Loose Ends in the Cortinarius Phylogeny: Five New Myxotelamonoid Species Indicate a High Diversity of These Ectomycorrhizal Fungi with South American Nothofagaceae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Work
2.2. Morphological Study
2.3. DNA Extraction, PCR Amplification and Sequencing
2.4. Data Analysis
3. Results
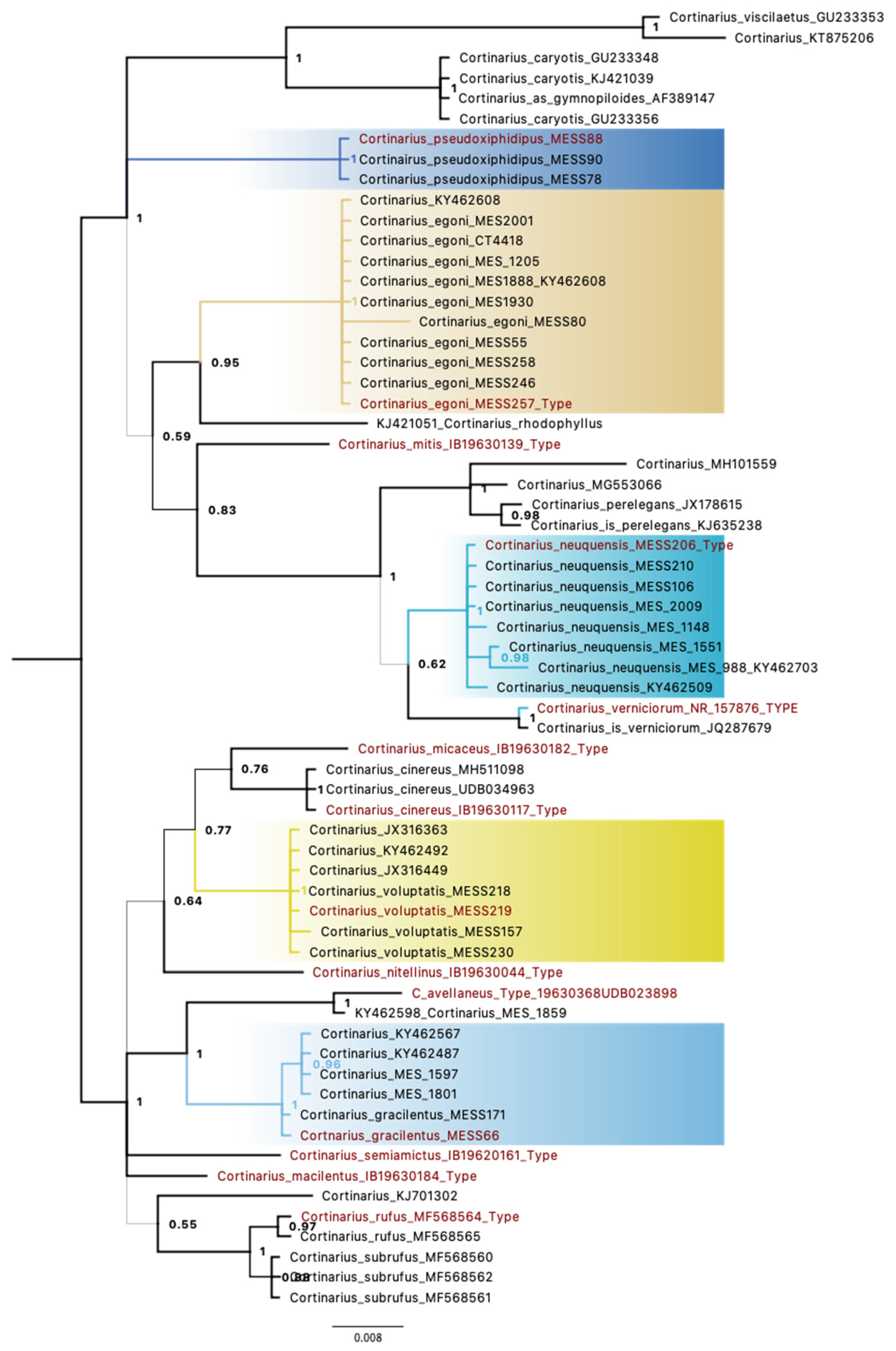
3.1. Molecular Data
3.2. Taxonomic Data
3.3. Taxonomy
3.3.1. Cortinarius egonii Salgado Salomón, Peintner, Liimat. and Niskanen spp. nov.
MycoBank MB 836828
Etymology
Diagnosis
Type
Macrocharacters
Microcharacters
Ecology and Distribution
3.3.2. Cortinarius gracilentus Salgado Salomón and Peintner spp. nov.
MycoBank MB 836579
Etymology
Diagnosis
Type
Macrocharacters
Microcharacters
Ecology and Distribution
3.3.3. Cortinarius neuquensis Salgado Salomón, Peintner, Liimat. and Niskanen spp. nov.
MycoBank MB 836823
Etymology
Diagnosis
Type
Macrocharacters
Microcharacters
Ecology and Distribution
3.3.4. Cortinarius pseudoxiphidipus Salgado Salomón and Peintner spp. nov.
MycoBank MB 836577
Etymology
Diagnosis
Type
Macrocharacters
Microcharacters
Ecology and Distribution
3.3.5. Cortinarius voluptatis Salgado Salomón and Peintner spp. nov.
MycoBank MB 836578
Etymology
Diagnosis
Type
Macrocharacters
Microcharacters
Ecology and Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barroetaveña, C.; Salgado Salomón, M.E.; Bassani, V. Rescuing the ectomycorrhizal biodiversity associated with South American Nothofagaceae forest, from the 19th century naturalists up to molecular biogeography. Forestry 2019, 92, 500–511. [Google Scholar] [CrossRef]
- Bödeker, I.T.M.; Clemmensen, K.E.; de Boer, W.; Martin, F.; Olson, A.; Lindahl, B.D. Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol. 2014, 203, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Arnold, N.; Palfner, G.; Schmidt, J.; Kuhnt, C.; Becerra, J. Chemistry of the aroma bouquet of the edible mushroom “lebre” (Cortinarius lebre, Basdiomycota, Agaricales) from Chile. J. Chil. Chem. Soc. 2012, 57, 1333–1335. [Google Scholar] [CrossRef] [Green Version]
- Gamundí, I.J.; Horak, E. Hongos De Los Bosques Andino Patagónicos; Vázquez Mazzini Editores: Buenos Aires, Argentina, 1993. [Google Scholar]
- Moser, M.; Horak, E. Cortinarius Fr. und nahe verwandte gattungen in südamerika. Nova Hedwig. Beih. 1975, 52, 1–628. [Google Scholar]
- Toledo, C.V.; Barroetaveña, C.; Rajchenberg, M. Phenology and environmental variables associated to the fruiting of wild edible mushrooms from Andean-Patagonia forests in Argentina. Rev. Mex. Biodivers. 2014, 85, 1093–1103. [Google Scholar] [CrossRef] [Green Version]
- Toledo, C.V.; Barroetaveña, C.; Fernandes, A.; Barros, L.; Ferreira, I. Chemical and antioxidant properties of wild edible mushrooms from native Nothofagus spp. forest, argentina. Molecules 2016, 21, 1201. [Google Scholar] [CrossRef]
- Garnica, S.; Weiß, M.; Oberwinkler, F. New Cortinarius species from Nothofagus forests in South Chile. Mycologia 2002, 94, 136–145. [Google Scholar] [CrossRef]
- Garrido, N.N. Agaricales s. l. und ihre Mykorrhizen in den Nothofagus- Waldern Mittelchiles. In Bibliotheca Mycologica: Band 120; J. Cramer: Berlin/Stuttgart, Germany, 1988. [Google Scholar]
- Horak, E.; Moser, M. Fungi austroamericani. 12. Studien zur Gattung Thaxterogaster Singer R. Nova Hedwig. 1965, 10, 211–242. [Google Scholar]
- Horak, E. Flora criptogámica de Tierra del Fuego. Orden Agaricales; Tomo 11: Buenos Aires, Argentina, 1979; pp. 1–524. [Google Scholar]
- Liimatainen, K.; Niskanen, T.; San-Fabian, B.; Mujic, A.B.; Peintner, U.; Dresch, P.; Furci, G.; Nouhra, E.; Matheny, P.B.; Smith, M.E. Cortinarius section Thaumasti in South American Nothofagaceae forests. Mycologia 2020, 112, 329–341. [Google Scholar] [CrossRef]
- Nouhra, E.; Urcelay, C.; Longo, S.; Tedersoo, L. Ectomycorrhizal fungal communities associated to Nothofagus species in Northern Patagonia. Mycorrhiza 2013, 23, 487–496. [Google Scholar] [CrossRef]
- Salgado Salomón, M.E.; Dresch, P.; Horak, E.; Galleguillos, F.; Barroetaveña, C.; Peintner, U. The enigmatic Cortinarius magellanicus complex occurring in Nothofagaceae forests of the Southern Hemisphere. Fun. Biol. 2018, 22, 1077–1097. [Google Scholar] [CrossRef]
- San Fabian, B.; Niskanen, T.; Liimatainen, K.; Kooij, P.W.; Mujic, A.B.; Truong, C.; Peintner, U.; Dresch, P.; Nouhra, E.; Matheny, P.B.; et al. New species of Cortinarius sect. Austroamericani, sect. nov., from South American Nothofagaceae forests. Mycologia 2018, 11, 1127–1144. [Google Scholar] [CrossRef]
- Singer, R. The agaric of the argentine sector of Tierra del Fuego and limitrophous regions of the Magallanes area. Part I. White and pink species groups. Sydowia 1952, 6, 165–226. [Google Scholar]
- Singer, R. Forest mycology and forest communities in South America II: Mycorrhiza sociology and fungus succession in the Nothofagus dombeyi-Austrocedrus chilensis woods of Patagonia. In Mycorrhizae; Hacskaylo, R., Ed.; USDA Forest Service, Miscellaneous Publication: Washington, DC, USA, 1971; Volume 1189, pp. 204–215. [Google Scholar]
- Singer, R.; Moser, R. Forest mycology and forest communities in South America. Mycopathol. Mycol. Appl. 1965, 26, 130–191. [Google Scholar] [CrossRef]
- Spegazzini, C. Fungi patagonici. Boletín Acad. Nac. Cienc. Córdoba 1887, 21, 5–64. [Google Scholar]
- Spegazzini, C. Fungi fuegiani. Boletín Acad. Nac. Cienc. Córdoba 1887, 21, 135–308. [Google Scholar]
- Valenzuela Flores, E. Estudio Sistemático Corológico y Ecológico de los Agaricales Sensu Lato de los Bosques Autóctonos de la Región de los Lagos en Chile. Ph.D. Thesis, Sección Biológicas, Departamento de Biología Vegetal, Facultad de Ciencias, Universidad de Alcalá de Henares, Alcalá de Henares, Madrid, España, 1993. [Google Scholar]
- Garnica, S.; Weiß, M.; Oertel, B.; Oberwinkler, F. A framework for a phylogenetic classification in the genus Cortinarius (Basidiomycota, Agaricales) derived from morphological and molecular data. Can. J. Bot. 2005, 83, 1457–1477. [Google Scholar] [CrossRef]
- Peintner, U.; Moncalvo, J.M.; Vilgalys, R. Toward a better understanding of the infrageneric relationships in Cortinarius (Agaricales, Basidiomycota). Mycologia 2004, 96, 1042–1058. [Google Scholar] [CrossRef]
- Garnica, S.; Weiß, M.; Oberwinkler, F. Morphological and molecular phylogenetic studies in South American Cortinarius species. Mycol. Res. 2003, 107, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Horak, E. Fungi Austroamericani IV. Revisión de los hongos superiores de Tierra del Fuego, Patagonia en el Herbario de C. Spegazzini en La Plata. Darwiniana 1967, 14, 355–385. [Google Scholar]
- Horak, E.; Wood, A.E. Cortinarius Fr. (Agaricales) in Australasia. 1. Subgen. Myxacium and subgenus Paramyxacium. Sydowia 1990, 42, 88–168. [Google Scholar]
- Palfner, G. Taxonomische Studien an Ektomykorrhizen aus den Nothofagus—Wäldern Mittelsüdchiles. Bibl. Mycol. 2001, 190, 1–243. [Google Scholar]
- Segedin, B.; Pennycook, S. A nomenclatural checklist of agarics, boletes, and related secotioid and gasteromycetous fungi recorded from New Zealand. N. Z. J. Bot. 2001, 39, 285–348. [Google Scholar] [CrossRef]
- Frøslev, T.G.; Jeppesen, T.S.; Læssøe, T. Seven new calochroid and fulvoid species of Cortinarius. Mycol. Res. 2006, 110, 1148–1160. [Google Scholar] [CrossRef]
- Garnica, S.; Weiß, M.; Oertel, B.; Ammirati, J.; Oberwinkler, F. Phylogenetic relationships in Cortinarius, section Calochroi, inferred from nuclear DNA sequences. BMC Evol. Biol. 2009, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, M.; Peintner, U. Die phylogenetischen Beziehungen der Cortinarius aureopluverulentus Gruppe. Micol. Veget. Medit. 2002, 17, 3–17. [Google Scholar]
- Niskanen, T.; Liimatainen, K.; Mahiques, R.; Ballarà, J.; Kytövuori, I. Cortinarius badiolaevis, a new conifer-associated, darkening species in the subgenus Telamonia (Basidiomycota, Agaricales). Mycol. Prog. 2011, 10, 101–105. [Google Scholar] [CrossRef]
- Niskanen, T.; Liimatainen, K.; Ammirati, J.F.; Hughes, K. Cortinarius section Sanguinei in North America. Mycologia 2013, 105, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Niskanen, T.; Liimatainen, K.; Ammirati, J.F. Five new Telamonia species (Cortinarius, Agaricales) from western North America. Botany 2013, 91, 478–485. [Google Scholar] [CrossRef]
- Peintner, U.; Horak, E.; Moser, M.; Vilgalys, R. Phylogeny of Rozites, Cuphocybe and Rapacea inferred from ITS and LSU rDNA sequences. Mycologia 2002, 94, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Peintner, U.; Moser, M.M.; Thomas, K.A.; Manimohan, P. First records of ectomycorrhizal Cortinarius species (Agaricales, Basidiomycetes) from tropical India and their phylogenetic position based on rDNA ITS sequences. Myc. Res. 2003, 107, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Soop, K.; Dima, B.; Cooper, J.A.; Park, D.; Oertel, B. A phylogenetic approach to a global supraspecific taxonomy of Cortinarius (Agaricales) with an emphasis on the southern mycota. Persoonia 2019, 42, 261–290. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Santiago, V.N.; Ortega, A.; Peintner, U.; López-Flores, I. Study on Cortinarius subgenus Telamonia section Hydrocybe in Europe, with especial emphasis on Mediterranean taxa. Mycol. Res. 2009, 113, 1070–1090. [Google Scholar] [CrossRef] [PubMed]
- Frøslev, T.G.; Matheny, P.B.; Hibbett, D. Lower level relationships in the mushroom genus Cortinarius (Basidiomycota, Agaricales): A comparison of RPB1, RPB2, and ITS phylogenies. Mol. Phylogenet. Evol. 2005, 37, 602–618. [Google Scholar] [CrossRef]
- Høiland, K.; Holst-Jensen, A. Cortinarius phylogeny and possible taxonomic implications of ITS rDNA sequences. Mycologia 2000, 92, 694–710. [Google Scholar] [CrossRef]
- Liu, Y.J.; Rogers, S.O.; Ammirati, J.F. Phylogenetic relationships in Dermocybe and related Cortinarius taxa based on nuclear ribosomal DNA internal transcribed spacers. Can. J. Bot. 1997, 75, 519–532. [Google Scholar] [CrossRef]
- Peintner, U.; Bougher, N.L.; Castellano, M.A.; Moncalvo, J.M.; Moser, M.; Trappe, J.M.; Vilgalys, R. Multiple origins of sequestrate fungi related to Cortinarius (Cortinariaceae). Am. J. Bot. 2001, 88, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.L.; Willink, A. Biogeografía de América Latina. In Serie de Biología, Monografía 13; The General Secretariat of the Organization of American States: Washington, DC, USA, 1980; p. 122. [Google Scholar]
- Maerz, A.; Paul, M.R. A Dictionary of Color, 1st ed.; Mc Graw-Hill Book Company Inc.: New York, NY, USA, 1930; p. 207. [Google Scholar]
- Largent, D.L. How to Identify Mushrooms to Genus I: Macroscopic Features; Mad River Press: Eureka, CA, USA, 1986; p. 166. [Google Scholar]
- Largent, D.L.; Johnson, D.; Watling, R. How to Identify Mushrooms to Genus III: Microscopic Features; Mad River Press: Eureka, CA, USA, 1986; p. 146. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.S., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Matheny, P.B.; Liu, Y.J.J.; Ammirati, J.F.; Hall, B.D. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). Am. J. Bot. 2002, 89, 688–698. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Everitt, B.S. An R and S-PLUS® Companion to Multivariate Analysis; USA. British Library Cataloguing in Publication Data; Springer: London, UK, 2005. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat versión 2017. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. 2017. Available online: http://www.infostat.com.ar (accessed on 31 March 2021).
- Soop, K.; Wallace, M.; Dima, B. New Cortinarius (Agaricales) species described from New Zealand. N. Z. J. Bot. 2018, 56, 163–182. [Google Scholar] [CrossRef]
- Valenzuela, E.; Moreno, G.; Garnica, S.; Ramirez, C. Micosociología en bosques nativos de Nothofagus y plantaciones de Pinus radiata en la X Región de Chile: Diversidad y rol ecológico. Rev. Chil. Hist. Nat. 1998, 71, 133–146. [Google Scholar]
- Niskanen, T. Cortinarius subgenus Telamonia p.p. in North Europe. Ph.D. Thesis, Helsinki University, Helsinki, Finland, 2008. Available online: https://helda.helsinki.fi/handle/10138/22011 (accessed on 5 January 2020).
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal lifestyle in fungi: Global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef] [PubMed]
- Kuhar, F.; Smith, M.E.; Mujic, A.; Truong, C.; Nouhra, E. A systematic overview of Descolea (Agaricales) in the Nothofagaceae forests of Patagonia. Fungal Biol. 2017, 121, 876–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, C.; Sanchez-Ramirez, S.; Kuhar, F.; Kaplan, Z.; Smith, M.E. The Gondwanan connection-Southern temperate Amanita lineages and the description of the first sequestrate species from the Americas. Fungal Biol. 2017, 121, 638–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trierveiler-Pereira, L.; Smith, M.E.; Trappe, J.M.; Nouhra, E.R. Sequestrate fungi from Patagonian Nothofagus forests: Cystangium (Russulaceae, Basidiomycota). Mycologia 2015, 107, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Truong, C.; Mujic, A.B.; Healy, R.; Kuhar, F.; Furci, G.; Torres, D.; Niskanen, T.; Sandoval-Leiva, P.A.; Fernandez, N.; Escobar, J.M.; et al. How to know the fungi: Combining field inventories and DNA-barcoding to document fungal diversity. New Phytol. 2017, 214, 913–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matheny, P.B.; Aime, M.C.; Bougher, N.L.; Buyck, B.; Desjardin, D.E.; Horak, E.; Kropp, B.R.; Lodge, D.J.; Soytong, K.; Trappe, J.M.; et al. Out of the Palaeotropics? Historical biogeography and diversification of the cosmopolitan ectomycorrhizal mushroom family Inocybaceae. J. Biogeogr. 2009, 36, 577–592. [Google Scholar] [CrossRef]
- Bruns, T.D.; White, T.J.; Taylor, J.W. Fungal molecular systematics. Annu. Rev. Ecol. Syst. 1991, 22, 525–564. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for Basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Tedersoo, L.; Kõljalg, U.; Hallenberg, N.; Larsson, K.-H. Fine scale distribution of ectomycorrhizal fungi and roots across substrate layers including coarse woody debris in a mixed forest. New Phytol. 2003, 159, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, A.C.; Chen, W.; Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor, N.; Chiapella, J.; Kuhar, F.; Mujic, A.B.; Crespo, E.M.; Nouhra, E.R. Unveiling new sequestrate Cortinarius species from northern Patagonian Nothofagaceae forests based on molecular and morphological data. Mycologia 2019, 111, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Garnica, S.; Schön, M.E.; Abarenkov, K.; Riess, K.; Liimatainen, K.; Niskanen, T.; Dima, B.; Soop, K.; Frøslev, T.G.; Jeppesen, T.S.; et al. Determining threshold values for barcoding fungi: Lessons from Cortinarius (Basidiomycota), a highly diverse and widespread ectomycorrhizal genus. FEMS Microbiol. Ecol. 2016, 92, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Truong, C.; Gabbarini, L.A.; Corrales, A.; Mujic, A.B.; Escobar, J.M.; Moretto, A.; Smith, M.E. Ectomycorrhizal fungi and soil enzymes exhibit contrasting patterns along elevation gradients in southern Patagonia. New Phytol. 2019, 222, 1936–1950. [Google Scholar] [CrossRef]
- Liimatainen, K.; Niskanen, T.; Dima, B.; Kytövuori, I.; Ammirati, J.F.; Frøslev, T.G. The largest type study of Agaricales species to date: Bringing identification and nomenclature of Phlegmacium (Cortinarius) into the DNA era. Persoonia 2014, 33, 98–140. [Google Scholar] [CrossRef] [Green Version]
- Ammirati, J.F.; Smith, A.H. Studies in the genus Cortinarius, III: Section Dermocybe, new North American species. Mycotaxon 1977, 5, 381–397. [Google Scholar]
- Moser, M. Die Gattung Dermocybe (Fr.) Wünsche, (Die Hautköpfe). Schweiz. Z. Pilzkd. 1974, 52, 129–142. [Google Scholar]
- Danks, M.; Lebel, T.; Vernes, K. ‘Cort short on a mountaintop’—Eight new species of sequestrate Cortinarius from sub-alpine Australia and affinities to sections within the genus. Persoonia 2010, 24, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Stefani, F.O.P.; Jones, R.H.; May, T.W. Concordance of seven gene genealogies compared to phenotypic data reveals multiple cryptic species in Australian dermocyboid Cortinarius (Agaricales). Mol. Phylogenet. Evol. 2014, 71, 249–260. [Google Scholar] [CrossRef]
- Horak, E. New species of Dermocybe (Agaricales) from New Zealand. Sydowia 1988, 40, 81–112. [Google Scholar]
- Soop, K. Contribution à l’étude de la mycoflore cortinarioïde de Nouvelle-Zélande. Bull. Soc. Mycol. France 2001, 117, 91–132. [Google Scholar]




| Species | Site | Associated Species | GenBank/UNITE Number | Herbaria Number | Type | Sampling Date |
|---|---|---|---|---|---|---|
| Cortinarius avellaneus | Argentina, Neuquén, PNNH, Puerto Manzano | Nothofagus dombeyi + N. pumilio | UDB023898 | IB 19630368 | Type | 18/4/1963 |
| Cortinarius caryotis | Unknown | Unknown | KJ421039 | F44422 | Unknown | |
| Cortinarius caryotis | New Zealand, Hawdon (Cass) | Nothofagus spp. | GU233348 | PDD 71004 | Holotype | 21/4/1999 |
| Cortinarius caryotis | New Zealand, UNP *, Lake Waikareiti Track | Unknown | GU233356 | PDD 74305 | 11/5/2001 | |
| Cortinarius cinereus | Chile, Coyhaique | Nothofagus dombeyi | MH511098 | CONCF0650 | 15/3/2007 | |
| Cortinarius cinereus | Argentina, Río Negro, PNNH **, Valle Frías | Nothofagus dombeyi | UDB023853 | IB 19630117 | Type | 21/3/1963 |
| Cortinarius cinereus | Chile | Unknown | UDB034963 | IBFFG 650 | Unknown | |
| Cortinarius dulcamarus | New Zealand, North Canterbury, Medbury Scientific Reserve | Kunzea ericoides | MH101559 | PDD 96951 | 26/5/2013 | |
| Cortinarius dulcamarus | New Zealand, Craigieburn | Nothofagus spp. | KJ635238 | PDD 97534 | Type | 5/5/2009 |
| Cortinarius egonii | Chile, Aysen, Carretera Austral, south of Bertrand port. | Nothofagus pumilio + N. dombeyi | MT925625 | CT4418/FLAS-F-63487 | 3/5/2016 | |
| Cortinarius egonii | Argentina, Río Negro, PNNH, Steffen lake | Nothofagus dombeyi | MN707588 | HCFC C246/IB 20170447 | 16/5/2017 | |
| Cortinarius egonii | Argentina, Río Negro, PNNH, Steffen lake | Nothofagus dombeyi | MN707589 | HCFC C257/IB 20170257 | Holotype/Isotype | 16/5/2017 |
| Cortinarius egonii | Argentina, Río Negro, PNNH, Steffen lake | Nothofagus dombeyi | MN707590 | HCFC C258/IB 20170258 | 16/5/2017 | |
| Cortinarius egonii | Argentina, Chubut, PNLA ***, Colihual stream | Nothofagus dombeyi | MN707571, MW405257 | HCFC C52/IB 20170324 | 11/4/2017 | |
| Cortinarius egonii | Argentina, Chubut, PNLA, Colihual stream | Nothofagus dombeyi | MN707574, MW405256, MW546832 | HCFC C80/IB 20170342 | 18/4/2017 | |
| Cortinarius egonii | Argentina, Río Negro, Bariloche, Nahuel Huapi National Park, Los Rápidos | Nothofagus dombeyi + N. antarctica | MT925623 | MES-1205 CORDC00006881 | 11/5/2016 | |
| Cortinarius egonii | Argentina, Río Negro, Bariloche, PNNH, Goye stream, near Colonia Suiza | Nothofagus dombeyi + N. pumilio | KY462608 | MES-1888/CORDC00005629 | 12/5/2016 | |
| Cortinarius egonii | Argentina, Río Negro, Bariloche, PNNH, Los Rápidos | Nothofagus antarctica | MES-1930/CORDC00005614 | 13/5/2016 | ||
| Cortinarius egonii | Argentina, Río Negro, Bariloche, PNNH, Road to Tronador | Nothofagus pumilio | MT925622 | MES-2001 CORDC00005551 | 14/5/2016 | |
| Cortinarius gracilentus | Argentina, Chubut, PNLA, Camping area | Nothofagus antarctica | MN707580, MW405251 | HCFC C171/IB 20170235 | 25/4/2017 | |
| Cortinarius gracilentus | Argentina, Chubut, PNLA, Rivadavia river | Nothofagus dombeyi | MN707572 | HCFC C66/IB 20170334 | Holotype/Isotype | 18/4/2017 |
| Cortinarius ‘gymnopiloides’ | New Zealand | Unknown | AF389147 | ZT NZ68501 | Type | Unknown |
| Cortinarius macilentus | Argentina, Río Negro, PNNH, Valle Frías | Nothofagus dombeyi | UDB023869 | IB19630184 | Type | 24/3/1963 |
| Cortinarius micaceus | Argentina, Río Negro, PNNH, Valle Frías | Nothofagus dombeyi + N. antarctica + N. pumilio | UDB023868 | IB 19630182 | Type | 20/3/1963 |
| Cortinarius mitis | Argentina, Río Negro, PNNH, Valle Frías | Nothofagus dombeyi | UDB023858 | IB19630139 | Type | 22/3/1963 |
| Cortinarius neuquensis | Argentina, Bariloche, PNNH, along road halfway to Tronador. | Nothofagus antarctica | MT925952 | MES-1148 CORDC00005190 | 9/5/2015 | |
| Cortinarius neuquensis | Argentina, Neuquén, PNL ****, Ñorquinco Lake | Lophozonia alpina+ L. obliqua | MN707581 | HCFC C196 IB 20170218 | 3/5/2017 | |
| Cortinarius neuquensis | Argentina, Neuquén, Chañy Protected Area, Chañy stream | Nothofagus antarctica + A. araucana | MN707582 | HCFC C206 IB 20170222 | Holotype/Isotype | 4/5/2017 |
| Cortinarius neuquensis | Argentina, Neuquén, Chañy Protected Area, Chañy stream | N. antarctica + A. araucana | MN707583, MW405255, MW546831 | HCFC C210 IB 20170224 | 4/5/2017 | |
| Cortinarius neuquensis | Chile, Osorno, PNP *****, last stop near Aguas Calientes | Nothofagus dombeyi | MT925953 | MES-1551 FLAS-F-64363 | 3/5/2016 | |
| Cortinarius neuquensis | Chile, Osorno, PNP, foothills of Volcan Puyehue, up the road past El Caulle north of Rio Golgol | Nothofagus dombeyi | KY462509 | MES-1638 FLAS-F-64429 | 4/5/2016 | |
| Cortinarius neuquensis | Argentina, Bariloche, PNNH, Road to Tronador | Nothofagus antarctica | MT925951 | MES-2009 CORDC00005547 | 14/5/2016 | |
| Cortinarius neuquensis | Chile | Lophozonia alpina | KY462703 | MES-988 FLAS-F-63016 | Unknown | |
| Cortinarius nitellinus | Argentina, Neuquén, PNNH, Puerto Manzano | Nothofagus dombeyi | UDB023833 | IB19630044 | Type | 12/3/1963 |
| Cortinarius ‘perelegans’ | New Zealand | Unknown | JX178615 | OTA 60285 | Unknown | |
| Cortinarius pseudoxiphidipus | Argentina, Chubut, PNLA, Rivadavia river | Nothofagus dombeyi | MN707573, MW405254, MW546828 | HCFC C78 IB 20170340 | 18/4/2017 | |
| Cortinarius pseudoxiphidipus | Argentina, Chubut, PNLA, Rivadavia river | Nothofagus dombeyi | MN707575, MW405252 | HCFC C88 IB 20170347 | Holotype/Isotype | 18/4/2017 |
| Cortinarius pseudoxiphidipus | Argentina, Chubut, PNLA, Rivadavia river | Nothofagus dombeyi | MN707576, MW405253, MW546829 | HCFC C90 IB 20170441 | 18/4/2017 | |
| Cortinarius rhodophyllus | Unknown | Unknown | KJ421051 | TUB 020416 | Unknown | |
| Cortinarius rufus | Argentina, Neuquén, PNNH, Puerto Manzano | N. pumilio | MF568564 | IB19630369 | Type | 18/4/1963 |
| Cortinarius rufus | Argentina, Río Negro, PNNH, Arroyo Goye near Colonia Suiza | Nothofagus dombeyi + N. pumilio | MF568565 | K(M)234990 | 12/5/2016 | |
| Cortinarius semiamictus | Argentina, Río Negro, Paso de las Nubes, Frías Valley | Nothofagus dombeyi + N. antarctica | UDB023828 | IB 19620161 | Type | 7/4/1962 |
| Cortinarius sp. | Australia | Unknown | MG553066 | PERTH:06435416 FC393 | Unknown | |
| Cortinarius sp. | Chile, Osorno, PNP, foothills of Volcan Puyehue, up the road past El Caulle north of Rio Golgol | Nothofagus dombeyi | KY462487 | MES-1597 FLAS-F-64397 | Unknown | |
| Cortinarius sp. | Chile, Osorno, PNP, foothills of Volcan Puyehue, up the road past El Caulle north of Rio Golgol | Nothofagus dombeyi | KY462492 | MES-1602 FLAS-F-64401 | Unknown | |
| Cortinarius sp. | Chile, Osorno, PNP, foothills of Volcan Puyehue, up the road past El Caulle north of Rio Golgol | Nothofagus dombeyi | KY462567 | MES-1801 FLAS-F-64558 | Unknown | |
| Cortinarius sp. | Argentina, Nahuel Huapi National Park, Arroyo Goye, near Colonia Suiza | Unknown | KY462598 | MES-1859 CORDC00005597 | Unknown | |
| Cortinarius subrufus | Argentina, Río Negro, PNNH, Hess lake | Nothofagus antarctica | MF568560 | K(M)235093 | 17/5/2016 | |
| Cortinarius subrufus | Chile, Magallanes, Karukinka Reserve, Vicuña station | Nothofagus antarctica | MF568561 | K(M)235583 | 27/3/2017 | |
| Cortinarius subrufus | Chile, Magallanes, Karukinka Reserve, Vicuña station | Nothofagus antarctica | MF568562 | K(M)235584 | 26/3/2017 | |
| Cortinarius verniciorum | New Zealand, Fiordland, Te Anau Downs Motel | Leptospermum, possibly Nothofagus | JQ287679 | PDD 94010 | 25/4/2008 | |
| Cortinarius verniciorum | New Zealand, Fiordland, Te Anau Downs Motel | Leptospermum, possibly Nothofagus | NR157876 | PDD 94010 | Type | 25/4/2008 |
| Cortinarius viscilaetus | New Zealand, Totara, Milford Road | Nothofagus spp. | KT875206 | PDD 107734 | 18/5/2015 | |
| Cortinarius viscilaetus | New Zealand, Te Anau, Kepler Track | Nothofagus spp. | GU233353 | PDD 71010 | Type | 18/4/1997 |
| Cortinarius voluptatis | Argentina, Chubut, PNLA, Rivadavia Camping area | Nothofagus antarctica | MN707579 | CIEFAP157/IB 20170109 | 25/4/2017 | |
| Cortinarius voluptatis | Argentina, Neuquén, PNL, Yuco region | Lophozonia alpina + L. obliqua | MN707584, MW405260 | HCFC C218/IB 20170229 | 5/5/2017 | |
| Cortinarius voluptatis | Argentina, Neuquén, PNL, Yuco region | Lophozonia alpina + L. obliqua | MN707585, MW405258, MW546830 | HCFC C219/IB 20170231 | Holotype/Isotype | 5/5/2017 |
| Cortinarius voluptatis | Argentina, Neuquén, PNL, Yuco region | Lophozonia alpina + L. obliqua | MN707587, MW405259 | HCFC C230/IB 20170238 | 5/5/2017 | |
| Uncultured Cortinarius | Argentina, Neuquén, PNL, Yuco region | Lophozonia alpina | KJ701302 | Environmental | Unknown | |
| Uncultured fungus | Argentina | Nothofagus pumilio | JX316449/UDB008462 | Environmental | Unknown | |
| Uncultured fungus | Argentina | Lophozonia alpina | JX316363 | Environmental | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado Salomón, M.E.; Barroetaveña, C.; Niskanen, T.; Liimatainen, K.; Smith, M.E.; Peintner, U. Loose Ends in the Cortinarius Phylogeny: Five New Myxotelamonoid Species Indicate a High Diversity of These Ectomycorrhizal Fungi with South American Nothofagaceae. Life 2021, 11, 420. https://doi.org/10.3390/life11050420
Salgado Salomón ME, Barroetaveña C, Niskanen T, Liimatainen K, Smith ME, Peintner U. Loose Ends in the Cortinarius Phylogeny: Five New Myxotelamonoid Species Indicate a High Diversity of These Ectomycorrhizal Fungi with South American Nothofagaceae. Life. 2021; 11(5):420. https://doi.org/10.3390/life11050420
Chicago/Turabian StyleSalgado Salomón, María Eugenia, Carolina Barroetaveña, Tuula Niskanen, Kare Liimatainen, Matthew E. Smith, and Ursula Peintner. 2021. "Loose Ends in the Cortinarius Phylogeny: Five New Myxotelamonoid Species Indicate a High Diversity of These Ectomycorrhizal Fungi with South American Nothofagaceae" Life 11, no. 5: 420. https://doi.org/10.3390/life11050420
APA StyleSalgado Salomón, M. E., Barroetaveña, C., Niskanen, T., Liimatainen, K., Smith, M. E., & Peintner, U. (2021). Loose Ends in the Cortinarius Phylogeny: Five New Myxotelamonoid Species Indicate a High Diversity of These Ectomycorrhizal Fungi with South American Nothofagaceae. Life, 11(5), 420. https://doi.org/10.3390/life11050420






