Inflammation in Metabolic and Cardiovascular Disorders—Role of Oxidative Stress
Abstract
:1. Introduction
2. Innate Immune Activation Contributes to Metabolic Disorders
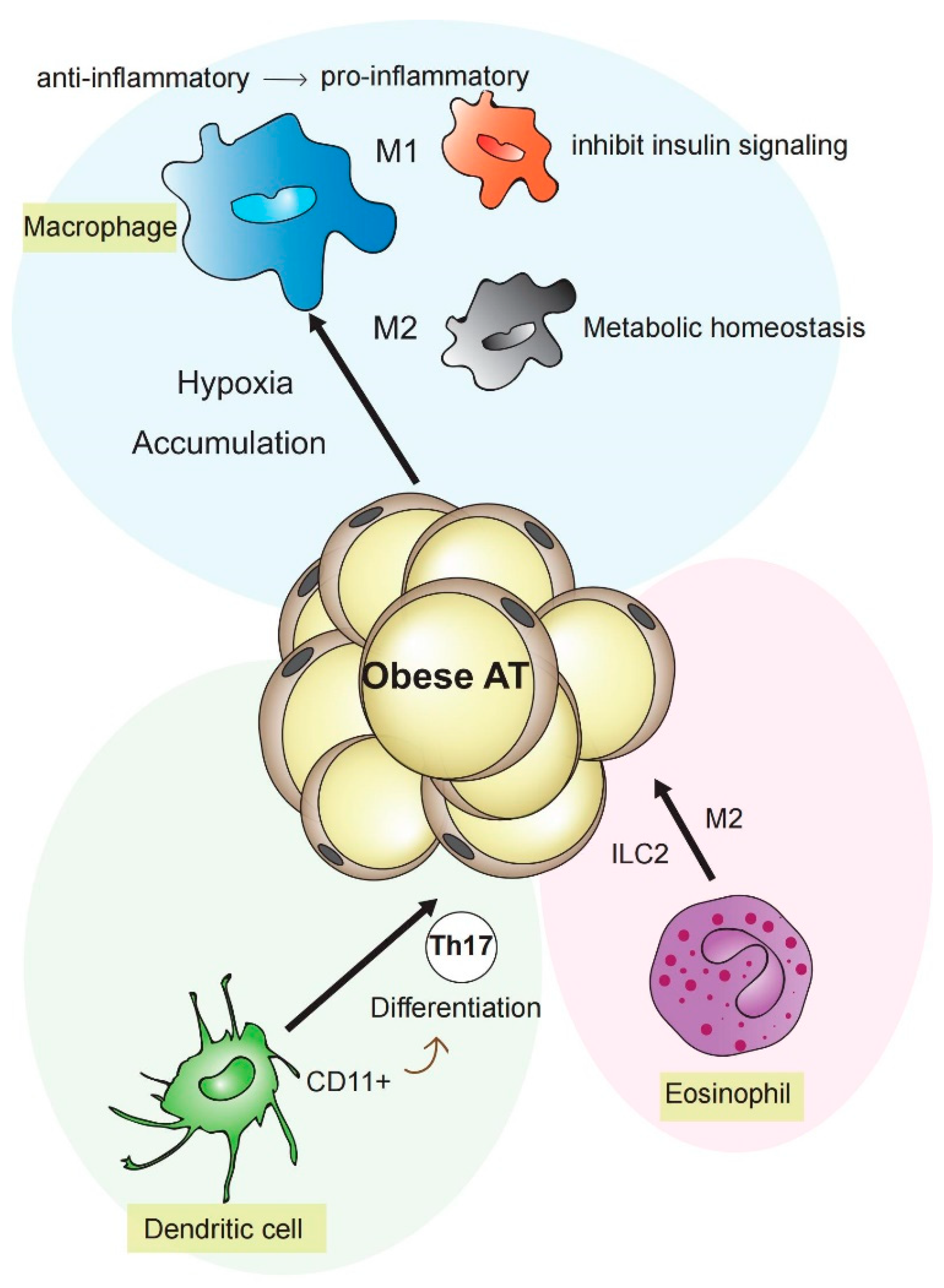
2.1. Macrophages, Dendritic Cells, and Further Immune Cells Contributing to Inflammation in Metabolic Disorders
2.2. Different Types of Adipose Tissue
2.3. Platelets, Diabetes, and Its Sequel
2.4. Platelets and the Fat Tissue
3. Oxidative Stress as a Mediator of Tissue Damage and Promotor of Inflammation
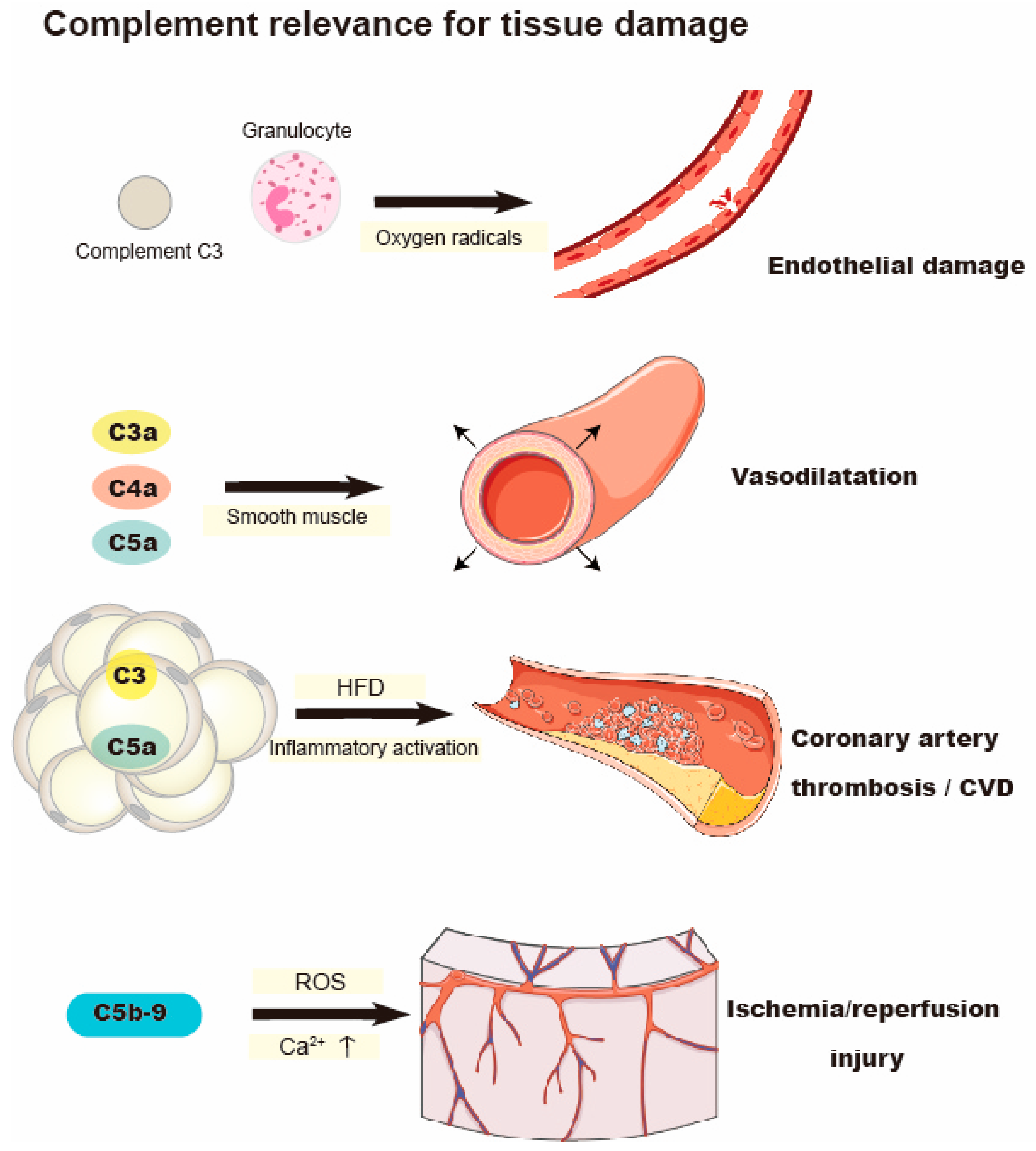
4. Complement Activation and Oxidative Stress
5. Oxidative Stress Causes Hypothalamic Dysfunction in Metabolic Disease
6. Translational Implications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, L.A.J.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative Activation of Macrophages: Mechanism and Functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Fujisaka, S.; Usui, I.; Bukhari, A.; Ikutani, M.; Oya, T.; Kanatani, Y.; Tsuneyama, K.; Nagai, Y.; Takatsu, K.; Urakaze, M.; et al. Regulatory Mechanisms for Adipose Tissue M1 and M2 Macrophages in Diet-Induced Obese Mice. Diabetes 2009, 58, 2574–2582. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Choi, Y.J.; Joung, S.M.; Lee, B.H.; Jung, Y.-S.; Lee, J.Y. Hypoxic stress up-regulates the expression of Toll-like receptor 4 in macrophages via hypoxia-inducible factor. Immunology 2010, 129, 516–524. [Google Scholar] [CrossRef]
- Lumeng, C.N.; DelProposto, J.B.; Westcott, D.J.; Saltiel, A.R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008, 57, 3239–3246. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Liu, Y.-J. Development of Dendritic-Cell Lineages. Immunity 2007, 26, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Krawczyk, C.M.; Holowka, T.; Sun, J.; Blagih, J.; Amiel, E.; DeBerardinis, R.J.; Cross, J.R.; Jung, E.; Thompson, C.B.; Jones, R.G.; et al. Toll-like Receptor–Induced Changes in Glycolytic Metabolism Regulate Dendritic Cell Activation. Blood 2010, 115, 4742–4749. [Google Scholar] [CrossRef] [Green Version]
- Wculek, S.K.; Khouili, S.C.; Priego, E.; Heras-Murillo, I.; Sancho, D. Metabolic Control of Dendritic Cell Functions: Digesting Information. Front. Immunol. 2019, 10, 775. [Google Scholar] [CrossRef] [Green Version]
- Pearce, E.J.; Everts, B. Dendritic cell metabolism. Nat. Rev. Immunol. 2015, 15, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.G.; O’Neill, L.A.J. Krebs cycle rewired for macrophage and dendritic cell effector functions. FEBS Lett. 2017, 591, 2992–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thwe, P.M.; Pelgrom, L.R.; Cooper, R.; Beauchamp, S.; Reisz, J.A.; D’Alessandro, A.; Everts, B.; Amiel, E. Cell-Intrinsic Glycogen Metabolism Supports Early Glycolytic Reprogramming Required for Dendritic Cell Immune Responses. Cell Metab. 2017, 26, 558–567.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanovic-Racic, M.; Yang, X.; Turner, M.S.; Mantell, B.S.; Stolz, D.B.; Sumpter, T.L.; Sipula, I.J.; Dedousis, N.; Scott, D.K.; Morel, P.A.; et al. Dendritic Cells Promote Macrophage Infiltration and Comprise a Substantial Proportion of Obesity-Associated Increases in CD11c+ Cells in Adipose Tissue and Liver. Diabetes 2012, 61, 2330–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertola, A.; Ciucci, T.; Rousseau, D.; Bourlier, V.; Duffaut, C.; Bonnafous, S.; Blin-Wakkach, C.; Anty, R.; Iannelli, A.; Gugenheim, J.; et al. Identification of Adipose Tissue Dendritic Cells Correlated With Obesity-Associated Insulin-Resistance and Inducing Th17 Responses in Mice and Patients. Diabetes 2012, 61, 2238–2247. [Google Scholar] [CrossRef] [Green Version]
- Macdougall, C.E.; Longhi, M.P. Adipose tissue dendritic cells in steady-state. Immunology 2019, 156, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Rudemiller, N.P.; Privratsky, J.R.; Ren, J.; Wen, Y.; Griffiths, R.; Crowley, S.D. Classical Dendritic Cells Mediate Hypertension by Promoting Renal Oxidative Stress and Fluid Retention. Hypertension 2020, 75, 131–138. [Google Scholar] [CrossRef]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef]
- Kano, G.; Almanan, M.; Bochner, B.S.; Zimmermann, N. Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: Role for reactive oxygen species-enhanced MEK/ERK activation. J. Allergy Clin. Immunol. 2013, 132, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molofsky, A.B.; Nussbaum, J.C.; Liang, H.-E.; Van Dyken, S.J.; Cheng, L.E.; Mohapatra, A.; Chawla, A.; Locksley, R.M. Innate Lymphoid Type 2 Cells Sustain Visceral Adipose Tissue Eosinophils and Alternatively Activated Macrophages. J. Exp. Med. 2013, 210, 535–549. [Google Scholar] [CrossRef]
- Niccoli, G.; Montone, R.A.; Sabato, V.; Crea, F. Role of Allergic Inflammatory Cells in Coronary Artery Disease. Circulation 2018, 138, 1736–1748. [Google Scholar] [CrossRef]
- Haley, K.J.; Lilly, C.M.; Yang, J.H.; Feng, Y.; Kennedy, S.P.; Turi, T.G.; Thompson, J.F.; Sukhova, G.H.; Libby, P.; Lee, R.T. Overexpression of Eotaxin and the CCR3 Receptor in Human Atherosclerosis: Using Genomic Technology to Identify a Potential Novel Pathway of Vascular Inflammation. Circulation 2000, 102, 2185–2189. [Google Scholar] [CrossRef] [Green Version]
- Boshuizen, M.C.S.; Hoeksema, M.A.; Neele, A.E.; van der Velden, S.; Hamers, A.A.J.; Van den Bossche, J.; Lutgens, E.; de Winther, M.P.J. Interferon-β Promotes Macrophage Foam Cell Formation by Altering Both Cholesterol Influx and Efflux Mechanisms. Cytokine 2016, 77, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Wang, L.; Li, F.; Yang, M.; Song, L.; Tian, F.; Yukht, A.; Shah, P.K.; Rothenberg, M.E.; Sharifi, B.G. Oxidized LDL Activated Eosinophil Polarize Macrophage Phenotype from M2 to M1 through Activation of CD36 Scavenger Receptor. Atherosclerosis 2017, 263, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Chatzigeorgiou, A.; Chavakis, T. Immune Cells and Metabolism. Handb. Exp. Pharmacol. 2016, 233, 221–249. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Park, Y.J.; Ham, M.; Kim, J.B. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol. Cells 2014, 37, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, T.P.; Kogan, S.; Aouadi, M.; Hendricks, G.M.; Straubhaar, J.; Czech, M.P. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1425–H1437. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Nitta, T.; Maruno, K.; Yeh, Y.-S.; Kuwata, H.; Tomita, K.; Goto, T.; Takahashi, N.; Kawada, T. Macrophage Infiltration into Obese Adipose Tissues Suppresses the Induction of UCP1 Level in Mice. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E676–E687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts-Toler, C.; O’Neill, B.T.; Cypess, A.M. Diet-induced obesity causes insulin resistance in mouse brown adipose tissue. Obesity 2015, 23, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Picard, F. Brown fat biology and thermogenesis. Front. Biosci. 2011, 16, 1233–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P.; Spiegelman, B.M. Brown and Beige Fat: Molecular Parts of a Thermogenic Machine. Diabetes 2015, 64, 2346–2351. [Google Scholar] [CrossRef] [Green Version]
- Neinast, M.D.; Frank, A.P.; Zechner, J.F.; Li, Q.; Vishvanath, L.; Palmer, B.F.; Aguirre, V.; Gupta, R.K.; Clegg, D.J. Activation of Natriuretic Peptides and the Sympathetic Nervous System Following Roux-En-Y Gastric Bypass Is Associated with Gonadal Adipose Tissues Browning. Mol. Metab. 2015, 4, 427–436. [Google Scholar] [CrossRef]
- den Hartigh, L.J.; Wang, S.; Goodspeed, L.; Wietecha, T.; Houston, B.; Omer, M.; Ogimoto, K.; Subramanian, S.; Gowda, G.A.N.; O’Brien, K.D.; et al. Metabolically Distinct Weight Loss by 10, 12 CLA and Caloric Restriction Highlight the Importance of Subcutaneous White Adipose Tissue for Glucose Homeostasis in Mice. PLoS ONE 2017, 12, e0172912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, M.; Himms-Hagen, J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Sidossis, L.S.; Porter, C.; Saraf, M.K.; Børsheim, E.; Radhakrishnan, R.S.; Chao, T.; Ali, A.; Chondronikola, M.; Mlcak, R.; Finnerty, C.C.; et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015, 22, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Fukui, Y.; Masui, S.; Osada, S.; Umesono, K.; Motojima, K. A new thiazolidinedione, NC-2100, which is a weak PPAR-gamma activator, exhibits potent antidiabetic effects and induces uncoupling protein 1 in white adipose tissue of KKAy obese mice. Diabetes 2000, 49, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Gaiz, A.; Mosawy, S.; Colson, N.; Singh, I. Thrombotic and cardiovascular risks in type two diabetes; Role of platelet hyperactivity. Biomed. Pharmacother. 2017, 94, 679–686. [Google Scholar] [CrossRef]
- Weber, C. Platelets and chemokines in atherosclerosis: Partners in crime. Circ. Res. 2005, 96, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Ferroni, P.; Basili, S.; Falco, A.; Davì, G. Platelet activation in type 2 diabetes mellitus. J. Thromb. Haemost. 2004, 2, 1282–1291. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, M.; Singh, J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: Molecular insights and therapeutic strategies. Cardiovasc. Diabetol. 2018, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.D.; Frenette, P.S. The vessel wall and its interactions. Blood 2008, 111, 5271–5281. [Google Scholar] [CrossRef]
- Nording, H.; Baron, L.; Langer, H.F. Platelets as therapeutic targets to prevent atherosclerosis. Atherosclerosis 2020, 307, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Kakouros, N.; Rade, J.J.; Kourliouros, A.; Resar, J.R. Platelet Function in Patients with Diabetes Mellitus: From a Theoretical to a Practical Perspective. Int. J. Endocrinol. 2011, 2011, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.-i.; Takahashi, M.; Ishida, M.; Lee, J.D.; Berk, B.C. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J. Biol. Chem. 1997, 272, 20389–20394. [Google Scholar] [CrossRef] [Green Version]
- Redondo, P.C.; Ben-Amor, N.; Salido, G.M.; Bartegi, A.; Pariente, J.A.; Rosado, J.A. Ca2+-independent activation of Bruton’s tyrosine kinase is required for store-mediated Ca2+ entry in human platelets. Cell. Signal. 2005, 17, 1011–1021. [Google Scholar] [CrossRef]
- Senis, Y.A.; Mazharian, A.; Mori, J. Src family kinases: At the forefront of platelet activation. Blood 2014, 124, 2013–2024. [Google Scholar] [CrossRef] [Green Version]
- Rawish, E.; Nording, H.; Münte, T.; Langer, H.F. Platelets as Mediators of Neuroinflammation and Thrombosis. Front. Immunol. 2020, 11, 548631. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Varghese, S.; Vitseva, O.; Tanriverdi, K.; Freedman, J.E. CD40 ligand influences platelet release of reactive oxygen intermediates. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2428–2434. [Google Scholar] [CrossRef] [Green Version]
- Vinik, A.I.; Erbas, T.; Park, T.S.; Nolan, R.; Pittenger, G.L. Platelet dysfunction in type 2 diabetes. Diabetes Care 2001, 24, 1476–1485. [Google Scholar] [CrossRef] [Green Version]
- Jennings, P.E.; McLaren, M.; Scott, N.A.; Saniabadi, A.R.; Belch, J.J. The relationship of oxidative stress to thrombotic tendency in type 1 diabetic patients with retinopathy. Diabet. Med. 1991, 8, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Jennings, P.E. From hemobiology to vascular disease: A review of the potential of gliclazide to influence the pathogenesis of diabetic vascular disease. J. Diabetes Complicat. 1994, 8, 226–230. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C. Regulation of Microvascular Function by Adipose Tissue in Obesity and Type 2 Diabetes: Evidence of an Adipose-Vascular Loop. Am. J. Biomed. Sci. 2009, 1, 133. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Maenhaut, N.; Van de Voorde, J. Regulation of vascular tone by adipocytes. BMC Med. 2011, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Tozawa, K.; Ono-Uruga, Y.; Yazawa, M.; Mori, T.; Murata, M.; Okamoto, S.; Ikeda, Y.; Matsubara, Y. Megakaryocytes and Platelets from a Novel Human Adipose Tissue-Derived Mesenchymal Stem Cell Line. Blood 2019, 133, 633–643. [Google Scholar] [CrossRef]
- Griendling, K.K.; FitzGerald, G.A. Oxidative stress and cardiovascular injury: Part I: Basic mechanisms and in vivo monitoring of ROS. Circulation 2003, 108, 1912–1916. [Google Scholar] [CrossRef] [Green Version]
- Carresi, C.; Mollace, R.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Ruga, S.; Zito, M.C.; et al. Oxidative Stress Triggers Defective Autophagy in Endothelial Cells: Role in Atherothrombosis Development. Antioxidants 2021, 10, 387. [Google Scholar] [CrossRef]
- McCann, S.K.; Dusting, G.J.; Roulston, C.L. Early increase of Nox4 NADPH oxidase and superoxide generation following endothelin-1-induced stroke in conscious rats. J. Neurosci. Res. 2008, 86, 2524–2534. [Google Scholar] [CrossRef]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durrant, J.R.; Seals, D.R.; Connell, M.L.; Russell, M.J.; Lawson, B.R.; Folian, B.J.; Donato, A.J.; Lesniewski, L.A. Voluntary Wheel Running Restores Endothelial Function in Conduit Arteries of Old Mice: Direct Evidence for Reduced Oxidative Stress, Increased Superoxide Dismutase Activity and down-Regulation of NADPH Oxidase. J. Physiol. 2009, 587, 3271–3285. [Google Scholar] [CrossRef]
- Fang, J.; Seki, T.; Maeda, H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv. Drug Deliv. Rev. 2009, 61, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Khandrika, L.; Kumar, B.; Koul, S.; Maroni, P.; Koul, H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009, 282, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricklin, D.; Lambris, J.D. Complement in immune and inflammatory disorders: Pathophysiological mechanisms. J. Immunol. 2013, 190, 3831–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, T.; Moldow, C.F.; Craddock, P.R.; Bowers, T.K.; Jacob, H.S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J. Clin. Investig. 1978, 61, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Stahl, G.L.; Shernan, S.K.; Smith, P.K.; Levy, J.H. Complement activation and cardiac surgery: A novel target for improving outcomes. Anesth. Analg. 2012, 115, 759–771. [Google Scholar] [CrossRef] [Green Version]
- Berger, H.J.; Taratuska, A.; Smith, T.W.; Halperin, J.A. Activated complement directly modifies the performance of isolated heart muscle cells from guinea pig and rat. Am. J. Physiol. 1993, 265 Pt 2, H267–H272. [Google Scholar] [CrossRef]
- Vakeva, A.P.; Agah, A.; Rollins, S.A.; Matis, L.A.; Li, L.; Stahl, G.L. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: Role of the terminal complement components and inhibition by anti-C5 therapy. Circulation 1998, 97, 2259–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, G.; Li, B.; Liu, X.; Zhang, M.; Gao, F.; Zhao, Y.; An, F.; Zhang, Y.; Zhang, C. Overexpression of Complement Component C5a Accelerates the Development of Atherosclerosis in ApoE-Knockout Mice. Oncotarget 2016, 7, 56060–56070. [Google Scholar] [CrossRef] [Green Version]
- Humbles, A.A.; Lu, B.; Nilsson, C.A.; Lilly, C.; Israel, E.; Fujiwara, Y.; Gerard, N.P.; Gerard, C. A Role for the C3a Anaphylatoxin Receptor in the Effector Phase of Asthma. Nature 2000, 406, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Shibata, K.; Akatsu, H.; Shimizu, N.; Sakata, N.; Katsuragi, T.; Okada, H. Contribution of Anaphylatoxin C5a to Late Airway Responses after Repeated Exposure of Antigen to Allergic Rats. J. Immunol. 2001, 167, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, K.S.; Friedrichs, G.S.; Homeister, J.W.; Lucchesi, B.R. The complement system in myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 1994, 28, 437–444. [Google Scholar] [CrossRef]
- Braquet, P.; Paubert-Braquet, M.; Bourgain, R.H.; Bussolino, F.; Hosford, D. PAF/cytokine auto-generated feedback networks in microvascular immune injury: Consequences in shock, ischemia and graft rejection. J. Lipid Mediat. 1989, 1, 75–112. [Google Scholar]
- Suffritti, C.; Tobaldini, E.; Schiavon, R.; Strada, S.; Maggioni, L.; Mehta, S.; Sandrone, G.; Toschi-Dias, E.; Cicardi, M.; Montano, N. Complement and Contact System Activation in Acute Congestive Heart Failure Patients. Clin. Exp. Immunol. 2017, 190, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Muscari, A.; Antonelli, S.; Bianchi, G.; Cavrini, G.; Dapporto, S.; Ligabue, A.; Ludovico, C.; Magalotti, D.; Poggiopollini, G.; Zoli, M.; et al. Serum C3 Is a Stronger Inflammatory Marker of Insulin Resistance than C-Reactive Protein, Leukocyte Count, and Erythrocyte Sedimentation Rate: Comparison Study in an Elderly Population. Diabetes Care 2007, 30, 2362–2368. [Google Scholar] [CrossRef] [Green Version]
- Weyer, C.; Tataranni, P.A.; Pratley, R.E. Insulin action and insulinemia are closely related to the fasting complement C3, but not acylation stimulating protein concentration. Diabetes Care 2000, 23, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertle, E.; van Greevenbroek, M.M.; Arts, I.C.; van der Kallen, C.J.; Geijselaers, S.L.; Feskens, E.J.; Jansen, E.H.; Schalkwijk, C.G.; Stehouwer, C.D. Distinct Associations of Complement C3a and Its Precursor C3 with Atherosclerosis and Cardiovascular Disease. The CODAM Study. Thromb. Haemost. 2014, 111, 1102–1111. [Google Scholar] [CrossRef]
- Shim, K.; Begum, R.; Yang, C.; Wang, H. Complement activation in obesity, insulin resistance, and type 2 diabetes mellitus. World J. Diabetes 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Onat, A.; Uzunlar, B.; Hergenç, G.; Yazici, M.; Sari, I.; Uyarel, H.; Can, G.; Sansoy, V. Cross-Sectional Study of Complement C3 as a Coronary Risk Factor among Men and Women. Clin. Sci. 2005, 108, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Rawish, E.; Nickel, L.; Schuster, F.; Stölting, I.; Frydrychowicz, A.; Saar, K.; Hübner, N.; Othman, A.; Kuerschner, L.; Raasch, W. Telmisartan Prevents Development of Obesity and Normalizes Hypothalamic Lipid Droplets. J. Endocrinol. 2020, 244, 95–110. [Google Scholar] [CrossRef]
- Kjaergaard, M.; Nilsson, C.; Nielsen, M.O.; Grove, K.; Raun, K. Hypothalamic oxidative stress and inflammation, and peripheral glucose homeostasis in Sprague-Dawley rat offspring exposed to maternal and postnatal chocolate and soft drink. Nutr. Diabetes 2018, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, G.; Viggiano, E.; Trinchese, G.; De Filippo, C.; Messina, A.; Monda, V.; Valenzano, A.; Cincione, R.I.; Zammit, C.; Cimmino, F.; et al. Long Feeding High-Fat Diet Induces Hypothalamic Oxidative Stress and Inflammation, and Prolonged Hypothalamic AMPK Activation in Rat Animal Model. Front. Physiol. 2018, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008, 135, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Git, K.C.G.; Adan, R.A.H. Leptin resistance in diet-induced obesity: The role of hypothalamic inflammation. Obes. Rev. 2015, 16, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.-Y.; Chen, P.-N.; Hsieh, Y.-S. Targeting oxidative stress in the hypothalamus: The effect of transcription factor STAT3 knockdown on endogenous antioxidants-mediated appetite control. Arch. Toxicol. 2015, 89, 87–100. [Google Scholar] [CrossRef]
- Samodien, E.; Johnson, R.; Pheiffer, C.; Mabasa, L.; Erasmus, M.; Louw, J.; Chellan, N. Diet-Induced Hypothalamic Dysfunction and Metabolic Disease, and the Therapeutic Potential of Polyphenols. Mol. Metab. 2019, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.J.; Murphy, M.P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N. Y. Acad. Sci. 2010, 1201, 96–103. [Google Scholar] [CrossRef]
- Mercer, J.R.; Yu, E.; Figg, N.; Cheng, K.-K.; Prime, T.A.; Griffin, J.L.; Masoodi, M.; Vidal-Puig, A.; Murphy, M.P.; Bennett, M.R. The Mitochondria-Targeted Antioxidant MitoQ Decreases Features of the Metabolic Syndrome in ATM+/−/ApoE−/− Mice. Free Radic. Biol. Med. 2012, 52, 841–849. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Feng, S.; Yang, Q.; Liu, M.; Li, W.; Yuan, W.; Zhang, S.; Wu, B.; Li, J. Edaravone for Acute Ischaemic Stroke. Cochrane Database Syst. Rev. 2011, CD007230. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Altenhöfer, S.; Radermacher, K.A.; Kleikers, P.W.M.; Wingler, K.; Schmidt, H.H.H.W. Evolution of NADPH Oxidase Inhibitors: Selectivity and Mechanisms for Target Engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, A.M.; Russell, J.W.; Sullivan, K.A.; Backus, C.; Hayes, J.M.; McLean, L.L.; Feldman, E.L. SOD2 Protects Neurons from Injury in Cell Culture and Animal Models of Diabetic Neuropathy. Exp. Neurol. 2007, 208, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Otero, P.; Bonet, B.; Herrera, E.; Rabano, A. Development of atherosclerosis in the diabetic BALB/c mice. Prevention with Vitamin E administration. Atherosclerosis 2005, 182, 259–265. [Google Scholar] [CrossRef]
- Zhang, Y.; Wada, J.; Hashimoto, I.; Eguchi, J.; Yasuhara, A.; Kanwar, Y.S.; Shikata, K.; Makino, H. Therapeutic Approach for Diabetic Nephropathy Using Gene Delivery of Translocase of Inner Mitochondrial Membrane 44 by Reducing Mitochondrial Superoxide Production. J. Am. Soc. Nephrol. 2006, 17, 1090–1101. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, V.; Xiong, Y.; Ho, Y.-S. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic. Biol. Med. 2006, 41, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- DeRubertis, F.R.; Craven, P.A.; Melhem, M.F. Acceleration of diabetic renal injury in the superoxide dismutase knockout mouse: Effects of tempol. Metabolism 2007, 56, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Rawish, E.; Nording, H.M.; Langer, H.F. Inflammation in Metabolic and Cardiovascular Disorders—Role of Oxidative Stress. Life 2021, 11, 672. https://doi.org/10.3390/life11070672
Sun Y, Rawish E, Nording HM, Langer HF. Inflammation in Metabolic and Cardiovascular Disorders—Role of Oxidative Stress. Life. 2021; 11(7):672. https://doi.org/10.3390/life11070672
Chicago/Turabian StyleSun, Ying, Elias Rawish, Henry M. Nording, and Harald F. Langer. 2021. "Inflammation in Metabolic and Cardiovascular Disorders—Role of Oxidative Stress" Life 11, no. 7: 672. https://doi.org/10.3390/life11070672
APA StyleSun, Y., Rawish, E., Nording, H. M., & Langer, H. F. (2021). Inflammation in Metabolic and Cardiovascular Disorders—Role of Oxidative Stress. Life, 11(7), 672. https://doi.org/10.3390/life11070672





