenase-2, ENB: Enterotoxin B, FBP: Fibronectin-binding protein, Green dotted arrow (
): regular process/pathway, Black dotted rectangle area: AG Induction of Salmonella-specific cell-mediated immune response in Salmonella typhimurum, Blue dotted rectangle area: AG inhibits cellular inflammatory factor in Propionibacterium acnes, COX-2: Cyclooxygenase-2, ENB: Enterotoxin B, FBP: Fibronectin-binding protein, Green dotted arrow ( ): expected regulation process, Green dotted arrow with a flat bottom (
): expected regulation process, Green dotted arrow with a flat bottom ( ): attachment of AG to the target site, Green down arrow (
): attachment of AG to the target site, Green down arrow ( ): downregulation and expected outcomes, Green up arrow (
): downregulation and expected outcomes, Green up arrow ( ): upregulation and expected outcomes, HaCaT: Human Epidermal Keratinocyte line, HLN: Hemolysin, IFN-γ: Interferon-gamma, IgG: Immunoglobulin G, IL-6: Interleukin 6, IL-8: Interleukin 8, NF-κB: Nuclear factor-kappa-light-chain-enhancer of activated B cell, Orange dotted rectangle area: AG analogue influences the quorum sensing system and inhibits exopolysaccharides generation in Pseudomonas aeruginosa. psl production is also significantly inhibited in this biofilm-forming bacteria, (
): upregulation and expected outcomes, HaCaT: Human Epidermal Keratinocyte line, HLN: Hemolysin, IFN-γ: Interferon-gamma, IgG: Immunoglobulin G, IL-6: Interleukin 6, IL-8: Interleukin 8, NF-κB: Nuclear factor-kappa-light-chain-enhancer of activated B cell, Orange dotted rectangle area: AG analogue influences the quorum sensing system and inhibits exopolysaccharides generation in Pseudomonas aeruginosa. psl production is also significantly inhibited in this biofilm-forming bacteria, ( ): Phosphorylation, Purple dotted rectangle area: AG influences quorum sensing system and reduces the expression of F1 pili, P pili and Tsh by downregulating fimA, papC and tsh in Escherichia coli. All these virulence genes help bacteria to the adherence cell surface. The red arrow (
): Phosphorylation, Purple dotted rectangle area: AG influences quorum sensing system and reduces the expression of F1 pili, P pili and Tsh by downregulating fimA, papC and tsh in Escherichia coli. All these virulence genes help bacteria to the adherence cell surface. The red arrow ( ): Inhibition/downregulation of the process, Red up arrow (
): Inhibition/downregulation of the process, Red up arrow ( ): upregulation (at disease or infection stage), TNF-α: Tumor Necrosis Factor- alpha, TSST-1: Toxic Shock Syndrome Toxin-1.
): upregulation (at disease or infection stage), TNF-α: Tumor Necrosis Factor- alpha, TSST-1: Toxic Shock Syndrome Toxin-1.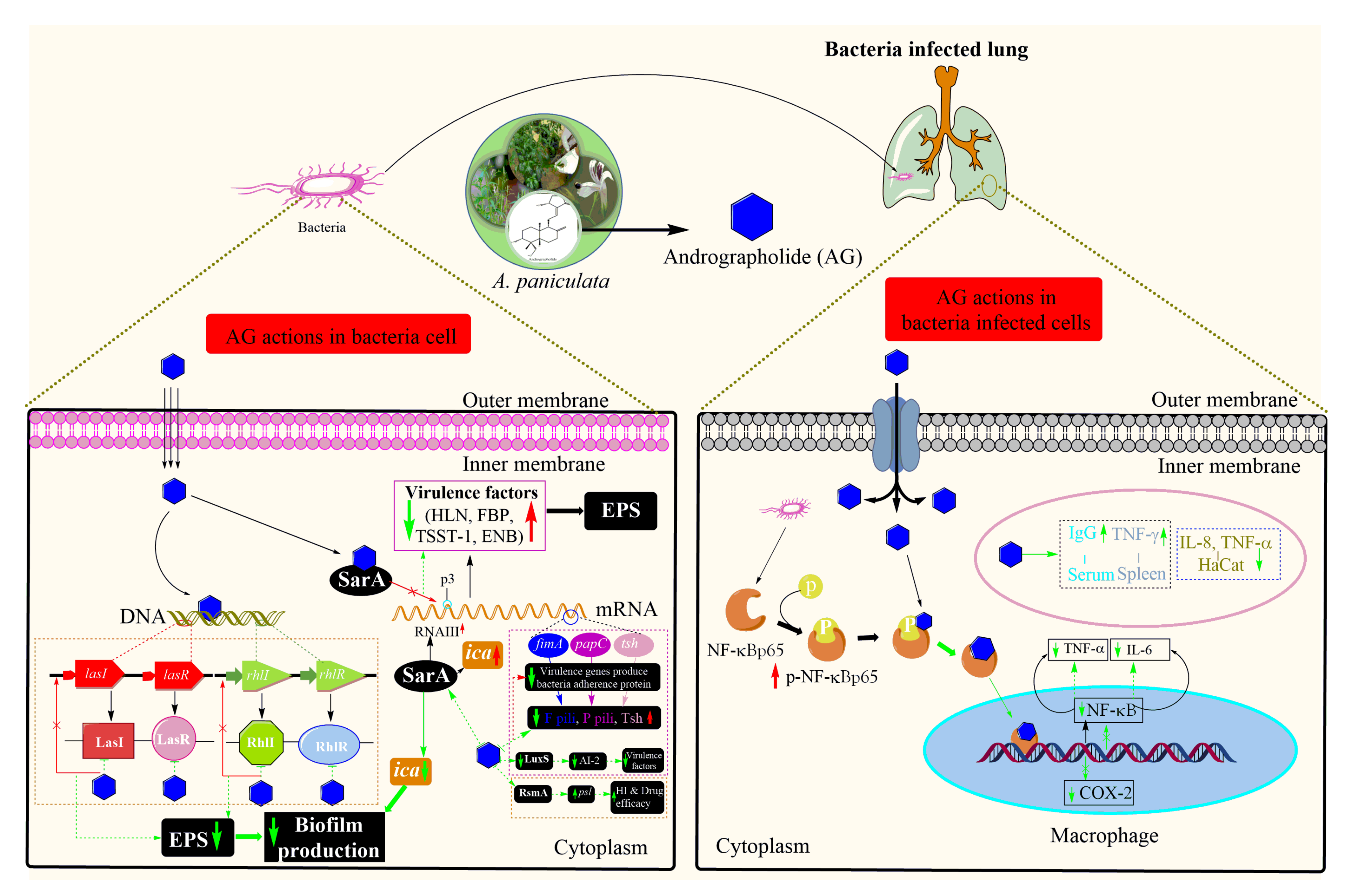
| Study ID; Year; Country | Study Design | Gender & Age | Recruitment(n)/Analyzed(n) | Diagnosis | Study Medications | Daily Dosage (Duration) | Active Ingredients | Salient Outcomes |
|---|---|---|---|---|---|---|---|---|
| Thamlikitkul, et al. [60]; 1991; Thailand | R, DB | G: M&F A: 12 y or older | n = 152/142 CR = (93.43%) AP (LDG) = 48/46 AP (HDG) = 51/47 Paracetamol = 53/49 BCS = NSD (p > 0.05) | Pharyngotons- illitis | AP dried leaves extract (250 or 500 mg/capsule) and paracetamol (325 mg/capsule) | 3 capsules 4 xD (7 d) | 6% of AND | There was NSD in the efficacy of relieving fever (p = 0.16) and sore throat (p = 0.49) among 3 groups on day 7. The majority of the Paracetamol and AP (HDG) group patients stopped taking medication on the 3rd day due to relief of symptoms. |
| Hancke, et al. [56]; 1995; Chile | R, DBPC | G: M&F A: 18–60 y | n = 59/59 CR = 100% AP = 33/33 P = 28/28 BCS = NSD (p > 0.05) | Common cold | Monodrug Kan Jang: AP dried extract (100 mg/tablet) | 1.2 g daily (4 d) | 4% of AND | AP extract attenuates the signs of a common cold significantly at day 4 after treatment which is not observed with the placebo (p < 0.05). |
| Caceres, et al. [241]; 1997; Chile | R, DBPC | G: M&F A: ~18 y | n = 107/107 CR = 100% AP = 54/54 P = 53/53 | Healthy volunteer * | Monodrug Kan Jang: AP dried extract (100 mg/tablet) | Daily 2 tablets, 5 d/w (3 m) | 5.6% of AND | After the third month of treatment, a significant decrease in common colds in the AP group was observed compared to the P group (p < 0.05). |
| Melchior, et al. [58]; 1997; Sweden | R, DBPC | G: M&F A: 18–55 y | n = 50/50 CR = 100% AP = 25/25 P = 25/25 BCS = NSD (p > 0.05) | Common cold sand sinusitis | AP leaves hydroalcoholic extract (85 mg/tablet) | 4 tablets 3 xD (5 d) | AND & DAND | The total recovery rate was 67.5% and 36% in Kan Jang and placebo group, respectively (p < 0.046). |
| Melchior, et al. [240]; 2000; Sweden | R, DBPC | G: M&F A: 18–55 y | n = 47/46 CR = 97.87% AP = 23/23 P = 24/23 BCS = NSD (p > 0.05) | Uncomplicated acute URTI | Combination of APE and AS extract (85 mg/tablet) | 3 tablets 4 xD (5–6 d) | 5.25 mg AND & DAND; 9.7 mg per tablet EB and EE | Much improvement in the patient’s overall symptoms cores in TrG was observed compared to P (p = 0.08). |
| Melchior, et al. [240]; 2000; Russia | R, DBPC | G: M&F A: 18–55 y | n = 180/179 CR = 99.44% AP = 90/89 P = 90/90 BCS = NSD (p > 0.05) | Uncomplicated acute URTI | Combination of APE and AS extract (85 mg/tablet) | 3 tablets 4 xD (5–6 d) | 55.25 mg AND & DAND; 9.7 mg per tablet EB and EE | The difference between TrG and P groups was significant for total diagnosis score (p = 0.003) and total symptom score (p = 0.0006). |
| Caceres, et al. [242]; 1999; Chile | R, DBPC | G: M&F A: 25–50 y | n = 208/158 CR = 87.78% AP = 102/79 P = 106/79 BCS = NSD (p > 0.05) | Common colds | APE (100 mg/tablet) | 4 tablets 3 xD (5 d) | 5% of total AND & DAND | On day 4 of treatment, the decrease in the intensity and duration of symptoms was highly significant between TrG and P groups (p < 0.001). |
| Gabrielian, et al. [239]; 2002; Armenia | PG, DBPC | G: M&F A: 15–64 y | n = 200/185 CR = 95.45% AP = 100/95P = 100/90 BCS = NSD (p > 0.05) | Acute URTIs and sinusitis | Combination of APE and AS extract (85 mg/tablet) | 4 tablets 3 xD (5 d) | 5 mg AND & 10 mg per tablet | Headache, nasal, sore and dry throat, and general malaise showed the most significant improvement (p < 0.001), while cough and eye symptoms did not differ significantly between the groups. |
| Spasov, et al. [238]; 2004; Russia | RC3PG | G: M&F A: 4–11 y | n = 133/133 CR = 100% Group A (AP) = 53/53 Group B = 41/41 Group C = 39/39 BCS = SD; NSD (BP, p > 0.05) | Uncomplicated URTI | Combination of APE and AS extract (85 mg/tablet); Immunal drops: contain EP and ethanol (4:1) | A: 2 tablets 3 xD (10 d); B: 10 drops 3 xD (10 d);C: 500 mg paracetamol 3 xD (10 d) and othersℓ | 5.25 mg AND & DAND; 9.7 mg per tablet EB and EE | Compared with group B (Immunal), the AP extract group considerably improved the development of the disease and intensified children’s recovery with common colds. |
| Saxena, et al. [59]; 2010; India | R, DBPC | G: M&F A: 18–60 y | n = 223/220 CR = 98.65% AP = 112/112P = 111/108 BCS = NSD (p > 0.05) | Uncomplicated URTIs | KalmCold™ (dried AP leaves extract; formulated by mixing methanol and water extract) (100 mg/capsule) | 1 capsule 2 xD (5 d) | AND, IAND, NAND, AGN, DDHAND, SCF & MWN | The intervention group showed 2.1 folds higher improvement in reducing URTI symptoms than the placebo group (p ≤ 0.05). |
| Kulichenko, et al. [57]; 2003; Russia | RPG | G: M&F A: 19–63 y | n = 540/540 CR = 100% G (AP) = 71/71 Crt = 469/469 BCS = NSD (p > 0.05) | Influenza | AP: Combination of APE (88.8 mg) and AS (10 mg) extract (100 mg/tablet) Ctr: STD€ | AP: 2 tablets 3 xD (3–5 d); Ctr: STD€ | AP: 5.25 mg AND & DAND; 9.7 mg per tablet EB and EE;Ctr: amantadine | Kan Jang treated patients recovered (69.9%) very quickly and reduced the risk of post-influenza complications, while Ctr group recovered 32.2% only (p < 0.001). |
| Kulichenko, et al. [57]; 2003; Russia | SRC | G: M&F A: 20–63 y | n = 66/66 CR = 100% AP = 35/35 Ctr = 31/31 BCS = NSD (p > 0.05) | Influenza | AP: Combination of APE (88.8 mg) and AS (10 mg) extract (100 mg/tablet) Ctr: STD€ | AP: 3 tablets 3 xD (5 d); Ctr: Standard therapy | AP: 5.25 mg/tablet AND & DAND, EB and EE; Ctr: amantadine | AP extract significantly reduced clinical symptoms and sped up patients’ recovery, and significantly (p < 0.001) decreased the number of days off work and the number of cases with post-influenza complications. |
| Calabrese, et al. [54]; 2000; USA | NRCT | G: M&F A: 18 y and above | n = 18/17 CR = 94.44% AP = 13/13HV = 5/4 BCS = NSD (p > 0.05) | HIV | AND | 5, 10, 20 mg/kg BW 3 xD first 3 w, second 3 w and last 3 w, respectively | AND | A dose of 10 mg/kg andrographolide administration significantly (p = 0.002) increased the mean CD4+ lymphocyte count (405 cells/mm3 to 501 cells/mm3) in HIV patients. |
| Chuthaputti, et al. [55]; 2007; Thailand | RCOL | G: M&F A: 3–15 y | n = 25/25 CR = 100% AP = 15/15 Ctr = 10/10 BCS = NSD (p > 0.05) | Influenza | AP: Paracetamol and AP aerial part extract (400 mg/capsule); Ctr: Paracetamol (500 mg/tablet) | AP: 4 capsules 4 xD; Ctr: 2 tablets 4 xD (7 d) | 9% of AND, Paracetamol | The severity of cough, fatigue, and overall symptoms of the TrG were significantly lower than the Ctr group from Day 4 onwards. |
| Reference | Study Design | Recruitment(n)/Analyzed(n) | Diagnosis | Adverse Effects (Cases) | |
|---|---|---|---|---|---|
| Treatment | Placebo | ||||
| Thamlikitkul, et al. [60] | R, DB | n = 152/142 | Pharyngotonsillitis | Minimal and self-limiting side effects (i.e., nausea, vomiting, abdominal discomfort, dizziness, drowsiness, and malaise) were found about 20% in treatment (LDG & HDG) and paracetamol groups (9–11). | No placebo groups. |
| Hancke, et al. [56] | R, DBPC | n = 59/59 | Common cold | No adverse event reported | No adverse event reported. |
| Caceres, et al. [241] | R, DBPC | n = 107/107 | HV * | Not reported adverse effect information | Not reported adverse effect information |
| Melchior, et al. [58] | R, DBPC | n = 50/50 | Common colds and sinusitis | Urticaria (2) | No adverse event reported. |
| Melchior, et al. [240] | R, DBPC | n = 47/46 | Uncomplicated acute URTI | No adverse event information reported | No adverse event information reported |
| Melchior, et al. [240] | R, DBPC | n = 180/179 | Uncomplicated acute URTI | Unpleasant sensations in the chest and intensified headache (1) | No adverse event reported. |
| Caceres, et al. [242] | R, DBPC | n = 208/158 | Common colds | No adverse events were observed | No adverse event reported. |
| Gabrielian, et al. [239] | PG, DBPC | n = 200/185 | URTIs and sinusitis | Increase in nasal discharge and epigastric pain (1), nose blocked (1), and severe headache (1). They were excluded from the analysis data. | No adverse event reported. |
| Spasov, et al. [238] | RC3PG | n = 133/133 | Uncomplicated URTI | No side effects were observed | No placebo group |
| Saxena, et al. [59] | R, DBPC | n = 223/220 | URTIs | Mild adverse effect; vomiting (1), epistaxis (1), Urticaria (1) and diarrhoea (3). Except for vomiting (patient in AP group) and urticaria, all other effects stopped spontaneously without any medication. | No adverse event reported. |
| Kulichenko, et al. [57] | RPG | n = 540/540 | Influenza | AP group: Dry cough, rhinitis, and pain in the throat (22). Influenza complications were found in 30.1% of the AP group and 67.8% of the Ctr group (p < 0.01). | No placebo group |
| Kulichenko, et al. [57] | SRC | n = 66/66 | Influenza | Influenza complications were found in 31.43% of AP-treated patients and 70.97% of STD€-treated patients (p < 0.01). | No placebo group |
| Calabrese, et al. [54] | NRCT | n = 18/17 | HIV | Anaphylactic reaction (1). All but one (92%) reported at least one adverse event during the study. ¾ reported an adverse event by the healthy volunteer. All conditions were returned to normal by week 9. | No placebo group |
| Chuthaputti, et al. [55] | RCOL | n = 25/25 | Influenza | No adverse event information reported. | No placebo group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, S.; Urbi, Z.; Karuniawati, H.; Mohiuddin, R.B.; Moh Qrimida, A.; Allzrag, A.M.M.; Ming, L.C.; Pagano, E.; Capasso, R. Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy. Life 2021, 11, 348. https://doi.org/10.3390/life11040348
Hossain S, Urbi Z, Karuniawati H, Mohiuddin RB, Moh Qrimida A, Allzrag AMM, Ming LC, Pagano E, Capasso R. Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy. Life. 2021; 11(4):348. https://doi.org/10.3390/life11040348
Chicago/Turabian StyleHossain, Sanower, Zannat Urbi, Hidayah Karuniawati, Ramisa Binti Mohiuddin, Ahmed Moh Qrimida, Akrm Mohamed Masaud Allzrag, Long Chiau Ming, Ester Pagano, and Raffaele Capasso. 2021. "Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy" Life 11, no. 4: 348. https://doi.org/10.3390/life11040348
APA StyleHossain, S., Urbi, Z., Karuniawati, H., Mohiuddin, R. B., Moh Qrimida, A., Allzrag, A. M. M., Ming, L. C., Pagano, E., & Capasso, R. (2021). Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy. Life, 11(4), 348. https://doi.org/10.3390/life11040348