Description of a CSF-Enriched miRNA Panel for the Study of Neurological Diseases
Abstract
:1. Introduction
2. Material and Methods
2.1. Biological Samples
2.2. Circulating RNA Extraction and Purification
2.3. Circulating miRNA Retrotranscription and Preamplification
2.4. Circulating miRNA Profiling
2.5. Databases for Cellular/Tissue-Enriched Source Analyses and Disease Associations
2.6. Search of Candidate Normalizer miRNAs for CSF Samples
3. Results
3.1. Profiling of CSF Samples in fc-OA Plates
3.2. Selection of 215 miRNAs to Be Included in cc-OA Plates
- Previously associated with MS in tissue, serum/plasma or CSF;
- Particularly brain-enriched;
- Detectable in CSF based on existing literature and/or our previous experience;
- Potential endogenous normalizer;
- Negative control.
3.3. miRNA Classification According to Their Detectability
3.4. miRNA Abundance in CSF Samples and Disease Associations
3.5. Cellular/Tissue-Enriched Source Analysis of Most Abundant miRNAs
3.6. Search for Suitable Endogenous Normalizers for CSF Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misra, M.K.; Damotte, V.; Hollenbach, J.A. The immunogenetics of neurological disease. Immunology 2018, 153, 399–414. [Google Scholar] [CrossRef]
- World Health Organization. Mental Health: Neurological Disorders. Available online: https://www.who.int/news-room/q-a-detail/mental-health-neurological-disorders (accessed on 28 April 2021).
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- DiLuca, M.; Olesen, J. The cost of brain diseases: A burden or a challenge? Neuron 2014, 82, 1205–1208. [Google Scholar] [CrossRef] [Green Version]
- LeFeber, J. In Vitro Models of Brain Disorders. In Advances in Neurobiology; Springer New York LLC: New York, NY, USA, 2019; Volume 22, pp. 19–49. [Google Scholar] [CrossRef]
- Vernau, W.; Vernau, K.A.; Sue Bailey, C. Cerebrospinal Fluid. In Clinical Biochemistry of Domestic Animals; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 769–819. [Google Scholar] [CrossRef]
- Hrishi, A.P.; Sethuraman, M. Cerebrospinal fluid (CSF) analysis and interpretation in neurocritical care for acute neurological conditions. Indian J. Crit. Care Med. 2019, 23 (Suppl. 2), S115–S119. [Google Scholar] [CrossRef]
- Jagot, F.; Davoust, N. Is it worth considering circulating microRNAs in multiple sclerosis? Front. Immunol. 2016, 7, 129. [Google Scholar] [CrossRef] [Green Version]
- Sempere, L.F. Celebrating 25 years of microRNA research: From discovery to clinical application. Int. J. Mol. Sci. 2019, 20, 1987. [Google Scholar] [CrossRef] [Green Version]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 1231–1243. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Ding, H.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, P. Circulating MicroRNAs: Biogenesis and Clinical Significance in Acute Myocardial Infarction. Front. Physiol. 2020, 11, 1088. [Google Scholar] [CrossRef]
- Sohel, M.H. Extracellular/Circulating MicroRNAs: Release Mechanisms, Functions and Challenges. Achiev. Life Sci. 2016, 10, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusardi, T.A.; Phillips, J.I.; Wiedrick, J.T.; Harrington, C.A.; Lind, B.; Lapidus, J.A.; Quinn, J.F.; Saugstad, J.A. MicroRNAs in human cerebrospinal fluid as biomarkers for Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 55, 1223–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, B.; Peplow, P. MicroRNAs in blood and cerebrospinal fluid as diagnostic biomarkers of multiple sclerosis and to monitor disease progression. Neural Regen. Res. 2020, 15, 606–619. [Google Scholar] [CrossRef]
- Reed, E.R.; Latourelle, J.C.; Bockholt, J.H.; Bregu, J.; Smock, J.; Paulsen, J.S.; Myers, R.H.; De Soriano, I.; Hobart, C.; Miller, A.; et al. MicroRNAs in CSF as prodromal biomarkers for Huntington disease in the PREDICT-HD study. Neurology 2018, 90, E264–E272. [Google Scholar] [CrossRef]
- Raoof, R.; Jimenez-Mateos, E.M.; Bauer, S.; Tackenberg, B.; Rosenow, F.; Lang, J.; Onugoren, M.D.; Hamer, H.; Huchtemann, T.; Körtvélyessy, P.; et al. Cerebrospinal fluid microRNAs are potential biomarkers of temporal lobe epilepsy and status epilepticus. Sci. Rep. 2017, 7, 3328. [Google Scholar] [CrossRef]
- Yang, Q.; Shi, Q.; Fu, J. Applications of cerebrospinal miRNA in the detection and treatment of acute CNS injury. Front. Lab. Med. 2018, 2, 83–88. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA Expression Across Human Tissues. Nucleic Acids Res. 2016, 44, 3865–3877. Available online: https://pubmed.ncbi.nlm.nih.gov/26921406/ (accessed on 3 July 2020). [CrossRef]
- Mycko, M.P.; Baranzini, S.E. microRNA and exosome profiling in multiple sclerosis. Mult. Scler. J. 2020, 26, 599–604. [Google Scholar] [CrossRef]
- Lizio, M.; Harshbarger, J.; Shimoji, H.; Severin, J.; Kasukawa, T.; Sahin, S.; Abugessaisa, I.; Fukuda, S.; Hori, F.; Ishikawa-Kato, S.; et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 2015, 16, 22. [Google Scholar] [CrossRef] [Green Version]
- Hoye, M.L.; Koval, E.D.; Wegener, A.J.; Hyman, T.S.; Yang, C.; O’Brien, D.R.; Miller, R.L.; Cole, T.; Schoch, K.M.; Shen, T.; et al. MicroRNA profiling reveals marker of motor neuron disease in ALS models. J. Neurosci. 2017, 37, 5574–5586. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, J.; Gao, Y.; Cui, C.; Zhang, S.; Li, J.; Zhou, Y.; Cui, Q. HMDD v3.0: A database for experimentally supported human microRNA-disease associations. Nucleic Acids Res. 2019, 47, D1013–D1017. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [Green Version]
- Marabita, F.; de Candia, P.; Torri, A.; Tegnér, J.; Abrignani, S.; Rossi, R.L. Normalization of circulating microRNA expression data obtained by quantitative real-time RT-PCR. Brief. Bioinform. 2016, 17, 204–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inada, K.; Okoshi, Y.; Cho-Isoda, Y.; Ishiguro, S.; Suzuki, H.; Oki, A.; Tamaki, Y.; Shimazui, T.; Saito, H.; Hori, M.; et al. Endogenous reference RNAs for microRNA quantitation in formalin-fixed, paraffin-embedded lymph node tissue. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Huff, T.; Tadi, P.; Varacallo, M. Neuroanatomy, Cerebrospinal Fluid.StatPearlsPublishing. 2019. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29262203 (accessed on 14 October 2020).
- Stoicea, N.; Du, A.; Lakis, C.D.; Tipton, C.; Arias-Morales, C.E.; Bergese, S.D. The miRNA journey from theory to practice as a CNS biomarker. Front. Genet. 2016, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, M.; Magdy, R. MicroRNAs in central nervous system disorders: Current advances in pathogenesis and treatment. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 36. [Google Scholar] [CrossRef]
- Duffy, C.P.; McCoy, C.E. The Role of MicroRNAs in Repair Processes in Multiple Sclerosis. Cells 2020, 9, 1711. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Mushtaq, G.; Greig, N. Current Update on Synopsis of miRNA Dysregulation in Neurological Disorders. CNS Neurol. Disord. Drug Targets. 2015, 14, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.X.; Fardo, D.W.; Jicha, G.A.; Nelson, P.T. A Customized Quantitative PCR MicroRNA Panel Provides a Technically Robust Context for Studying Neurodegenerative Disease Biomarkers and Indicates a High Correlation Between Cerebrospinal Fluid and Choroid Plexus MicroRNA Expression. Mol. Neurobiol. 2017, 54, 8191–8202. [Google Scholar] [CrossRef]
- Farr, R.J.; Januszewski, A.S.; Joglekar, M.V.; Liang, H.; McAulley, A.K.; Hewitt, A.W.; Thomas, H.E.; Loudovaris, T.; Kay, T.W.H.; Jenkins, A.; et al. A comparative analysis of high-throughput platforms for validation of a circulating microRNA signature in diabetic retinopathy. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Thermo Fisher Scientific. TaqMan Advanced MiRNA Assays-Superior Performance for MiRNA Detection and Quantification; Thermo Fisher Scientific: Waltham, MA, USA, 2016. [Google Scholar]
- Quintana, E.; Ortega, F.J.; Robles-Cedeño, R.; Villar, M.L.; Buxó, M.; Mercader, J.M.; Alvarez-Cermeño, J.C.; Pueyo, N.; Perkal, H.; Fernández-Real, J.M.; et al. miRNAs in cerebrospinal fluid identify patients with MS and specifically those with lipid-specific oligoclonalIgM bands. Mult. Scler. J. 2017, 23, 1716–1726. [Google Scholar] [CrossRef]
- Bergman, P.; Piket, E.; Khademi, M.; James, T.; Brundin, L.; Olsson, T.; Piehl, F.; Jagodic, M. Circulating miR-150 in CSF is a novel candidate biomarker for multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Min, W.; Li, M.; Han, G.; Dai, D.; Zhang, L.; Chen, X.; Wang, X.; Zhang, Y.; Yue, Z.; et al. Identification of hub genes and regulatory factors of glioblastomamultiforme subgroups by RNA-seq data analysis. Int. J. Mol. Med. 2016, 38, 1170–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Li, Y.; Wang, C.; Xu, F.; Wang, M.; Liu, Y. Serum miR-221 serves as a biomarker for Parkinson’s disease. Cell Biochem. Funct. 2016, 34, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Geekiyanage, H.; Jicha, G.A.; Nelson, P.T.; Chan, C. Blood serum miRNA: Non-invasive biomarkers for Alzheimer’s disease. Exp. Neurol. 2012, 235, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Zhu, Z.; Hao, J.; Wan, Z.; Guo, X. Decreased plasma miR-335 expression in patients with acute ischemic stroke and its association with calmodulin expression. J. Int. Med. Res. 2016, 44, 1331–1338. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Sang, W.; Wang, Y.; Zhang, C.; Zhang, D.; Sun, C.; Niu, M.; Zhang, Z.; Wei, X.; Pan, B.; Chen, W.; et al. MiR-150 impairs inflammatory cytokine production by targeting ARRB-after blocking CD28/B7 costimulatory pathway. Immunol. Lett. 2016, 172, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Krishnan Sundaram, V.; Kumar Sampathkumar, N.; Massaad, C.; Grenier, J. Optimal use of statistical methods to validate reference gene stability in longitudinal studies. PLoS ONE 2019. [Google Scholar] [CrossRef] [Green Version]
- Solayman, M.H.M.; Langaee, T.; Patel, A.; El-Wakeel, L.; El-Hamamsy, M.; Badary, O.; Johnson, J.A. Identification of Suitable Endogenous Normalizers for qRT-PCR Analysis of Plasma microRNA Expression in Essential Hypertension. Mol. Biotechnol. 2016, 58, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Li, Y.; Ye, F.; Wang, F.; Wan, X.; Lu, W.; Xie, X. Identification of miR-23a as a novel microRNA normalizer for relative quantification in human uterine cervical tissues. Exp. Mol. Med. 2011, 43, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Dai, J.; Tang, H.; Xu, T.; Yu, S.; Si, L.; Cui, C.; Sheng, X.; Chi, Z.; Mao, L.; et al. MicroRNA-23a-3p inhibits mucosal melanoma growth and progression through targeting adenylate cyclase 1 and attenuating cAMP and MAPK pathways. Theranostics 2019, 9, 945–960. [Google Scholar] [CrossRef]
- Baraniskin, A.; Kuhnhenn, J.; Schlegel, U.; Maghnouj, A.; Zöllner, H.; Schmiegel, W.; Hahn, S.; Schroers, R. Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro-Oncology 2012, 14, 29–33. [Google Scholar] [CrossRef]
- Sørensen, S.S.; Nygaard, A.-B.; Carlsen, A.L.; Heegaard, N.H.H.; Bak, M.; Christensen, T. Elevation of brain-enriched miRNAs in cerebrospinal fluid of patients with acute ischemic stroke. Biomark. Res. 2017, 5, 24. [Google Scholar] [CrossRef]
- De Ronde, M.W.J.; Ruijter, J.M.; Moerland, P.D.; Creemers, E.E.; Pinto-Sietsma, S.-J. Study Design and qPCR Data Analysis Guidelines for Reliable Circulating miRNA Biomarker Experiments: A Review. Clin. Chem. 2018, 64, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
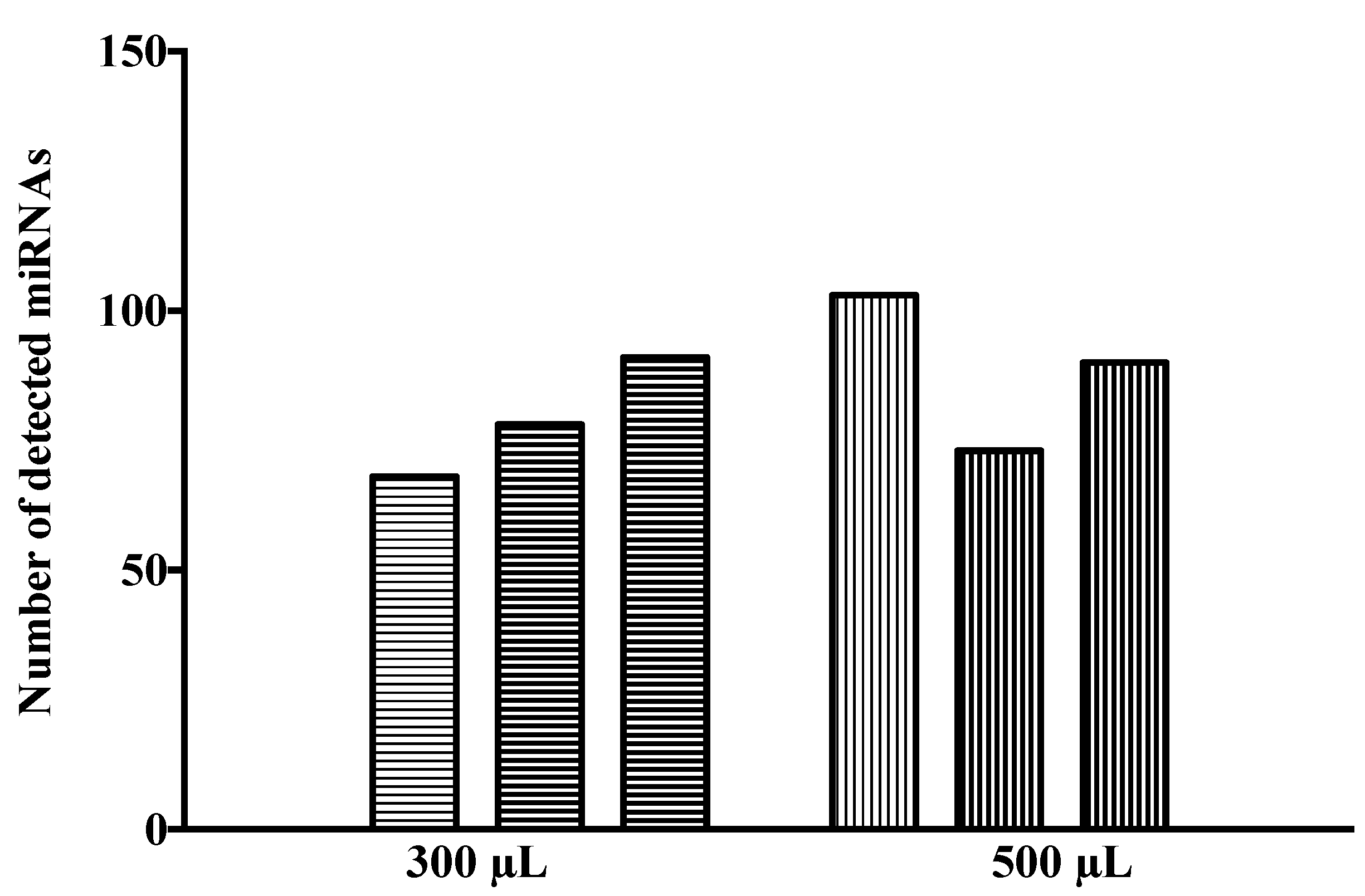
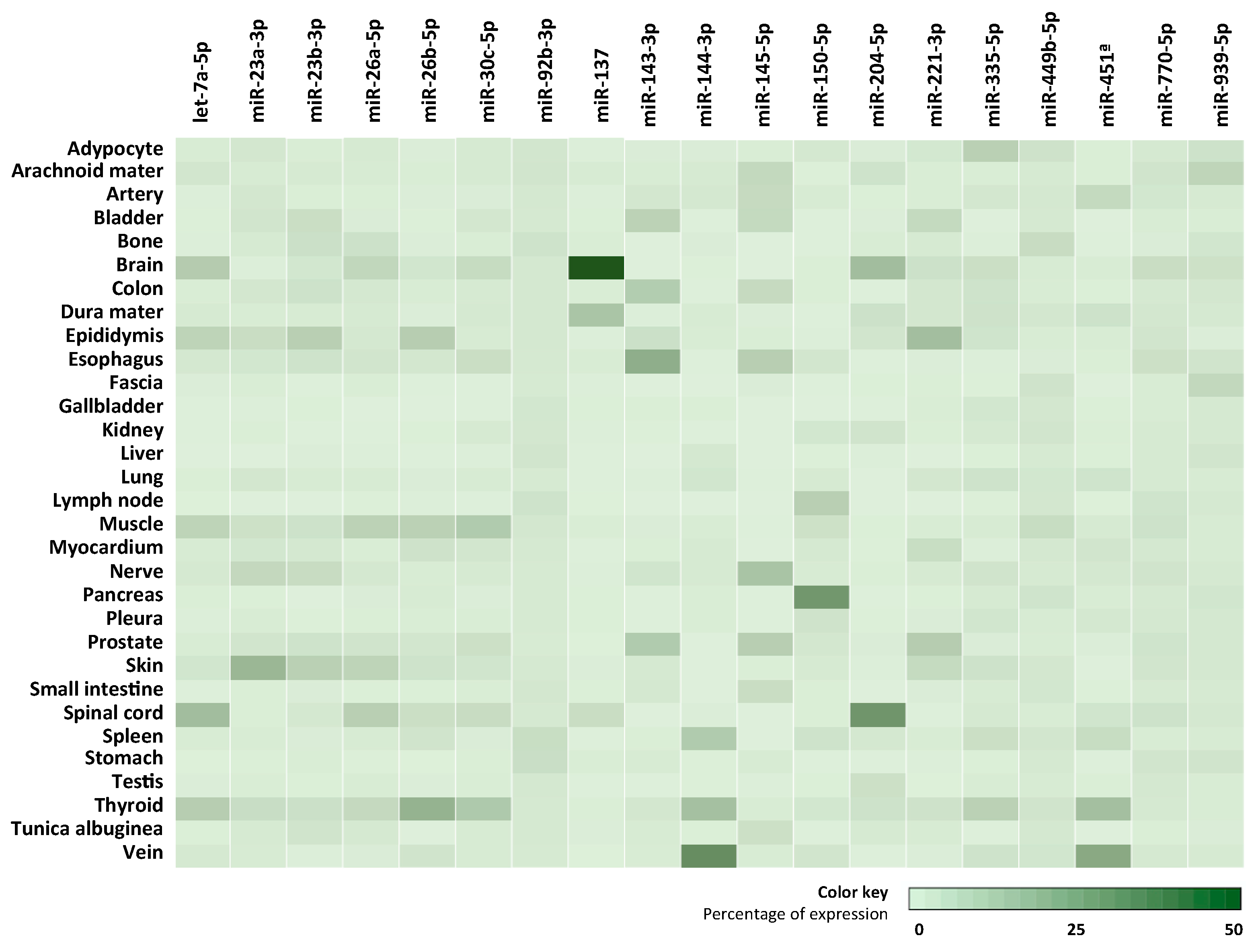
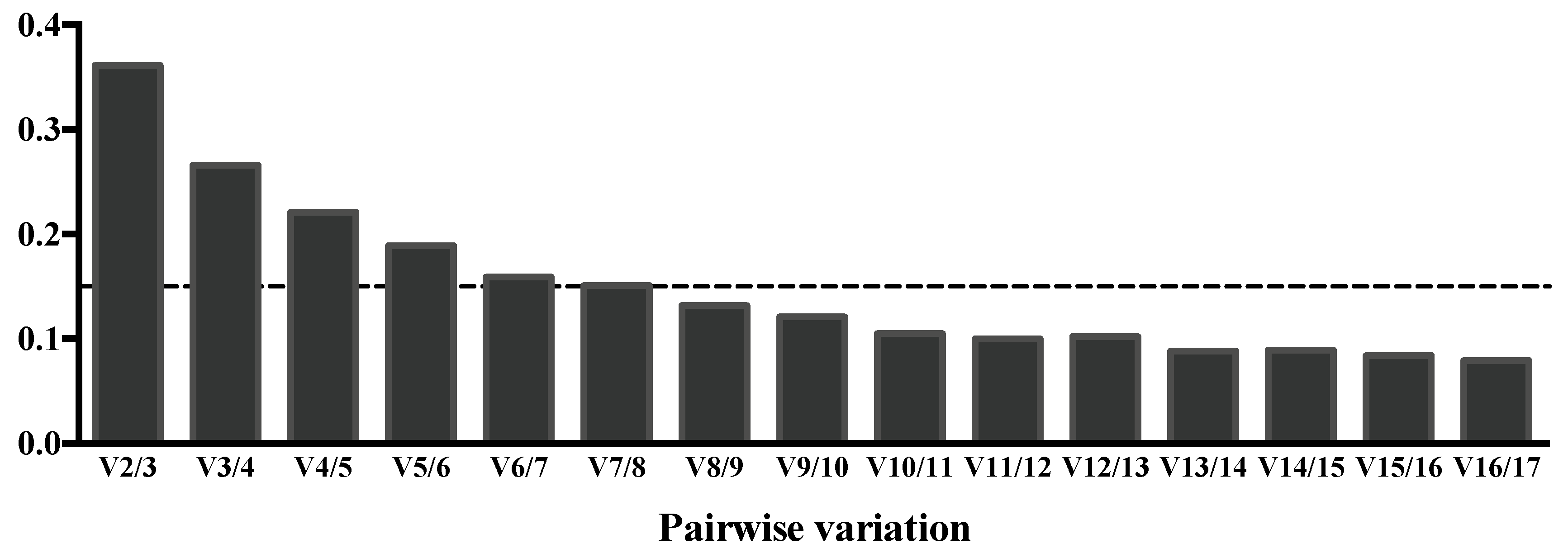
| Group | n | Age (Mean ± SD) | Sex (F/M) |
|---|---|---|---|
| SAS | 7 | 50.2 ± 5.8 | 5/2 |
| OND | 6 | 51.7 ± 5.5 | 4/2 |
| Vascular origin | 2 | 50.5 ± 0.7 | 2/0 |
| Migraines | 2 | 52 ± 5.7 | 2/0 |
| Dementia | 1 | 60 | 0/1 |
| Dizziness | 1 | 45 | 0/1 |
| PPMS | 11 | 52.6 ± 7.0 | 6/5 |
| RRMS | 40 | 32.1 ± 13.0 | 30/10 |
| Detection | Number of miRNAs (%) | miRNAs |
|---|---|---|
| 100% | 2 (0.9) | miR-143-3p; miR-23a-3p |
| 99–70% | 80 (37.2) | let-7a-5p; let-7b-5p; let-7c-5p; let-7f-5p; let-7g-5p; let-7i-5p; miR-100-3p; miR-100-5p; miR-101-3p; miR-10b-5p; miR-124-3p; miR-125a-5p; miR-125b-5p; miR-1260a; miR-1298-5p; miR-130a-3p; miR-137; miR-142-3p; miR-144-3p; miR-145-5p; miR-146a-5p; miR-148a-3p; miR-148b-3p; miR-150-5p; miR-151a-3p; miR-15a-5p; miR-181a-5p; miR-181c-5p; miR-185-5p; miR-186-5p; miR-195-5p; miR-199a-3p; miR-199a-5p; miR-19a-3p; miR-204-5p; miR-20a-5p; miR-21-5p; miR-219a-5p; miR-22-3p; miR-221-3p; miR-223-3p; miR-23b-3p; miR-24-3p; miR-25-3p; miR-26a-5p; miR-26b-5p; miR-27a-3p; miR-27b-3p; miR-29a-3p; miR-29c-5p; miR-30c-5p; miR-30d-5p; miR-320a; miR-320b; miR-335-5p; miR-338-3p; miR-342-3p; miR-34a-5p; miR-34c-5p; miR-361-5p; miR-374b-5p; miR-376a-3p; miR-378a-3p; miR-423-5p; miR-448; miR-449b-5p; miR-450b-3p; miR-451a; miR-452-3p; miR-497-5p; miR-645; miR-652-3p; miR-653-3p; miR-660-5p; miR-664a-3p; miR-770-5p; miR-885-5p; miR-9-5p; miR-92b-3p; miR-939-5p |
| 69–50% | 31 (14.4) | let-7b-3p; let-7e-5p; miR-1-3p; miR-103a-3p; miR-107; miR-128-3p; miR-133a-3p; miR-133b; miR-135a-5p; miR-151a-5p; miR-15b-5p; miR-17-5p; miR-1911-5p; miR-193a-5p; miR-196a-5p; miR-222-3p; miR-28-5p; miR-30c-2-3p; miR-34b-3p; miR-34b-5p; miR-34c-3p; miR-378a-5p; miR-424-5p; miR-501-3p; miR-516b-5p; miR-525-3p; miR-633; miR-9-3p; miR-93-5p; miR-99a-3p; miR-99b-5p |
| 49–30% | 31 (14.4) | let-7f-2-3p; miR-106b-3p; miR-106b-5p; miR-126-5p; miR-1264; miR-132-3p; miR-155-5p; miR-181b-5p; miR-190a-5p; miR-205-5p; miR-210-3p; miR-302b-3p; miR-302d-3p; miR-31-5p; miR-32-5p; miR-339-5p; miR-361-3p; miR-376c-3p; miR-411-5p; miR-412-3p; miR-425-5p; miR-483-3p; miR-484; miR-502-3p; miR-505-3p; miR-518f-3p; miR-524-3p; miR-576-3p; miR-583; miR-92a-3p; miR-937-3p |
| 30–1% | 70 (32.6) | miR-103a-2-5p; miR-10a-5p; miR-122-5p; miR-1247-5p; miR-1249-3p; miR-125a-3p; miR-127-3p; miR-129-2-3p; miR-1292-5p; miR-142-5p; miR-145-3p; miR-146b-5p; miR-153-3p; miR-181d-5p; miR-183-3p; miR-191-3p; miR-191-5p; miR-194-5p; miR-19b-3p; miR-200c-3p; miR-203a-3p; miR-206; miR-216a-5p; miR-218-5p; miR-27b-5p; miR-30a-3p; miR-30c-1-3p; miR-30e-3p; miR-323a-3p; miR-325; miR-326; miR-328-3p; miR-34a-3p; miR-363-3p; miR-369-3p; miR-369-5p; miR-373-3p; miR-375; miR-383-5p; miR-410-3p; miR-449a; miR-450b-5p; miR-452-5p; miR-454-3p; miR-455-3p; miR-483-5p; miR-486-5p; miR-487a-3p; miR-489-3p; miR-490-3p; miR-513a-5p; miR-515-3p; miR-518d-3p; miR-518e-3p; miR-520h; miR-523-3p; miR-532-3p; miR-532-5p; miR-548d-5p; miR-548e-3p; miR-548k; miR-548n; miR-551a; miR-570-3p; miR-593-5p; miR-615-3p; miR-628-3p; miR-642a-5p; miR-656-3p; miR-876-3p |
| 0% | 1 (0.5) | miR-211-5p |
| miRNA | Mean Cqvalue | Neurologicaldisease | PMID |
|---|---|---|---|
| miR-770-5p | 20.9 | GBM | 27572852 |
| miR-939-5p | 24.1 | Complex regional painsyndrome | 31489147 |
| miR-450b-3p | 24.1 | PD | 23938262 |
| miR-26b-5p | 24.4 | AD, hypoxia/ischemia, diffuseintrinsicpontine glioma, ALS | 23895045, 29937716, 0124166, 29543360, 30210287, 24027266 |
| miR-145-5p | 24.7 | Myastheniagravis, MS, stroke, seizure, GBM | 24043548, 23773985, 26096228, 27833019, 28284220, 23745809, 27752929, |
| miR-204-5p | 25.0 | Frontotemporaldementia, SPI, mesial temporal lobeepilepsy, GBM | 29434051, 29547407, 25410734, 30008822 |
| miR-30c-5p | 25.1 | ALS, MS | 30210287, 29551498 |
| miR-451a | 25.1 | Depression, ALS, GBM | 26343596, 30210287, 18765229 |
| miR-335-5p | 25.4 | Stroke, astrocytoma, neuroblastoma, majordepressiondisorder | 27856935, 21592405, 23806264, 26314506 |
| let-7a-5p | 25.8 | PD, GBM, ALS, MS | 30267378, 23600457, 26502847, 30210287, 25487315 |
| miR-23a-3p | 25.9 | MS, epilepsy, HD, SPI, GBM | 24277735, 26382856, 30359470, 27725128, 27907012, 20711171 |
| miR-221-3p | 26.0 | Stroke, PD, GBM, neuropathicpain | 23860376, 27748571, 28381184, 27059231, 18759060, 24055409 |
| miR-449b-5p | 26.0 | Stroke, PD | 30135469, 29935433 |
| miR-144-3p | 26.0 | Bipolar disorder, GBM, AD | 19849891, 26250785, 23546882 |
| miR-143-3p | 26.2 | AD, GBM | 26078483, 22490015, 23376635, 21211035 |
| miR-137 | 26.2 | AD, schizophrenia, GBM, HD | 22155483, 26899870, 29684772, 26187071, 21926974, 25044277, 18577219, 23965969, 21994399 |
| miR-150-5p | 26.4 | MS, stroke, HD | 28067602, 27144214, 27246008, 22048026 |
| miR-26a-5p | 26.5 | Migraine, PD, GBM | 26333279, 30267378, 20080666 |
| miR-92b-3p | 27.3 | Neuroblastoma, GBM | 21572098, 22829753 |
| miR-23b-3p | 27.4 | GBM | 22745829, 23152062 |
| Immune Cell Subset | miR-26a-5p | miR-26b-5p | miR-144-3p | miR-150-5p | miR-450b-3p |
|---|---|---|---|---|---|
| Circulating cell | 4.87 | 6.47 | 53.67 | 25.63 | 0.00 |
| Dendritic cell | 1.47 | 3.03 | 0.65 | 0.71 | 0.00 |
| Lymphocyte B lineage | 4.77 | 6.71 | 1.76 | 3.35 | 0.00 |
| Macrophage | 1.69 | 2.84 | 0.00 | 0.03 | 0.00 |
| Mastcell | 3.41 | 7.97 | 0.60 | 0.08 | 0.00 |
| Monocyte | 3.58 | 5.75 | 0.90 | 5.08 | 0.00 |
| Natural Killer cell | 3.65 | 7.26 | 0.24 | 17.09 | 0.00 |
| Neutrophil | 5.91 | 8.69 | 23.93 | 0.28 | 27.95 |
| T cell | 4.25 | 4.85 | 0.23 | 46.94 | 0.00 |
| miRNA | geNorm | NormFinder | CV Score | SSS Score |
|---|---|---|---|---|
| miR-101-3p | 1.63 (12) | 0.83 (12) | 0.98 (17) | 2.07 (14) |
| miR-125a-5p | 1.46 (4) | 0.63 (3) | 0.59 (3) | 1.69 (3) |
| miR-143-3p | 1.59 (8) | 0.79 (8) | 0.73 (9) | 1.92 (9) |
| miR-151a-3p | 1.71 (15) | 0.90 (15) | 0.85 (12) | 2.11 (15) |
| miR-15a-5p | 1.71 (16) | 0.91 (16) | 0.93 (16) | 2.15 (17) |
| miR-181a-5p | 1.67 (16) | 0.87 (14) | 0.80 (11) | 2.05 (13) |
| miR-186-5p | 1.72 (17) | 0.92 (17) | 0.88 (13) | 2.14 (16) |
| miR-21-5p | 1.66 (6) | 0.74 (6) | 0.62 (5) | 1.82 (6) |
| miR-221-3p | 1.61 (11) | 0.81 (11) | 0.61 (4) | 1.90 (8) |
| miR-23a-3p | 1.43 (1) | 0.61 (1) | 0.65 (6) | 1.68 (2) |
| miR-26b-5p | 1.46 (3) | 0.64 (4) | 0.46 (1) | 1.66 (1) |
| miR-27a-3p | 1.58 (7) | 0.77 (7) | 0.71 (8) | 1.89 (7) |
| miR-335-5p | 1.52 (5) | 0.72 (5) | 0.56 (2) | 1.77 (5) |
| miR-652-3p | 1.60 (10) | 0.79 (9) | 0.91 (15) | 2.00 (12) |
| miR-653-3p | 1.60 (9) | 0.80 (10) | 0.89 (14) | 1.99 (11) |
| miR-9-5p | 1.64 (13) | 0.83 (13) | 0.76 (10) | 1.99 (10) |
| miR-92b-3p | 1.44 (2) | 0.62 (2) | 0.66 (7) | 1.70 (4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-San Martín, M.; Gomez, I.; Miguela, A.; Belchí, O.; Robles-Cedeño, R.; Quintana, E.; Ramió-Torrentà, L. Description of a CSF-Enriched miRNA Panel for the Study of Neurological Diseases. Life 2021, 11, 594. https://doi.org/10.3390/life11070594
Muñoz-San Martín M, Gomez I, Miguela A, Belchí O, Robles-Cedeño R, Quintana E, Ramió-Torrentà L. Description of a CSF-Enriched miRNA Panel for the Study of Neurological Diseases. Life. 2021; 11(7):594. https://doi.org/10.3390/life11070594
Chicago/Turabian StyleMuñoz-San Martín, María, Imma Gomez, Albert Miguela, Olga Belchí, René Robles-Cedeño, Ester Quintana, and Lluís Ramió-Torrentà. 2021. "Description of a CSF-Enriched miRNA Panel for the Study of Neurological Diseases" Life 11, no. 7: 594. https://doi.org/10.3390/life11070594
APA StyleMuñoz-San Martín, M., Gomez, I., Miguela, A., Belchí, O., Robles-Cedeño, R., Quintana, E., & Ramió-Torrentà, L. (2021). Description of a CSF-Enriched miRNA Panel for the Study of Neurological Diseases. Life, 11(7), 594. https://doi.org/10.3390/life11070594






