Clinical Markers of Chronic Hypoxemia in Respiratory Patients Residing at Moderate Altitude
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Protein Determination
2.2. Ancestry Informative Markers (AIMs) Genotyping
2.3. Statistical Analysis
3. Results
3.1. Protein Measurements
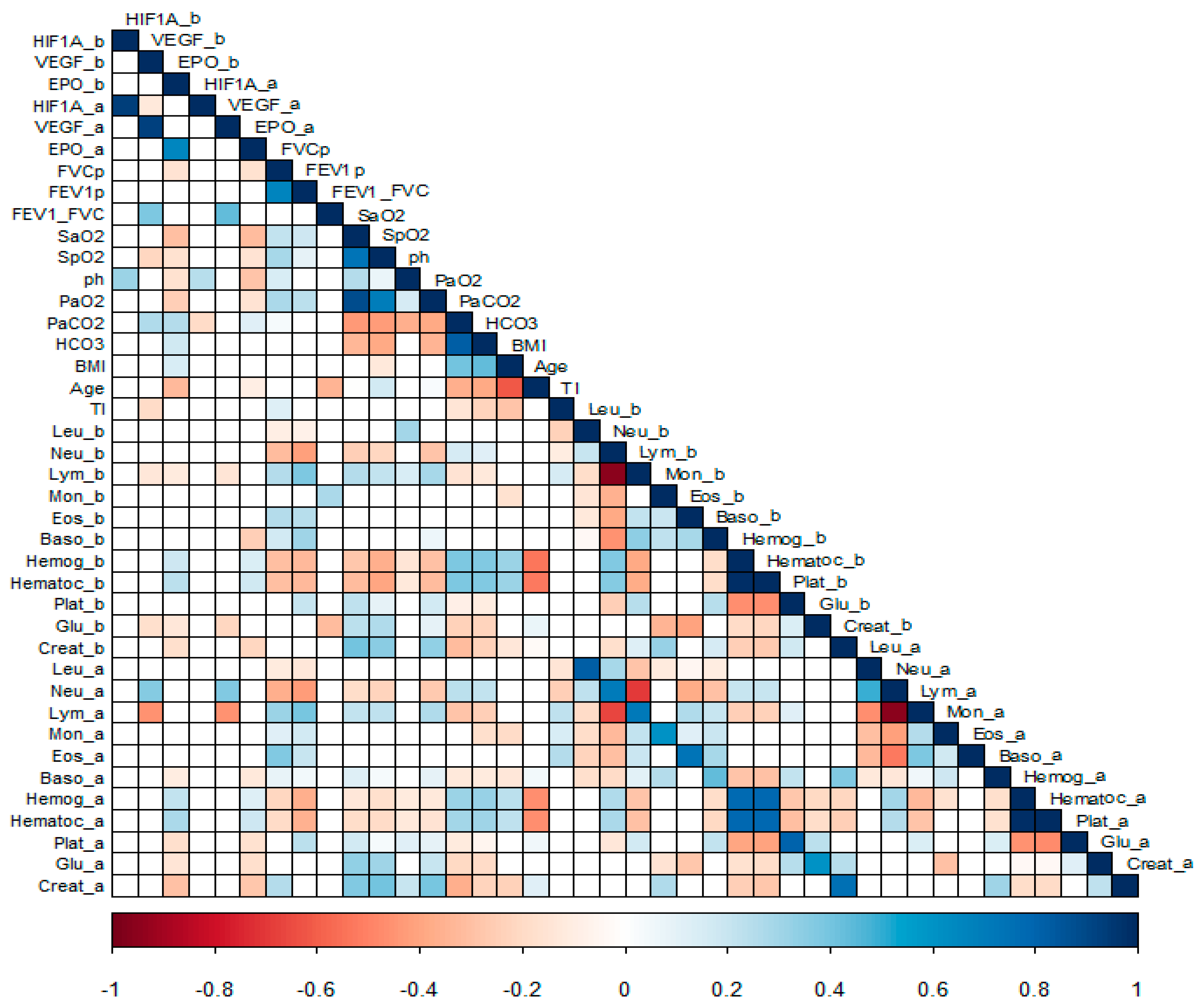
3.1.1. Correlation Analysis
3.1.2. Ancestral Contribution According to AIMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Ann. Intern. Med. 1980, 93, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Stuart Harris, C.; Bishop, J.; Clark, T.J.H.; Dornhorst, A.C.; Cotes, J.E.; Flenley, D.C.; Howard, P.; Oldham, P.D. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the medical research council working party. Lancet 1981, 1, 681–686. [Google Scholar]
- West, J.B. Physiological Effects of Chronic Hypoxia. N. Engl. J. Med. 2017, 376, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Saglam, M.; Vardar-Yagli, N.; Savci, S.; Inal-Ince, D.; Calik-Kutukcu, E.; Arikan, H.; Coplu, L. Functional capacity, physical activity, and quality of life in hypoxemic patients with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 423–428. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and Maladaptive Cardiorespiratory Responses to Continuous and Intermittent Hypoxia Mediated by Hypoxia-Inducible Factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001, 13, 167–171. [Google Scholar] [CrossRef]
- Turnbull, C.D.; Lee, L.Y.W.; Starkey, T.; Sen, D.; Stradling, J.; Petousi, N. Transcriptomics identify a unique intermittent hypox-ia-mediated profile in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2020, 201, 247–250. [Google Scholar] [CrossRef]
- Garcia, N.; Hopkins, S.R.; Powell, F.L. Effects of intermittent hypoxia on the isocapnic hypoxic ventilatory response and erythro-poiesis in humans. Respir. Physiol. 2000, 123, 39–49. [Google Scholar] [CrossRef]
- Zielinski, J. Effects of intermittent hypoxia on pulmonary hemodynamics: Animal models versus studies in humans. Eur. Respir. J. 2005, 25, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Perez-Padilla, R.; Torre-Bouscoulet, L.; Muiño, A.; Marquez, M.N.; Lopez, M.V.; De Oca, M.M.; Tálamo, C.; Menezes, A.M.B. Prevalence of oxygen desaturation and use of oxygen at home in adults at sea level and at moderate altitude. Eur. Respir. J. 2006, 27, 594–599. [Google Scholar] [CrossRef]
- American Thoracic Society. Standardization of spirometry, 1994 update. Am. J. Respir. Crit. Care Med. 1995, 152, 1107–1136. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 13.0; Stata Corporation: College Station, TX, USA, 2012. [Google Scholar]
- Rong, B.; Liu, Y.; Li, M.; Fu, T.; Gao, W.; Liu, H. Correlation of serum levels of HIF-1α and IL-19 with the disease progression of COPD: A retrospective study. Int. J. Chron. Obstruct Pulmon. Dis. 2018, 13, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Szmyd, B.; Michał, P.; Janusz, S.; Piotr, K.; Białasiewicz, P. Serum hypoxia-inducible factor-1α protein level as a diagnostic marker of obstructive sleep apnea. Pol. Arch. Intern. Med. 2020, 130, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Raguso, C.A.; Guinot, S.L.; Janssens, J.P.; Kayser, B.; Pichard, C. Chronic hypoxia: Common traits between chronic obstructive pulmonary disease and altitude. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, M.F.; Mackay, T.; Whyte, K.F.; Allen, M.; Tam, R.C.; Dore, C.J.; Henley, M.; Cotes, P.M.; Douglas, N.J. Nocturnal desaturation and serum erythropoietin: A study in patients with chronic obstructive pulmonary disease and in normal subjects. Clin. Sci. 1993, 84, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Sosnin, D.Y.; Khovaeva, Y.B.; Podyanova, A.I.; Syromyatnikova, T.N.; Nenasheva, O.Y. Eritropoetin as laboratory index of the degree of respiratory insufficiency in chronic obstructive pulmonary diseases. Klin. Lab. Diagn. 2018, 63, 691–695. [Google Scholar]
- Sharma, R.K.; Chakrabarti, S. Anaemia secondary to erythropoietin resistance: Important predictor of adverse outcomes in chronic obstructive pulmonary disease. Postgrad. Med. J. 2016, 92, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Pavlisa, G.; Vrbanic, V.; Kusec, V.; Jaksic, B. Erythropoietin response after correction of severe hypoxaemia due to acute respiratory failure in chronic obstructive pulmonary disease patients. Clin. Sci. (Lond.) 2004, 106, 43–51. [Google Scholar] [CrossRef]
- Winnicki, M.; Shamsuzzaman, A.; Lanfranchi, P.; Accurso, V.; Olson, E.; Davison, D.; Somers, V.K. Erythropoietin and obstructive sleep apnea. Am. J. Hypertens 2004, 17, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Hoepers, A.T.; Menezes, M.M.; Fröde, T.S. Systematic review of anaemia and inflammatory markers in chronic obstructive pulmonary disease. Clin. Exp. Pharmacol. Physiol. 2015, 42, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Pavlisa, G.; Kusec, V.; Kolonic, S.O.; Markovic, A.S.; Jaksic, B. Serum levels of VEGF and FGF in hypoxic patients with exacerbated COPD. Eur. Cytokine Netw. 2010, 21, 92–98. [Google Scholar] [PubMed]
| COPD | OSA | ILD | Controls | |
|---|---|---|---|---|
| Sex (M|F) | 12|8 | 27|12 | 1|1 | 44|26 |
| Age (years) | 68 (63–71) | 53 (42–57) | 64 (62–63) | 56 (45–69) |
| BMI (kg/m2) | 24.50 (22.23–27.25) | 39 (36.50–44.50) | 24 (23–28) | 26 (24–28.15) |
| Pulse Oximetry (%) | 89 (87–91) | 88 (87–90) | 94 (93-94) | 94 (93.25–95.90) |
| PaO2 (mmHg) | 54.95 (51.25–56.40) | 53.95 (49.67–56.77) | 47.90 (47.70–55.60) | NT |
| SpO2 (%) | 88 (86–88) | 87 (77–88) | 85 (77–87) | NT |
| SaO2 (%) | 87.47 (84.47–88.78) | 86.90 (84.79–88.35) | 83.90 (82.10–85.00) | NT |
| FEV1 (%) | 48 (46.50–64.50) | 81.5 (72.75–94.0) | 61 (39.5–83.5) | 108 (98–117) |
| FVC (%) | 79 (72.50–96.50) | 88 (76–94) | 54.50 (37.00–72.75) | 112.50 (97.25–119.75) |
| DLCO (%) | 82.50 (64.75–104.50) | 123 (103–139.8) | 45 (39–46) | 113.5 (105.8–127) |
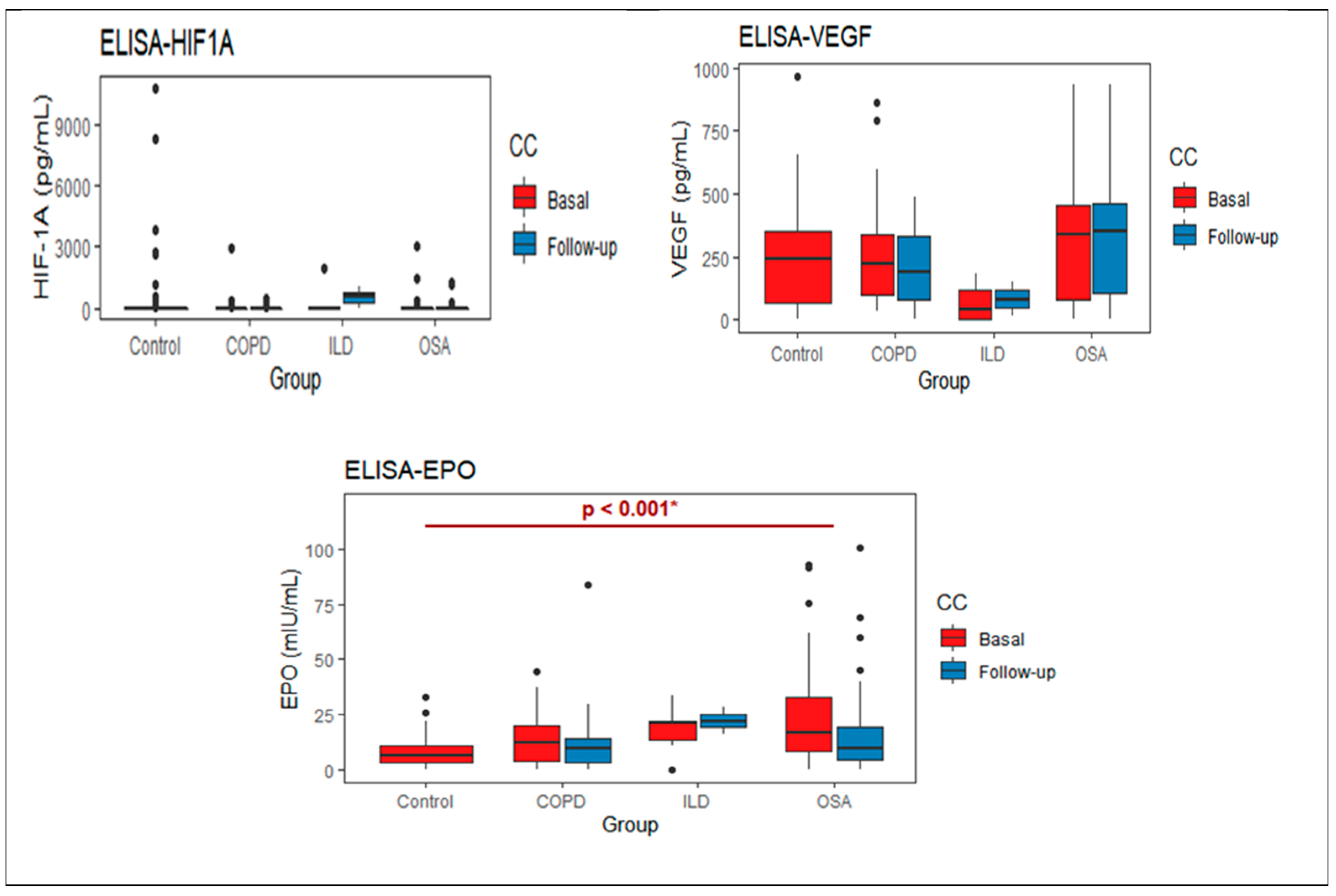
| Basal | Follow Up | p-Value | ||
|---|---|---|---|---|
| HIF1A | 0.270 |  | ||
| COPD | 146.5 (0–2917.1) | 39.62 (0–491.03) | 0.324 | |
| OSA | 125.0 (0–3071.8)) | 71.96 (0–1260.26) | 0.389 | |
| ILD | 384.87 (0–1917.95) | 526.3 (263.1–789.4) | 0.665 | |
| VEGF | 0.677 |  | ||
| COPD | 216.07 (78.83–328.81) | 192.47 (83.36–331.61) | 0.331 | |
| OSA | 342.0 (78–457.20) | 350.4 (104.1–464.1) | 0.678 | |
| ILD | 12.6 (1.1–137.46) | 83.36 (48.13–118.59) | 0.809 | |
| EPO | 0.033 |  | ||
| COPD | 12.39 (4.19–21.98) | 9.44 (2.93–14.19) | 0.023 | |
| OSA | 16.80 (7.96–32.56) | 9.20 (4.04–19.01) | 0.025 | |
| ILD | 20.72 (10.62–21.04) | 21.98 (18.98–24.98) | 0.809 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Plata, R.; Thirion-Romero, I.; Nava-Quiroz, K.J.; Pérez-Rubio, G.; Rodríguez-Llamazares, S.; Pérez-Kawabe, M.; Rodríguez-Reyes, Y.; Guerrero-Zuñiga, S.; Orea-Tejeda, A.; Falfán-Valencia, R.; et al. Clinical Markers of Chronic Hypoxemia in Respiratory Patients Residing at Moderate Altitude. Life 2021, 11, 428. https://doi.org/10.3390/life11050428
Fernández-Plata R, Thirion-Romero I, Nava-Quiroz KJ, Pérez-Rubio G, Rodríguez-Llamazares S, Pérez-Kawabe M, Rodríguez-Reyes Y, Guerrero-Zuñiga S, Orea-Tejeda A, Falfán-Valencia R, et al. Clinical Markers of Chronic Hypoxemia in Respiratory Patients Residing at Moderate Altitude. Life. 2021; 11(5):428. https://doi.org/10.3390/life11050428
Chicago/Turabian StyleFernández-Plata, Rosario, Ireri Thirion-Romero, Karol J. Nava-Quiroz, Gloria Pérez-Rubio, Sebastián Rodríguez-Llamazares, Midori Pérez-Kawabe, Yadira Rodríguez-Reyes, Selene Guerrero-Zuñiga, Arturo Orea-Tejeda, Ramcés Falfán-Valencia, and et al. 2021. "Clinical Markers of Chronic Hypoxemia in Respiratory Patients Residing at Moderate Altitude" Life 11, no. 5: 428. https://doi.org/10.3390/life11050428
APA StyleFernández-Plata, R., Thirion-Romero, I., Nava-Quiroz, K. J., Pérez-Rubio, G., Rodríguez-Llamazares, S., Pérez-Kawabe, M., Rodríguez-Reyes, Y., Guerrero-Zuñiga, S., Orea-Tejeda, A., Falfán-Valencia, R., Pérez-Padilla, R., & on behalf of the Mexican Translational Research Hypoxemia Working Group. (2021). Clinical Markers of Chronic Hypoxemia in Respiratory Patients Residing at Moderate Altitude. Life, 11(5), 428. https://doi.org/10.3390/life11050428









