Alantolactone Suppresses Proliferation and the Inflammatory Response in Human HaCaT Keratinocytes and Ameliorates Imiquimod-Induced Skin Lesions in a Psoriasis-Like Mouse Model
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Viability Assay
2.2. RNA Extraction and Quantitative Real-Time PCR Analysis
2.3. Western Blotting
2.4. Preparation of Nuclear Extracts and Measurement of NF-κB Activity
2.5. Animals
2.6. Preparation of Cream
2.7. IMQ-Induced Psoriasis-Like Mouse Model and Topical Alantolactone Treatment
2.8. Histopathology and Immunohistochemistry
2.9. Statistical Analysis
3. Result
3.1. Alantolactone Inhibited Proliferation and Inflammatory Responses in M5 Cytokine-Stimulated Keratinocytes
3.2. Alantolactone Inhibited STAT3 Phosphorylation and NF-κB Activation in HaCaT Keratinocytes
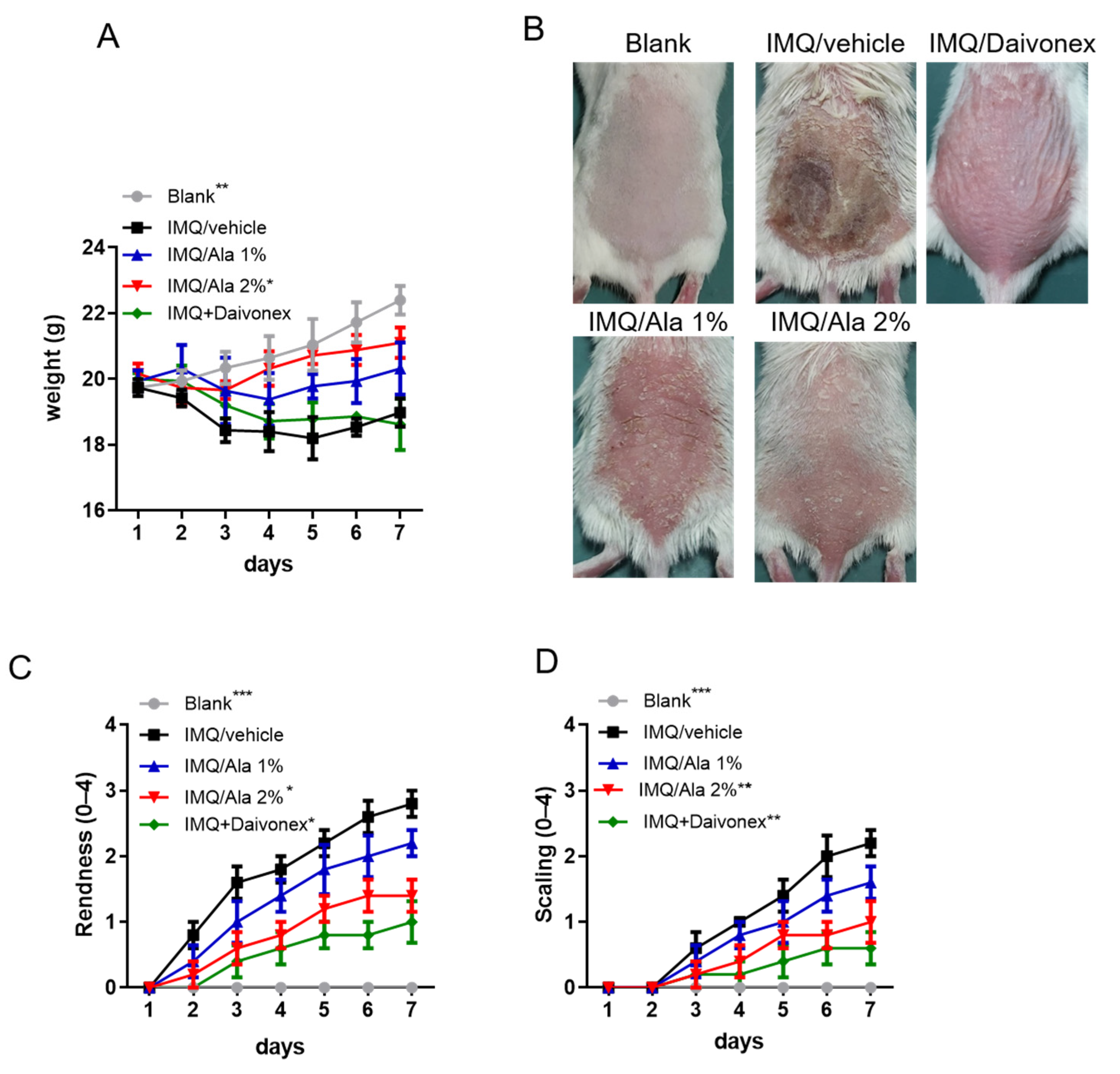
3.3. Alantolactone Decreased the Severity of IMQ-Induced Psoriasiform Dermatitis
3.4. Alantolactone Decreased Inflammatory Cytokines in the Skin Lesions of IMQ-Treated Mice
3.5. Alantolactone Suppressed STAT3 Phosphorylation and NF-κB Activation in the Skin Lesions of IMQ-Treated Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Pasquali, L.; Srivastava, A.; Meisgen, F.; Das Mahapatra, K.; Xia, P.; Xu Landen, N.; Pivarcsi, A.; Sonkoly, E. The keratinocyte transcriptome in psoriasis: Pathways related to immune responses, cell cycle and keratinization. Acta Derm.-Venereol. 2019, 99, 196–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanesi, C.; Scarponi, C.; Giustizieri, M.L.; Girolomoni, G. Keratinocytes in inflammatory skin diseases. Curr. Drug Targets Inflamm. Allergy 2005, 4, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; De Pita, O.; Girolomoni, G. Resident skin cells in psoriasis: A special look at the pathogenetic functions of keratinocytes. Clin. Dermatol. 2007, 25, 581–588. [Google Scholar] [CrossRef]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Editorial Board of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; China Chemical Industry Press: Beijing, China, 2015. [Google Scholar]
- Tripathi, Y.B.; Tripathi, P.; Upadhyay, B.N. Assessment of the adrenergic beta-blocking activity of Inula racemosa. J. Ethnopharmacol. 1988, 23, 3–9. [Google Scholar] [CrossRef]
- Tan, R.X.; Tang, H.Q.; Hu, J.; Shuai, B. Lignans and sesquiterpene lactones from Artemisia sieversiana and Inula racemosa. Phytochemistry 1998, 49, 157–161. [Google Scholar] [CrossRef]
- Xin, X.-L.; Ma, X.-C.; Liu, K.-X.; Han, J.; Wang, B.-R.; Guo, D.-A. Microbial transformation of alantolactone by Mucor polymorphosporus. J. Asian Nat. Prod. Res. 2008, 10, 933–937. [Google Scholar] [CrossRef]
- Stojanovic-Radic, Z.; Comic, L.; Radulovic, N.; Blagojevic, P.; Denic, M.; Miltojevic, A.; Rajkovic, J.; Mihajilov-Krstev, T. Antistaphylococcal activity of Inula helenium L. root essential oil: Eudesmane sesquiterpene lactones induce cell membrane damage. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Brun, R.; Willuhn, G.; Khalid, S.A. Anti-trypanosomal activity of helenalin and some structurally related sesquiterpene lactones. Planta Med. 2002, 68, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Choi, R.J.; Khan, S.; Lee, D.S.; Kim, Y.-C.; Nam, Y.J.; Lee, D.-U.; Kim, Y.S. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, Mapk and Ap-1 via the Myd88 signaling pathway in LPS-activated RAW 264.7 cells. Int. Immunopharmacol. 2012, 14, 375–383. [Google Scholar] [CrossRef]
- Konishi, T.; Shimada, Y.; Nagao, T.; Okabe, H.; Konoshima, T. Antiproliferative sesquiterpene lactones from the roots of Inula helenium. Biol. Pharm. Bull. 2002, 25, 1370–1372. [Google Scholar] [CrossRef] [Green Version]
- Pal, H.C.; Sehar, I.; Bhushan, S.; Gupta, B.D.; Saxena, A.K. Activation of caspases and poly (ADP-ribose) polymerase cleavage to induce apoptosis in leukemia HL-60 cells by Inula racemosa. Toxicol. In Vitro 2010, 24, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, Y.-L.; Xing, J.-S.; Lan, X.-Q.; Wang, L.-T.; Zhang, B. Alantolactone plays neuroprotective roles in traumatic brain injury in rats via anti-inflammatory, anti-oxidative and anti-apoptosis pathways. Am. J. Transl. Res. 2018, 10, 368–380. [Google Scholar] [PubMed]
- Dang, X.; He, B.; Ning, Q.; Liu, Y.; Guo, J.; Niu, G.; Chen, M. Alantolactone suppresses inflammation, apoptosis and oxidative stress in cigarette smoke-induced human bronchial epithelial cells through activation of Nrf2/HO-1 and inhibition of the NF-κB pathways. Respir. Res. 2020, 21, 95. [Google Scholar] [CrossRef]
- Lee, B.-K.; Park, S.-J.; Nam, S.-Y.; Kang, S.; Hwang, J.; Lee, S.-J.; Im, D.S. Anti-allergic effects of sesquiterpene lactones from Saussurea costus (Falc.) Lipsch. determined using in vivo and in vitro experiments. J. Ethnopharmacol. 2018, 213, 256–261. [Google Scholar] [CrossRef]
- Guilloteau, K.; Paris, I.; Pedretti, N.; Boniface, K.; Juchaux, F.; Huguier, V.; Guillet, G.; Bernard, F.-X.; Lecron, J.-C.; Morel, F. Skin inflammation induced by the synergistic action of IL-17a, IL-22, Oncostatin M, IL-1α, and TNF-α recapitulates some features of psoriasis. J. Immunol. 2010, 184, 5263–5270. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, Y.; Xu, M.; Li, J.; Teng, X.; Cheng, H.; Xia, Y. Zinc finger protein A20 is involved in the antipsoriatic effect of calcipotriol. Br. J. Dermatol. 2016, 175, 314–324. [Google Scholar] [CrossRef]
- Chen, C.; Wu, N.; Duan, Q.; Yang, H.; Wang, X.; Yang, P.; Zhang, M.; Liu, J.; Liu, Z.; Shao, Y.; et al. C10orf99 contributes to the development of psoriasis by promoting the proliferation of keratinocytes. Sci. Rep. 2018, 8, 8590. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Xiao, L.; Jia, J.; Li, F.; Wang, X.; Duan, Q.; Jing, H.; Yang, P.; Chen, C.; Wang, Q.; et al. Cornulin is induced in psoriasis lesions and promotes keratinocyte proliferation via phosphoinositide 3-kinase/Akt pathways. J. Investig. Dermatol. 2019, 139, 71–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Li, Y.R.; Li, S.; Ho, C.-T.; Chang, Y.-H.; Tan, K.-T.; Chung, T.-W.; Wang, B.-Y.; Chen, Y.-K.; Lin, C.-C. Tangeretin derivative, 5-acetyloxy-6,7,8,4′-tetramethoxyflavone induces G2/M arrest, apoptosis and autophagy in human non-small cell lung cancer cells in vitro and in vivo. Cancer Biol. Ther. 2016, 7, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xu, J.; Xiong, X.; Deng, Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019, 24, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Brash, D.E.; Grossman, D. Keratinocyte apoptosis in epidermal development and disease. J. Investig. Dermatol. 2006, 126, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Kastelan, M.; Prpić-Massari, L.; Brajac, I. Apoptosis in psoriasis. Acta Dermatovenerol. Croat. 2009, 17, 182–186. [Google Scholar]
- Gottlieb, A.B.; Chamian, F.; Masud, S.; Cardinale, I.; Abello, M.V.; Lowes, M.A.; Chen, F.; Magliocco, M.; Krueger, J.G. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J. Immunol. 2005, 175, 2721–2729. [Google Scholar] [CrossRef] [Green Version]
- Johansen, C.; Funding, A.T.; Otkjaer, K.; Kragballe, K.; Jensen, U.B.; Madsen, M.; Binderup, L.; Skak-Nielsen, T.; Fjording, M.S.; Iversen, L. Protein expression of TNF-alpha in psoriatic skin is regulated at a posttranscriptional level by MAPK-activated protein kinase. J. Immunol. 2006, 176, 1431–1438. [Google Scholar] [CrossRef] [Green Version]
- Gillitzer, R.; Berger, R.; Mielke, V.; Muller, C.; Wolff, K.; Stingl, G. Upper keratinocytes of psoriatic skin lesions express high levels of NAP-1/IL-8 mRNA in situ. J. Investig. Dermatol. 1991, 97, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillitzer, R.; Wolff, K.; Tong, D.; Muller, C.; Yoshimura, T.; Hartmann, A.A.; Stingl, G.; Berger, R. MCP-1 mRNA expression in basal keratinocytes of psoriatic lesions. J. Investig. Dermatol. 1993, 101, 127–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qazi, B.S.; Tang, K.; Qazi, A. Recent advances in underlying pathologies provide insight into interleukin-8 expression-mediated inflammation and angiogenesis. Int. J. Inflamm. 2011, 2011, 908468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lembo, S.; Capasso, R.; Balato, A.; Cirillo, T.; Flora, F.; Zappia, V.; Balato, N.; Ingrosso, D.; Ayala, F. MCP-1 in psoriatic patients: Effect of biological therapy. J. Dermatol. Treat. 2014, 25, 83–86. [Google Scholar] [CrossRef]
- Sano, S.; Chan, K.S.; Carbajal, S.; Clifford, J.; Peavey, M.; Kiguchi, K.; Itami, S.; Nickoloff, B.J.; Digiovanni. J. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med. 2005, 11, 43–49. [Google Scholar] [CrossRef]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. NF-κB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef]
- Jiang, T.; Cheng, H.; Su, J.; Wang, X.; Wang, Q.; Chu, J.; Li, Q. Gastrodin protects against glutamate-induced ferroptosis in HT-22 cells through Nrf2/HO-1 signaling pathway. Toxicol. In Vitro 2020, 62, 104715. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-L.; Liang, S.; Li, J.; Napierata, L.; Brown, T.; Benoit, S.; Senices, M.; Gill, D.; Dunussi-Joannopoulos, K.; Collins, M.; et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Investig. 2008, 118, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Lynde, C.W.; Poulin, Y.; Vender, R.; Bourcier, M.; Khalil, S. Interleukin 17A: Toward a new understanding of psoriasis pathogenesis. J. Am. Acad. Dermatol. 2014, 71, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Piskin, G.; Sylva-Steenland, R.M.R.; Bos, J.D.; Teunissen, M.B.M. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: Enhanced expression in psoriatic skin. J. Immunol. 2006, 176, 1908–1915. [Google Scholar] [CrossRef]
- Kim, C.-H.; Kim, J.-Y.; Lee, A.-Y. Therapeutic and immunomodulatory effects of glucosamine in combination with low-dose cyclosporine a in a murine model of imiquimod-induced psoriasis. Eur. J. Pharmacol. 2015, 756, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Marson, J.W.; Snyder, M.L.; Lebwohl, M.G. Newer Therapies in Psoriasis. Med. Clin. N. Am. 2021, 105, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Leone, S.; Maraolo, A.E.; Berti, E.; Damiani, G. Liver Illness and Psoriatic Patients. BioMed Res Int. 2018, 2018, 3140983. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuo, W.-H.; Tung, Y.-T.; Wu, C.-L.; Bracci, N.R.; Chang, Y.-K.; Huang, H.-Y.; Lin, C.-C. Alantolactone Suppresses Proliferation and the Inflammatory Response in Human HaCaT Keratinocytes and Ameliorates Imiquimod-Induced Skin Lesions in a Psoriasis-Like Mouse Model. Life 2021, 11, 616. https://doi.org/10.3390/life11070616
Chuo W-H, Tung Y-T, Wu C-L, Bracci NR, Chang Y-K, Huang H-Y, Lin C-C. Alantolactone Suppresses Proliferation and the Inflammatory Response in Human HaCaT Keratinocytes and Ameliorates Imiquimod-Induced Skin Lesions in a Psoriasis-Like Mouse Model. Life. 2021; 11(7):616. https://doi.org/10.3390/life11070616
Chicago/Turabian StyleChuo, Wen-Ho, Yu-Tang Tung, Chao-Liang Wu, Nicole R. Bracci, Yu-Kang Chang, Hung-Yi Huang, and Chi-Chien Lin. 2021. "Alantolactone Suppresses Proliferation and the Inflammatory Response in Human HaCaT Keratinocytes and Ameliorates Imiquimod-Induced Skin Lesions in a Psoriasis-Like Mouse Model" Life 11, no. 7: 616. https://doi.org/10.3390/life11070616





