Photorhabdus sp. ETL Antimicrobial Properties and Characterization of Its Secondary Metabolites by Gas Chromatography–Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Optimal Nutrient Medium for Metabolite Production
2.2. Extraction of Secondary Metabolites
2.3. Determination of Anti-Microbial Activity
2.3.1. Minimum Inhibitory Concentrations (MIC)
2.3.2. Agar Disk-Diffusion Method
2.3.3. Agar Well Diffusion Method
2.3.4. Determination of Anti-Microbial Activity Methods’ Validation
2.4. Profiling of Volatile Compounds
Data Processing and Statistical Analysis
3. Results and Discussion
3.1. Selection of the Optimal Nutrient Medium for Metabolite Production
3.2. Determination of Anti-Microbial Activity
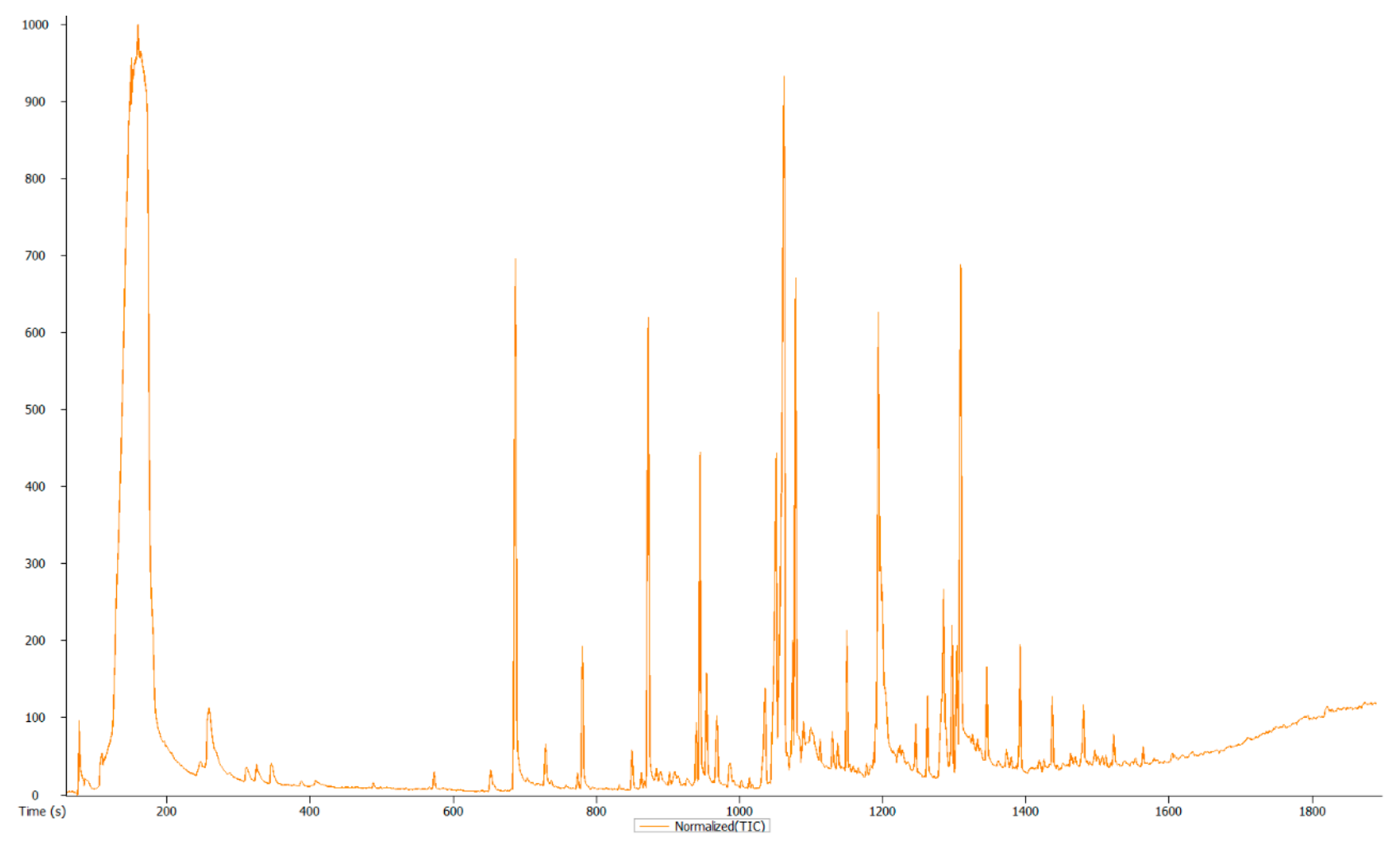
3.3. Overview and Exploration of the Acquired GC-MS Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales-López, S.; Yepes, J.A.; Prada-Herrera, J.C.; Torres-Jiménez, A. Enterobacteria in the 21st century: A review focused on taxonomic changes. J. Infect. Dev. Ctries. 2019, 13, 265–273. [Google Scholar] [CrossRef]
- Webster, J.M.; Genhui, C.; Kaiji, H.; Li, J. Bacterial metabolites. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI: New York, NY, USA, 2002; pp. 99–114. [Google Scholar]
- Ji, D.J.; Yi, Y.K.; Kang, G.H. Identification of an antibacterial compound, benzylideneacetone, from Xenorhabdus nematophila against major plant-pathogenic bacteria. FEMS Microbiol. Lett. 2004, 239, 241–248. [Google Scholar] [CrossRef]
- Clarke, D.J. The Regulation of secondary metabolism in Photorhabdus. In The Molecular Biology of Photorhabdus Bacteria. Current Topics in Microbiology and Immunology; Richard, H., Ed.; Springer: Cham, Switzerland, 2017; Volume 402, pp. 81–102. [Google Scholar]
- Salazar-Gutiérrez, J.D.; Castelblanco, A.; Rodríguez-Bocanegra, M.X.; Teran, W.; Sáenz-Aponte, A. Photorhabdus luminescens subsp. akhurstii SL0708 pathogenicity in Spodoptera frugiperda (Lepidoptera: Noctuidae) and Galleria mellonella (Lepidoptera: Pyralidae). J. Asia-Pac. Entomol. 2017, 20, 1112–1121. [Google Scholar] [CrossRef]
- Eckstein, S.; Heermann, R. Regulation of phenotypic switching and heterogeneity in Photorhabdus luminescens cell populations. J. Mol. Biol. 2019, 431, 4559–4568. [Google Scholar] [CrossRef]
- Dreyer, J.; Malan, A.P.; Dicks, L.M.T. Bacteria of the genus Xenorhabdus, a novel source of bioactive compounds. Front. Microbiol. 2018, 9, 3177. [Google Scholar] [CrossRef] [PubMed]
- Mukendi, J.P.K.; Kimbita, E.; Mbanzulu, K.M.; Maindo, P.P.M.; Misinzo, G. Morphological and molecular detection of canine dirofilarial species of veterinary and medical importance in Morogoro municipality, Tanzania. Vet. Parasitol. 2016, 220, 1–3. [Google Scholar] [CrossRef]
- Guschin, A.; Ryzhikh, P.; Rumyantseva, T.; Gomberg, M.; Unemo, M. Treatment efficacy, treatment failures and selection of macrolide resistance in patients with high load of Mycoplasma genitalium during treatment of maleurethritis with Josamycin. BMC Infect. Dis. 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Sawatzky, P.; Liu, G.; Mulvey, M.R. Antimicrobial resistance to Neisseria gonorrhoeae in Canada: 2009–2013. Can. Commun. Dis. Rep. 2015, 41, 40–41. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Bode, H.B. Entomopathogenic bacteria as a source of secondary metabolites. Curr. Opin. Chem. Biol. 2009, 13, 224–230. [Google Scholar] [CrossRef]
- Waterfield, N.; Ciche, T.; Clarke, D. Photorhabdus and a host of hosts. Annu. Rev. Microbiol. 2009, 63, 557–574. [Google Scholar] [CrossRef]
- Ehlers, R.-U.; Shapiro-Ilan, D.I. Mass production. In Nematodes as Biological Control Agents; Grewal, P.S., Ehlers, R., Shapiro-Ilan, D.I., Eds.; CABI: Cambridge, UK, 2005; pp. 65–78. [Google Scholar]
- Hazir, S.; Kaya, H.K.; Stock, S.P.; Keskün, N. Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) for biological control of soil pests. Turk. J. Biol. 2003, 27, 181–202. [Google Scholar]
- Derzelle, S.; Turlin, E.; Duchaud, S.; Pages, S.; Kunst, F.; Givaudan, A.; Danchin, A. The PhoPPhoQ two component regulatory system of Photorhabdus luminescens is essential for virulence in insects. J. Bacteriol. 2004, 186, 1270–1279. [Google Scholar] [CrossRef]
- Blackburn, D.; Wood, P.L., Jr.; Burk, T.J.; Crawford, B.; Wright, S.M.; Adams, B.J. Evolution of virulence in Photorhabdus spp., entomopathogenic nematode symbionts. Syst. Appl. Microbiol. 2016, 39, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Mabona, U.; Viljoen, A.; Shikanga, E.; Marston, A.; Van vuuren, S. Antimicrobial activity of Southern African medicinal plants with dermatological relevance: From an ethno-pharmacological screening approach, to combination studies and the isolation of a bioactive compound. J. Ethnopharmacol. 2013, 148, 45–55. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.J. Plant Secondary Metabolites. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Elsevier: Pergamon, Turkey, 2011; pp. 299–308. [Google Scholar]
- Ezra, D.; Hess, W.M.; Strobel, G. New endophytic isolates of Muscodor albus, a volatile-antibiotic-producing fungus. Microbiology 2004, 150, 4023–4031. [Google Scholar] [CrossRef]
- Tugizimana, F.; Piater, L.; Dubery, I. Plant metabolomics: A new frontier in phytochemical analysis. S. Afr. J. Sci. 2013, 109, 1–11. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yamazaki, M.; Saito, K. A polyhedral approach for understanding flavonoid biosynthesis in Arabidopsis. New Biotechnol. 2010, 27, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Hirai, M.Y.; Sasaki, E.; Akiyama, K.; Yonekura-Sakakibara, K.; Provart, N.J.; Sakurai, T.; Shimada, Y.; Saito, K. AtMetExpress development: A phytochemical atlas of Arabidopsis development. Plant Physiol. 2010, 152, 566–578. [Google Scholar] [CrossRef]
- Rukmini, K.; Devi, P.S. GC-MS Analysis and phytochemical screening of a rare Pteridophyte Nephrolepis Cardifolia (L.) Presl. From Tirumala Hills. Int. J. Pharm. Sci. Rev. Res. 2014, 3, 13–19. [Google Scholar]
- Torre, M. Challenges for mass production of nematodes in submerged culture. Biotechnol. Adv. 2003, 21, 407–416. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, X.; An, F.; Wang, G.; Zhang, X. Improvement of antibiotic activity of Xenorhabdus bovienii by medium optimization using response surface methodology. Microb. Cell Fact. 2011, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.S.; Kumar, S.; Aharwal, R.P.; Chaturvedi, S. Anti-bacterial potential of endophytic fungi isolated from Saraca indica. J. Biol. Chem. Sci. 2014, 1, 24–34. [Google Scholar]
- Maloney, K.N.; Macmillan, J.B.; Kauffman, C.A.; Jensen, P.R.; Dipasquale, A.G.; Rheingold, A.L.; Fenical, W. Lodopyridone, a structurally unprecedented alkaloid from a marine actinomycete. Org. Lett. 2009, 11, 5422. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tudela, J.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Donnelly, J.P.; Dupont, B.; Fegeler, W.; Moore, C.; et al. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Eur. Soc. Clin. Microbiol. Infect. Dis. 2002, 9, 1–8. [Google Scholar] [CrossRef]
- Rezende, N.; Jayme, C.C.; Brassesco, M.S.; Tedesco, A.C.; Oliveira, H.F. Standardization of a resazurin-based assay for the evaluation of metabolic activity in oral squamous carcinoma and glioblastoma cells. Photodiagn. Photodyn. Ther. 2019, 26, 371–374. [Google Scholar] [CrossRef]
- Thomashow, L.S. Biocontrol of plant root pathogens. Curr. Opin. Biotechnol. 1996, 7, 343–347. [Google Scholar] [CrossRef]
- Niño, J.; Correa, Y.M.; Mosquera, O.M. Antibacterial, antifungal, and cytotoxic activities of 11 Solanaceae plants from Colombian biodiversity. Pharm. Biol. 2006, 44, 14–18. [Google Scholar] [CrossRef]
- Kumar, S.N.; Mohandas, S.; Nambisan, B. Purification of an antifungal compound, cyclo(l-Pro-d-Leu) for cereals produced by Bacillus cereus subsp. thuringiensis associated with entomopathogenic nematode. Microbiol. Res. 2013, 168, 278–288. [Google Scholar] [CrossRef]
- Thomas, G.M.; Poinar, G.O. Xenorhabdus gen. nov., a genus of entomopathogenic, nematophilic bacteria of the family Enterobacteriaceae. Int. J. Syst. Evol. Microbiol. 1979, 29, 352–360. [Google Scholar] [CrossRef]
- Akhurst, R.J. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J. Gen. Microbiol. 1980, 121, 303–309. [Google Scholar] [CrossRef]
- Akhurst, R.J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J. Gen. Microbiol. 1982, 128, 3061–3065. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.; Duvic, B.; Givaudan, A.; Boemare, N. Isolation and entomotoxic properties of the Xenorhabdus nematophilus F1 lecithinase. Appl. Environ. Microbiol. 1998, 64, 2367–2373. [Google Scholar] [CrossRef]
- Diblasi, L.; Arrighi, F.; Silva, J.; Bardon, A.; Cartagena, E. Penicillium commune metabolic profile as a promising source of antipathogenic natural products. Nat. Prod. Res. 2015, 29, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Gherraf, N.; Zellagui, A.; Kabouche, A.; Lahouel, M.; Salhi, R.; Rhouati, S. Chemical constituents and antimicrobial activity of essential oils of Ammodaucus Leucotricus. Arab. J. Chem. 2013. [Google Scholar] [CrossRef]
- Chen, J.; Wei, J.H.; Cai, S.F.; Zhang, H.J.; Zhang, X.H.; Liang, W.J.; Wu, S.G. Chemical constituents in whole herb of Bidens pilosa var. radiata. J. Chin. Med. Mater. 2013, 36, 410–413. [Google Scholar]
- Molfetta, I.; Ceccarini, L.; Macchia, M.; Flamini, G.; Cioni, P.L. Abelmoschus esculentus (L.) Moench. and Abelmoschus moschatus Medik: Seeds production and analysis of the volatile compounds. Food Chem. 2013, 141, 34–40. [Google Scholar] [CrossRef]
- Begum, I.F.; Mohankumar, R.; Jeevan, M.; Ramani, K. GC–MS analysis of bio-active molecules derived from Paracoccus pantotrophus FMR19 and the antimicrobial activity against bacterial pathogens and MDROs. Indian J. Microbiol. 2016, 56, 426–432. [Google Scholar] [CrossRef]
- Yogeswari, S.; Ramalakshmi, S.; Neelavathy, R.; Muthumary, J. Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Glob. J. Pharmacol. 2012, 6, 65–71. [Google Scholar]
- Jonsson, S.; Vavilin, V.A.; Svensson, B.H. Phthalate hydrolysis under landfill conditions. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2006, 53, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Kurohane, K.; Imai, Y. Di-(2-ethylhexyl) phthalate enhances skin sensitization to isocyanate haptens in mice. Toxicol. Lett. 2010, 192, 97–100. [Google Scholar] [CrossRef]
- Ishido, M.; Suzuki, J. Classification of phthalates based on an in vitro neurosphere assay using rat mesencephalic neural stem cells. J. Toxicol. Sci. 2014, 39, 25–32. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Kannan, K. Phthalates and parabens in personal care products from China: Concentrations and human exposure. Arch. Environ. Contam. Toxicol. 2014, 66, 113–119. [Google Scholar] [CrossRef]
- Alrumman, S.A. Phytochemical and antimicrobial properties of Tamarix aphylla L. leaves growing naturally in the Abha Region, Saudi Arabia. Arab. J. Sci. Eng. 2016, 41, 2123–2129. [Google Scholar] [CrossRef]
- Chen, N.; Fang, G.; Zhou, D.; Gao, J. Effects of clay minerals on diethyl phthalate degradation in Fenton reactions. Chemosphere 2016, 165, 52–58. [Google Scholar] [CrossRef]
- Gardner, S.T.; Wood, A.T.; Lester, R.; Onkst, P.E.; Burnham, N.; Perygin, D.H.; Rayburn, J. Assessing differences in toxicity and teratogenicity of three phthalates, Diethyl phthalate, Di-n-propyl phthalate, and Di-n-butyl phthalate, using Xenopus laevis embryos. J. Toxicol. Environ. Health Part A 2016, 79, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sarmah, A.K.; Bolan, N.S.; He, L.; Lin, X.; Che, L.; Tang, C.; Wang, H. Effect of aging process on adsorption of diethyl phthalate in soils amended with bamboo biochar. Chemosphere 2016, 142, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, S.; Ahmadi, E.; Gholami, M.; Ghaffari, H.R.; Azari, A.; Ansari, M.; Miri, M.; Sharafi, K.; Rezaei, S. A comparative study of anaerobic fixed film baffled reactor and up-flow anaerobic fixed film fixed bed reactor for biological removal of diethyl phthalate from wastewater: A performance, kinetic, biogas, and metabolic pathway study. Biotechnol. Biofuels 2017, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Mao, C.; Du, D. Time-resolved immunoassay based on magnetic particles for the detection of diethyl phthalate in environmental water samples. Sci. Total Environ. 2017, 601–602, 723–731. [Google Scholar] [CrossRef]
- Phillips, S.; Rao, M.R.K.; Prabhu, K.; Priya, M.; Kalaivani, S.; Ravi, A.; Dinakar, S. Preliminary GC-MS analysis of an Ayurvedic medicine “Kulathadi Kashayam”. J. Chem. Pharm. Res. 2015, 7, 393–400. [Google Scholar]
- Kwak, A.-M.; Lee, I.-K.; Lee, S.-Y.; Yun, B.-S.; Kang, H.-W. Oxalic acid from Lentinula edodes culture filtrate: Antimicrobial activity on phytopathogenic bacteria and qualitative and quantitative analyses. Mycobiology 2016, 44, 338–342. [Google Scholar] [CrossRef]
- Bulathsinghala, A.T.; Shaw, I.C. The toxic chemistry of methyl bromide. Hum. Exp. Toxicol. 2014, 33, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Juergensmeyer, M.A.; Gingras, B.A.; Scheffrahn, R.H.; Weinberg, M.J. Methyl bromide fumigant lethal to Bacillus anthracis spores. J. Environ. Health 2007, 69, 24–26. [Google Scholar]
- Balachandara, R.; Karmegam, N.; Saravanan, M.; Subbaiya, R.; Gurumoorthy, P. Synthesis of bioactive compounds from vermicast isolated actinomycetes species and its antimicrobial activity against human pathogenic bacteria. Microb. Pathog. 2018, 121, 155–165. [Google Scholar] [CrossRef]
- Gopi, M.; Dhayanithi, N.B.; Devi, K.N.; Kumar, T.T.A. Marine natural product, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- (C7H10N202) of antioxidant properties from Bacillus species at Lakshadweep archipelago. J. Coast. Life Med. 2014, 2, 632–637. [Google Scholar]
- Awla, H.K.; Kadir, J.; Othman, R.; Rashid, T.S.; Wong, M.-Y. Bioactive compounds produced by Streptomyces sp. isolate UPMRS4 and antifungal activity against Pyricularia oryzae. Am. J. Plant Sci. 2016, 7, 1077–1085. [Google Scholar] [CrossRef]
- Gover, N.; Patni, V. Phytochemical characterization using various solvent extracts and GC-MS analysis of methanolic extract of Woodfordia Fruticosa (L.) kurz. Leaves. Int. J. Pharm. Pharm. Sci. 2013, 5, 4. [Google Scholar]
- Beulah, G.G.; Soris, P.T.; Mohan, V.R. GC-MS determination of bioactive compounds of Dendrophthoe falcata (L.F) Ettingsh: An epiphytic plant. Int. J. Health Sci. Res. 2018, 8, 261–269. [Google Scholar]
- Boussaada, O.; Ammar, S.; Saidana, D.; Chriaa, J.; Chraif, I.; Daami, M.; Helal, A.N.; Mighri, Z. Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiol. Res. 2008, 163, 87–95. [Google Scholar] [CrossRef]
- Brito-Madurro, A.G.; Prade, R.A.; Madurro, J.M.; Santos, M.A.; Peres, N.T.; Cursino-Santos, J.R.; Martinez-Rossi, N.M.; Rossi, A. A single amino acid substitution in one of the lipases of Aspergillus nidulans confers resistance to the antimycotic drug undecanoic acid. Biochem. Genet. 2008, 46, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Narra, N.; Kaki, S.S.; Prasad, R.B.N.; Misra, S.; Dhevendar, K.; Kontham, V.; Korlipara, P.V. Synthesis and evaluation of anti-oxidant and cytotoxic activities of novel 10-undecenoic acid methyl ester based lipoconjugates of phenolic acids. Beilstein J. Org. Chem. 2017, 13, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.Y.; Mohammed, G.J.; Hameed, I.H. Analysis of bioactive chemical compounds of Nigella sativa using gas chromatography-mass spectrometry. J. Pharmacogn. Phytother. 2016, 8, 8–24. [Google Scholar]
- Hussein, H.M.; Hameed, I.H.; Ibraheem, O.H. Antimicrobial activity and spectral chemical analysis of methanolic leaves extract of adiantum capillus-veneris using GC-MS and FT-IR spectroscopy. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 369–385. [Google Scholar]
- Poonsri, W.; Pluempanupat, W.; Chitchirachan, P.; Bullangpoti, V.; Koul, O. Insecticidal alkanes from Bauhinia scandens var. horsfieldii against Plutella xylostella L. (Lepidoptera: Plutellidae). Ind. Crops Prod. 2015, 65, 170–174. [Google Scholar] [CrossRef]
- Che, L.; Liu, B.; Ruan, C.; Tang, J.; Huang, D. Biocontrol of Lasiodiplodia theobromae, which causes black spot disease of harvested was apple fruit, using a strain of Breibacillus brevis FJAT-0809-GLX. Crop Prot. 2015, 67, 178–183. [Google Scholar] [CrossRef]
- Rajkumar, S.; Jebanesan, A. Mosquitocidal activities of octacosane from Moschosma polystachyum Linn. (lamiaceae). J. Ethnopharmacol. 2004, 90, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical water extraction of biological materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Pinto, M.E.A.; Araujo, S.G.; Morais, M.I.; Sa, N.O.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L.A.R.S. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An. Acad. Bras. Ciências 2017, 89, 1671–1681. [Google Scholar] [CrossRef]
- Knothe, G. Avocado and olive oil methyl esters. Biomass Bioenergy 2013, 58, 143–148. [Google Scholar] [CrossRef]
- Dowling, A.J. Identifying anti-host effectors in Photorhabdus. In The Molecular Biology of Photorhabdus Bacteria. Current Topics in Microbiology and Immunology; Richard, H., Ed.; Springer: Cham, Switzerland, 2017; Volume 402, pp. 25–38. [Google Scholar]
- Eleftherianos, I.; Shokal, U.; Yadav, S.; Kenney, E.; Maldonado, T. Insect immunity to entomopathogenic nematodes and their mutualistic bacteria. In The Molecular Biology of Photorhabdus Bacteria. Current Topics in Microbiology and Immunology; Richard, H., Ed.; Springer: Cham, Switzerland, 2017; Volume 402, pp. 123–156. [Google Scholar]
- Eleftherianos, I.; Ffrench-Constant, R.; Clarke, D.J.; Dowling, A.J. Dissecting the immune response to the entomopathogen Photorhabdus. Trends Microbiol. 2010, 18, 552–560. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Reynolds, S.; Castillo, J. Insect immune responses to nematode parasites. Trends Parasitol. 2011, 27, 537–547. [Google Scholar]
- Hoshizaki, D. Fat body. In The Insects: Structure and Function; Chapman, R., Ed.; Cambridge University Press: New York, NY, USA, 2013; p. 903. [Google Scholar]
- Raman, N.; Parameswari, S. Designing and synthesis of antifungal active macrocyclic ligand and its complexes derived from diethylphthalate and benzidine. Mycobiology 2007, 35, 65–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davies, J.; Spiegelman, G.B.; Yim, G. The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 2006, 9, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Ryan, K.S. Introducing the parvome: Bioactive compounds in the microbial world. ACS Chem. Biol. 2012, 7, 252–259. [Google Scholar] [CrossRef]

| Test Organism | Gram Reaction of Test Microorganisms | MIC (mg/mL) | Positive Control (Streptomycin) (mg/mL) |
|---|---|---|---|
| Bacteria | |||
| Pseudomonas aeruginosa | Negative | 0.83 ± 0.28 cd | 0.025 |
| Klebsiella oxytoca | Negative | 0.42 ± 0.14 ab | 1 |
| Escherichia coli | Negative | 0.062 ± 0 a | 1 |
| Staphylococcus aureus | Positive | 0.25 ± 0 ab | <0.031 |
| Staphylococcus epidermidis | Positive | 1.00 ± 0 d | 1.000 |
| Klebsiella pneumoniae | Negative | 0.42 ± 0.14 ab | 2.000 |
| Veillonella parvula | Negative | 4.00 ± 0 e | 0.062 |
| Enterococcus faecium | Positive | 4.00 ± 0 e | 0.025 |
| Bacillus cereus | Positive | 0.500 ± 0 bc | 0.5 |
| Staphylococcus saprophyticus | Positive | 0125 ± 0 a | 0.062 |
| Mycobacterium smegmatis | Positive | 0.25 ± 0 ab | 0.5 |
| Fungi | Fungal MIC (mg/mL) | ZI (diameter in mm) | Clotrimazole |
| Aspergillus flavus | 1.00 ± 0 d | 14 ± 1.2 | 1 |
| Aspergillus niger | 0.83 ± 0.28 cd | 12 ± 1 | 1 |
| Aspergillus parasiticus | 1.00 ± 0 d | 12 ± 1.3 | 1 |
| Rt (s) | m/z | Actual Masses | MF | Name | MC/Compound Nature | Activity/Function (References) | |
|---|---|---|---|---|---|---|---|
| 1. | 159.547 | 103.0652 | 43.018008 | C6H12O2 | 2-Pentanone, 4-hydroxy-4-methyl- | Alcohol | Strong antibacterial activity [41]. |
| 2. | 324.79 | 131.124 | 43.054469 | C13H28 | Tridecane | Long-chain alkane | Volatile oil component of various fuels and solvents; a distillation chaser in research laboratories [42,43]. |
| 3. | 702.79 | 206.1668 | 191.143147 | C14H22O | Phenol, 2,5-bis(1,1-dimethylethyl)- | Phenol/Aromatic hydro carbon | Antibacterial activity [44]. |
| 4. | 780.266 | 141.1627 | 57.070034 | C16H34 | Hexadecane | Alkane long chain hydrocarbon | Antimicrobial and antioxidant activity [45]. |
| 5. | 782.647 | 202.1097 | 149.023421 | C12H14O4 | Diethyl Phthalate | Diester of phthalic acid (FAEE), ethyl ester, Phthalate ester | Antimicrobial activity, a teratogenic agent, neurotoxin, plasticiser, and an endocrine disruptor [46,47,48,49,50,51,52,53,54,55]. |
| 6. | 849.532 | 147.0928 | 43.054409 | C19H39Cl | Nonadecane, 1-chloro- | Alkane (long-chain) | Antioxidant [56]. |
| 7. | 871.555 | 241.2753 | 58.065283 | C17H38BrN | Tetradonium Bromide | Nitrogen compound (germicidal detergent) | - |
| 8. | 883.831 | 140.1559 | 57.070068 | C22H42O4 | Oxalic acid, isobutyl hexadecyl ester | Ester, Organic acid | Oxalic acid has antimicrobial activity [57]. |
| 9. | 910.782 | 131.1138 | 69.057689 | C4BrF9 | Tris(trifluoromethyl) bromomethane | Bromomethane (or methyl bromide)/organobromine compound. | Pesticide [58,59]. |
| 10 | 939.259 | 131.0866 | 43.054488 | C18H36 | 3-Octadecene, (E)- | Alkene | Antimicrobial activity [60]. |
| 11 | 966.787 | 163.0868 | 70.065210 | C11H18N2O2 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | Antibiotic compound | Antioxidant properties, antimicrobial activity [40,61,62]. |
| 12. | 1046.92 | 292.2021 | 57.070074 | C18H28O3 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester | Aromatic acid ester (FAME)/Benzenepropanoic acid (carboxylic acid) | Fixative, or preservative agents used in foods, cosmetics, and medicines [63]. |
| 13. | 1060.08 | 233.1522 | 149.023469 | C25H40O4 | Phthalic acid, 6-ethyl-3-octyl heptyl ester | Ester/Plasticizer Compound | Antimicrobial, antifouling [64]. |
| 14. | 1066.93 | 124.1123 | 59.036837 | C9H19NO | Nonanamide | Amide | - |
| 15. | 1073.98 | 139.1472 | 43.054469 | C20H40 | 3-Eicosene, (E)- | Alkene/Long chain fatty acid | Antibacterial activity [44,65]. |
| 16. | 1074.22 | 192.982 | 55.054478 | C20H40 | 5-Eicosene, (E)- | Alkene/Long chain fatty acid | [44,65]. |
| 17. | 1077.64 | 183.2102 | 57.070030 | C20H42 | Eicosane | Alkane/Long chain fatty acid | Antibacterial activity [44,65]. |
| 18. | 1153.56 | 199.1687 | 74.036306 | C12H24O2 | Undecanoic acid, methyl ester | FAME/Medium-chain fatty acids | Antimicrobial, antioxidant [66,67]. |
| 19. | 1284.1 | 262.2527 | 59.036757 | C18H35NO | 9-Octadecenamide | Amide/Fatty acid amide | Anti-inflammatory and antibacterial. activities [68,69]. |
| 20. | 1296.83 | 219.0448 | 57.070020 | C27H56 | Heptacosane | Alkane | Insecticidal activity [70]. |
| 21. | 1302.48 | 244.1204 | 70.065251 | C33H37N5O5 | Ergotaman-3—,6′,18-trione, 9,10-dihydro-12′-hydroxy-2′-methyl-5′-(phenylmethyl)-, (5′a,10a)- | Ketone | Antimicrobial (antifungal) [71]. |
| 22. | 1437 | 225.2612 | BPI(57.070047) | C28H58 | Octacosane | Alkane | Insecticidal activity [72]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lulamba, T.E.; Green, E.; Serepa-Dlamini, M.H. Photorhabdus sp. ETL Antimicrobial Properties and Characterization of Its Secondary Metabolites by Gas Chromatography–Mass Spectrometry. Life 2021, 11, 787. https://doi.org/10.3390/life11080787
Lulamba TE, Green E, Serepa-Dlamini MH. Photorhabdus sp. ETL Antimicrobial Properties and Characterization of Its Secondary Metabolites by Gas Chromatography–Mass Spectrometry. Life. 2021; 11(8):787. https://doi.org/10.3390/life11080787
Chicago/Turabian StyleLulamba, Tshikala Eddie, Ezekiel Green, and Mahloro Hope Serepa-Dlamini. 2021. "Photorhabdus sp. ETL Antimicrobial Properties and Characterization of Its Secondary Metabolites by Gas Chromatography–Mass Spectrometry" Life 11, no. 8: 787. https://doi.org/10.3390/life11080787
APA StyleLulamba, T. E., Green, E., & Serepa-Dlamini, M. H. (2021). Photorhabdus sp. ETL Antimicrobial Properties and Characterization of Its Secondary Metabolites by Gas Chromatography–Mass Spectrometry. Life, 11(8), 787. https://doi.org/10.3390/life11080787






