Potential Role of Diabetes Mellitus-Associated T Cell Senescence in Epithelial Ovarian Cancer Omental Metastasis
Abstract
:1. Introduction
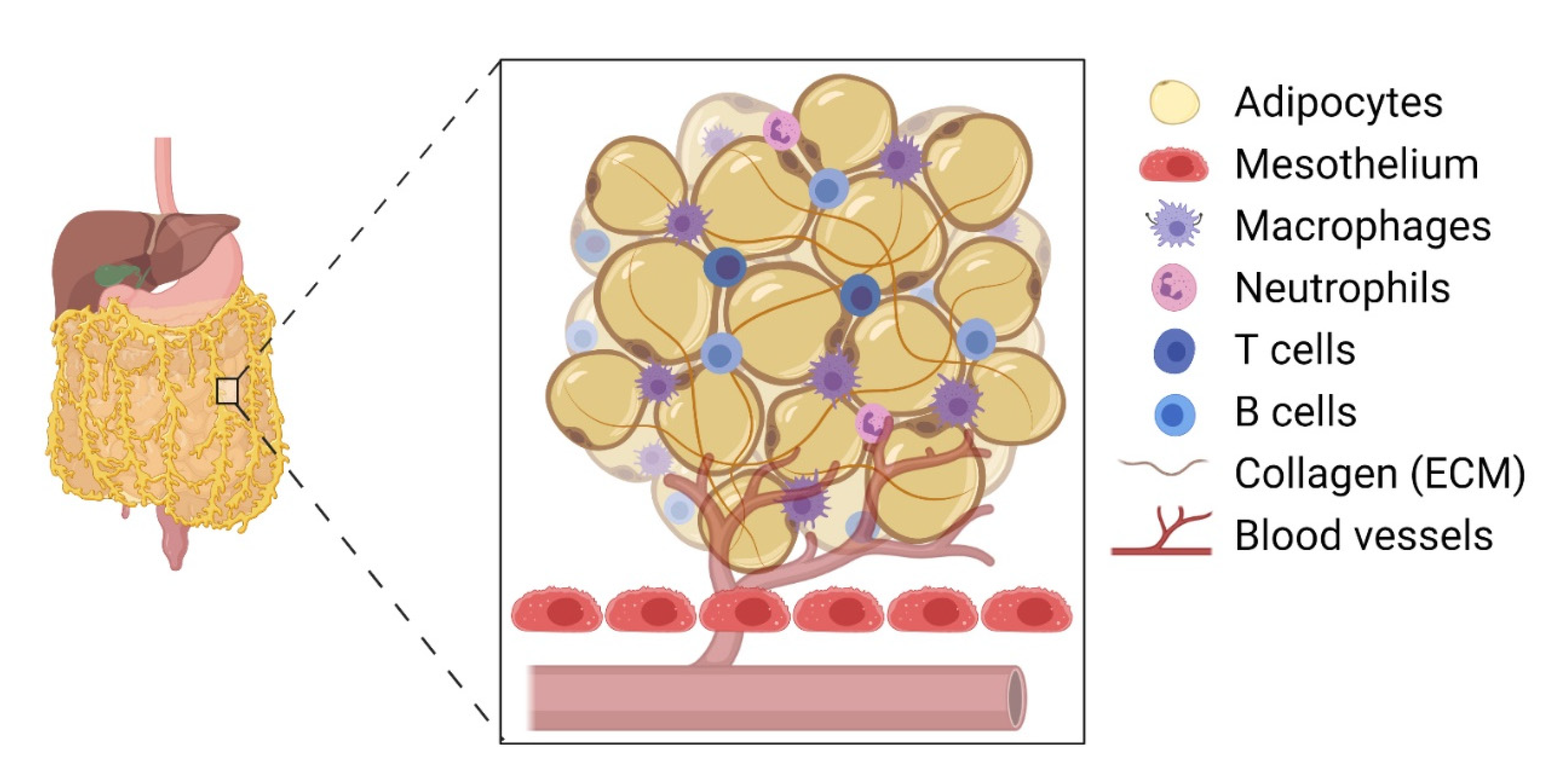
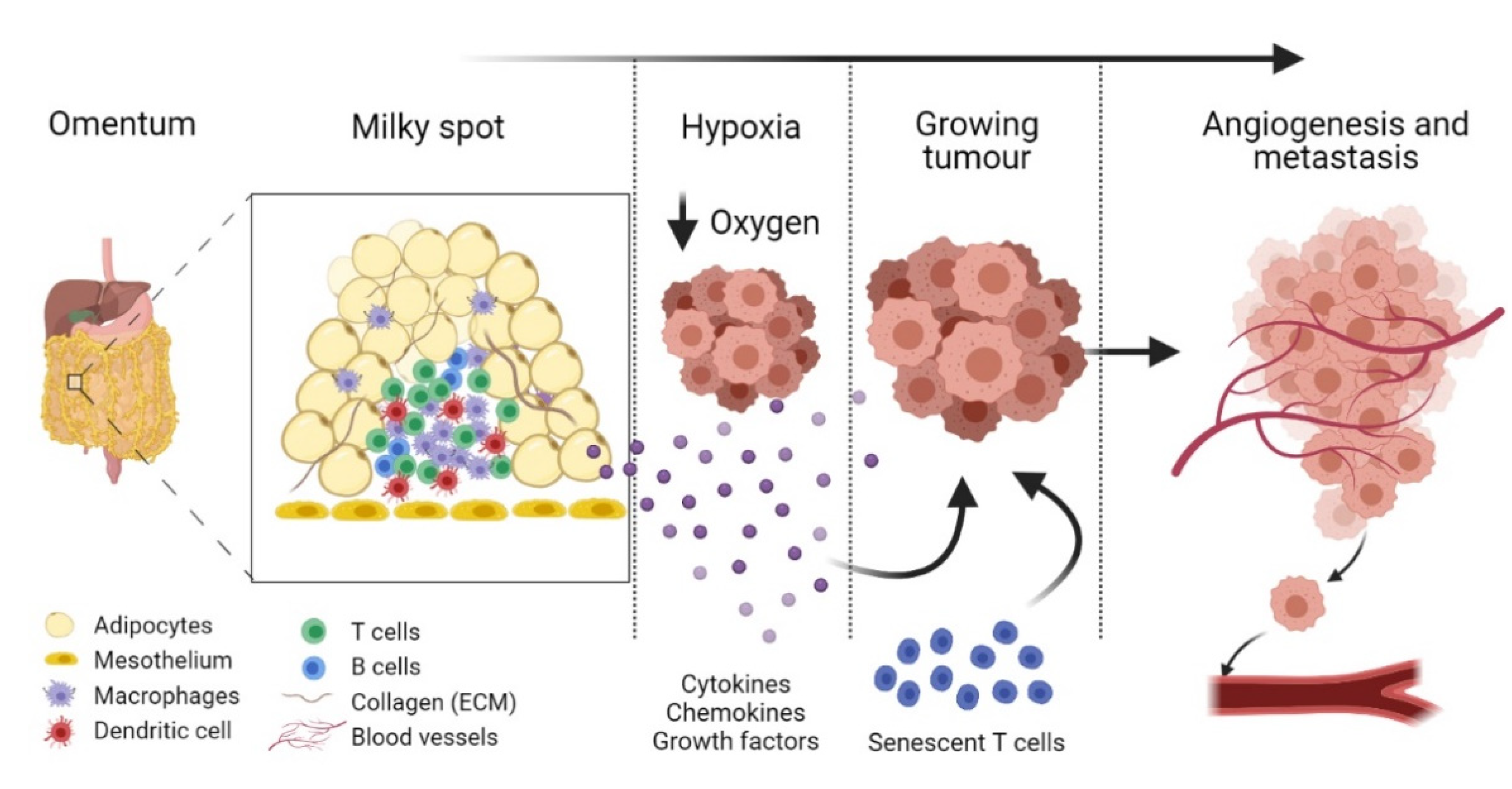
2. Omental Milky Spots as the Preferential Site for Colonisation by Epithelial Cancer Cells
3. Diabetes Mellitus-Associated T Cell Senescence
4. Age-Related Immunosenescence and Cancer
5. Could Premature Immunosenescence Contribute to EOC Progression in DM?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Acknowledgments
Conflicts of Interest
References
- Etzerodt, A.; Moulin, M.; Doktor, T.K.; Delfini, M.; Mossadegh-Keller, N.; Bajenoff, M.; Sieweke, M.; Moestrup, S.K.; Auphan-Anezin, N.; Lawrence, T. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J. Exp. Med. 2020, 217, e20191869. [Google Scholar] [CrossRef] [PubMed]
- Harper, E.I.; Sheedy, E.F.; Stack, M.S. With Great Age Comes Great Metastatic Ability: Ovarian Cancer and the Appeal of the Aging Peritoneal Microenvironment. Cancers 2018, 10, 230. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Geng, X.; Li, Y. Milky spots: Omental functional units and hotbeds for peritoneal cancer metastasis. Tumor Biol. 2016, 37, 5715–5726. [Google Scholar] [CrossRef] [Green Version]
- Frasca, D.; Blomberg, B.B. Adipose Tissue: A Tertiary Lymphoid Organ: Does It Change with Age? Gerontol. 2019, 66, 114–121. [Google Scholar] [CrossRef]
- Callender, L.; Carroll, E.C.; Beal, R.W.J.; Chambers, E.; Nourshargh, S.; Akbar, A.; Henson, S.M. Human CD8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell 2017, 17, e12675. [Google Scholar] [CrossRef]
- Bakhru, A.; Buckanovich, R.J.; Griggs, J.J. The impact of diabetes on survival in women with ovarian cancer. Gynecol. Oncol. 2011, 121, 106–111. [Google Scholar] [CrossRef]
- Shah, M.M.; Erickson, B.; Matin, T.; McGwin, G.; Martin, J.Y.; Daily, L.B.; Pasko, D.; Haygood, C.W.; Fauci, J.M.; Leath, C.A. Diabetes mellitus and ovarian cancer: More complex than just increasing risk. Gynecol. Oncol. 2014, 135, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Rao, X.; Zhong, J. Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. J. Diabetes Res. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Carroll, E.C.; Callender, L.A.; Hood, G.A.; Berryman, V.; Pattrick, M.; Finer, S.; Hitman, G.A.; Ackland, G.L.; Henson, S.M. Type 2 diabetes is associated with the accumulation of senescent T cells. Clin. Exp. Immunol. 2019, 197, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Craig, E.R.; Londoño, A.I.; Norian, L.A.; Arend, R.C. Metabolic risk factors and mechanisms of disease in epithelial ovarian cancer: A review. Gynecol. Oncol. 2016, 143, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Nagle, C.M.; Dixon, S.C.; Jensen, A.; Kjaer, S.K.; Modugno, F.; DeFazio, A.; Fereday, S.; Hung, J.; Johnatty, S.E.; Australian Ovarian Cancer Study Group; et al. Obesity and survival among women with ovarian cancer: Results from the Ovarian Cancer Association Consortium. Br. J. Cancer 2015, 113, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Ignacio, R.M.C.; Lee, E.-S.; Wilson, A.J.; Beeghly-Fadiel, A.; Whalen, M.M.; Son, D.-S. Obesity-Induced Peritoneal Dissemination of Ovarian Cancer and Dominant Recruitment of Macrophages in Ascites. Immune Netw. 2018, 18, e47. [Google Scholar] [CrossRef]
- Shirakawa, K.; Yan, X.; Shinmura, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yamamoto, T.; Anzai, A.; Isobe, S.; Yoshida, N.; et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J. Clin. Investig. 2016, 126, 4626–4639. [Google Scholar] [CrossRef] [Green Version]
- Conley, S.M.; Hickson, L.J.; Kellogg, T.A.; McKenzie, T.; Heimbach, J.K.; Taner, T.; Tang, H.; Jordan, K.L.; Saadiq, I.M.; Woollard, J.R.; et al. Human Obesity Induces Dysfunction and Early Senescence in Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells. Front. Cell Dev. Biol. 2020, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Savino, B.; Locati, M.; Zammataro, L.; Allavena, P.; Bonecchi, R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010, 21, 27–39. [Google Scholar] [CrossRef]
- Son, D.-S.; Kabir, S.M.; Dong, Y.-L.; Lee, E.; Adunyah, S.E. Inhibitory Effect of Tumor Suppressor p53 on Proinflammatory Chemokine Expression in Ovarian Cancer Cells by Reducing Proteasomal Degradation of IκB. PLoS ONE 2012, 7, e51116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, T.; Xue, L.; Guo, H. Senescent T cells: A potential biomarker and target for cancer therapy. EBioMedicine 2021, 68, 103409. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Migoni, S.; Caamaño, J. Fat-Associated Lymphoid Clusters in Inflammation and Immunity. Front. Immunol. 2016, 7, 612. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Kaur, S.; Coussens, L.M.; Lengyel, E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J. Clin. Investig. 2008, 118, 1367–1379. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Lengyel, E. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle 2009, 8, 683–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saman, H.; Raza, S.S.; Uddin, S.; Rasul, K. Inducing Angiogenesis, a Key Step in Cancer Vascularization, and Treatment Approaches. Cancers 2020, 12, 1172. [Google Scholar] [CrossRef]
- Pranjol, Z.I.; Gutowski, N.J.; Hannemann, M.M.; Whatmore, J.L. The Potential Role of the Proteases Cathepsin D and Cathepsin L in the Progression and Metastasis of Epithelial Ovarian Cancer. Biomolecules 2015, 5, 3260–3279. [Google Scholar] [CrossRef] [Green Version]
- Pranjol, Z.I.; Gutowski, N.J.; Hannemann, M.; Whatmore, J.L. Cathepsin L Induces Proangiogenic Changes in Human Omental Microvascular Endothelial Cells via Activation of the ERK1/2 Pathway. Curr. Cancer Drug Targets 2019, 19, 231–242. [Google Scholar] [CrossRef]
- Pranjol, Z.I.; Gutowski, N.J.; Hannemann, M.; Whatmore, J.L. Cathepsin D non-proteolytically induces proliferation and migration in human omental microvascular endothelial cells via activation of the ERK1/2 and PI3K/AKT pathways. Biochim. Biophys. Acta (BBA)-Bioenerg. 2018, 1865, 25–33. [Google Scholar] [CrossRef]
- Pranjol, Z.I.; Zinovkin, D.A.; Maskell, A.R.T.; Stephens, L.J.; Achinovich, S.L.; Los, D.M.; Nadyrov, E.A.; Hannemann, M.; Gutowski, N.J.; Whatmore, J.L. Cathepsin L-induced galectin-1 may act as a proangiogenic factor in the metastasis of high-grade serous carcinoma. J. Transl. Med. 2019, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Pranjol, Z.I.; Whatmore, J.L. Cathepsin D in the Tumor Microenvironment of Breast and Ovarian Cancers; Springer: Cham, Switzarland, 2020; pp. 1–16. [Google Scholar] [CrossRef]
- Meza-Perez, S.; Randall, T.D. Immunological Functions of the Omentum. Trends Immunol. 2017, 38, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Allen, L. The peritoneal stomata. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1936, 67, 89–103. [Google Scholar] [CrossRef]
- Motohara, T.; Masuda, K.; Morotti, M.; Zheng, Y.; El-Sahhar, S.; Chong, K.Y.; Wietek, N.; Alsaadi, A.; KaramiNejadRanjbar, M.; Hu, Z.; et al. An evolving story of the metastatic voyage of ovarian cancer cells: Cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 2018, 38, 2885–2898. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, A.; Takahashi, T.; Sawai, K.; Taniguchi, H.; Shimotsuma, M.; Okano, S.; Sakakura, C.; Tsujimoto, H.; Osaki, K.; Sasaki, S. Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res. 1993, 53, 687–692. [Google Scholar]
- Dux, K. Anatomy of the greater and lesser omentum in the mouse with some physiological implications. In The Omentum: Research and Clinical Applications; Goldsmith, H.S., Ed.; Springer: New York, NY, USA, 1990; pp. 19–43. [Google Scholar] [CrossRef]
- Havrlentová, L.; Faistová, H.; Mazur, M.; Humeňanská, A.; Polák, Š. Omentum majus and milky spots as an important part of the immune system. Rozhl. Chir. 2017, 96, 383–386. [Google Scholar] [PubMed]
- Clark, R.; Krishnan, V.; Schoof, M.; Rodriguez, I.; Theriault, B.; Chekmareva, M.; Rinker-Schaeffer, C. Milky Spots Promote Ovarian Cancer Metastatic Colonization of Peritoneal Adipose in Experimental Models. Am. J. Pathol. 2013, 183, 576–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, S.A.; Rybalko, V.Y.; Bigelow, C.E.; Lugade, A.A.; Foster, T.; Frelinger, J.G.; Lord, E.M. Preferential Attachment of Peritoneal Tumor Metastases to Omental Immune Aggregates and Possible Role of a Unique Vascular Microenvironment in Metastatic Survival and Growth. Am. J. Pathol. 2006, 169, 1739–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussios, S.; Karathanasi, A.; Cooke, D.; Neille, C.; Sadauskaite, A.; Moschetta, M.; Zakynthinakis-Kyriakou, N.; Pavlidis, N.; Kyriakou, Z. PARP Inhibitors in Ovarian Cancer: The Route to "Ithaca". Diagnostics 2019, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Tallapragada, S.; Schaar, B.; Kamat, K.; Chanana, A.M.; Zhang, Y.; Patel, S.; Parkash, V.; Rinker-Schaeffer, C.; Folkins, A.K.; et al. Omental macrophages secrete chemokine ligands that promote ovarian cancer colonization of the omentum via CCR1. Commun. Biol. 2020, 3, 1–13. [Google Scholar] [CrossRef]
- Ha, S.-A.; Tsuji, M.; Suzuki, K.; Meek, B.; Yasuda, N.; Kaisho, T.; Fagarasan, S. Regulation of B1 cell migration by signals through Toll-like receptors. J. Exp. Med. 2006, 203, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Lowery, E.; Braun, R.K.; Martín, A.; Huang, N.; Medina, M.; Sethupathi, P.; Seki, Y.; Takami, M.; Byrne, K.; et al. Cellular Basis of Tissue Regeneration by Omentum. PLoS ONE 2012, 7, e38368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litbarg, N.O.; Gudehithlu, K.P.; Sethupathi, P.; Arruda, J.A.L.; Dunea, G.; Singh, A.K. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res. 2007, 328, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murata, K.; Shibuya, H.; Morita, M.; Ishikawa, M.; Furu, M.; Ito, H.; Ito, J.; Matsuda, S.; Watanabe, T.; et al. A Distinct Human CD4+ T Cell Subset That Secretes CXCL13 in Rheumatoid Synovium. Arthritis Rheum. 2013, 65, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Moreno, J.; Moyron-Quiroz, J.E.; Carragher, D.; Kusser, K.; Hartson, L.; Moquin, A.; Randall, T.D. Omental Milky Spots Develop in the Absence of Lymphoid Tissue-Inducer Cells and Support B and T Cell Responses to Peritoneal Antigens. Immunity 2009, 30, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Duong, L.; Radley, H.; Lee, B.; Dye, D.; Pixley, F.; Grounds, M.D.; Nelson, D.; Jackaman, C. Macrophage function in the elderly and impact on injury repair and cancer. Immun. Ageing 2021, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Larbi, A. Markers of T Cell Senescence in Humans. Int. J. Mol. Sci. 2017, 18, 1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Zamudio, R.I.; Dewald, H.K.; Vasilopoulos, T.; Gittens-Williams, L.; Fitzgerald-Bocarsly, P.; Herbig, U. Conclusive Identification of Senescent T Cells Reveals Their Abundance in Aging Humans. bioRxiv 2020. [Google Scholar] [CrossRef]
- Macaulay, R.; Akbar, A.; Henson, S.M. The role of the T cell in age-related inflammation. AGE 2012, 35, 563–572. [Google Scholar] [CrossRef]
- Slaney, C.Y.; Kershaw, M.; Darcy, P.K. Trafficking of T Cells into Tumors. Cancer Res. 2014, 74, 7168–7174. [Google Scholar] [CrossRef] [Green Version]
- Saleh, R.; Nair, V.S.; Toor, S.M.; Taha, R.Z.; Murshed, K.; Al-Dhaheri, M.; Khawar, M.; Petkar, M.A.; Abu Nada, M.; Al-Ejeh, F.; et al. Differential gene expression of tumor-infiltrating CD8+ T cells in advanced versus early-stage colorectal cancer and identification of a gene signature of poor prognosis. J. Immunother. Cancer 2020, 8, e001294. [Google Scholar] [CrossRef]
- Hwang, W.-T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westergaard, M.; Andersen, R.; Chong, C.; Kjeldsen, J.W.; Pedersen, M.; Friese, C.; Hasselager, T.; Lajer, H.; Coukos, G.; Bassani-Sternberg, M.; et al. Tumour-reactive T cell subsets in the microenvironment of ovarian cancer. Br. J. Cancer 2019, 120, 424–434. [Google Scholar] [CrossRef] [Green Version]
- Haynes, L.; Eaton, S.M.; Burns, E.M.; Randall, T.D.; Swain, S.L. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc. Natl. Acad. Sci. USA 2003, 100, 15053–15058. [Google Scholar] [CrossRef] [Green Version]
- Linton, P.J.; Haynes, L.; Klinman, N.R.; Swain, S.L. Antigen-independent changes in naive CD4 T cells with aging. J. Exp. Med. 1996, 184, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Montfort, A.; Pearce, O.; Maniati, E.; Vincent, B.G.; Bixby, L.; Böhm, S.; Dowe, T.; Wilkes, E.H.; Chakravarty, P.; Thompson, R.; et al. A Strong B-cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases. Clin. Cancer Res. 2016, 23, 250–262. [Google Scholar] [CrossRef] [Green Version]
- Dumitriu, I.E. The life (and death) of CD4+CD28null T cells in inflammatory diseases. Immunology 2015, 146, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strioga, M.; Pasukoniene, V.; Characiejus, D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology 2011, 134, 17–32. [Google Scholar] [CrossRef]
- Yu, H.T.; Youn, J.-C.; Lee, J.; Park, S.; Chi, H.-S.; Lee, J.; Choi, C.; Park, S.; Choi, D.; Ha, J.-W.; et al. Characterization of CD8+CD57+ T cells in patients with acute myocardial infarction. Cell. Mol. Immunol. 2014, 12, 466–473. [Google Scholar] [CrossRef]
- Yi, H.-S.; Kim, S.Y.; Kim, J.T.; Lee, Y.-S.; Moon, J.S.; Kim, M.; Kang, Y.E.; Joung, K.H.; Lee, J.H.; Kim, H.J.; et al. T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callender, L.A.; Carroll, E.C.; Bober, E.; Akbar, A.N.; Solito, E.; Henson, S.M. Mitochondrial mass governs the extent of human T cell senescence. Aging Cell 2019, 19, e13067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farr, J.N.; Khosla, S. Cellular senescence in bone. Bone 2019, 121, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hoft, D.F.; Peng, G. Senescent T cells within suppressive tumor microenvironments: Emerging target for tumor immunotherapy. J. Clin. Investig. 2020, 130, 1073–1083. [Google Scholar] [CrossRef]
- Kasakovski, D.; Xu, L.; Li, Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J. Hematol. Oncol. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Lian, J.; Yue, Y.; Yu, W.; Zhang, Y.; Lian, J.; Yue, Y.; Yu, W.; Zhang, Y. Immunosenescence: A key player in cancer development. J. Hematol. Oncol. 2020, 13, 1–18. [Google Scholar] [CrossRef]
- Salas-Benito, D.; Eguren-Santamaria, I.; Sanmamed, M.F. Senescent T Cells as a Resistance Mechanism to Lung Cancer Immunotherapy. Clin. Cancer Res. 2020, 27, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, F.J.; Franzese, O.; Finney, H.M.; Fletcher, J.; Belaramani, L.L.; Salmon, M.; Dokal, I.; Webster, D.; Lawson, A.D.G.; Akbar, A.N. The Loss of Telomerase Activity in Highly Differentiated CD8+CD28−CD27− T Cells Is Associated with Decreased Akt (Ser473) Phosphorylation. J. Immunol. 2007, 178, 7710–7719. [Google Scholar] [CrossRef] [Green Version]
- Winterberg, P.; Ford, M.L. The effect of chronic kidney disease on T cell alloimmunity. Curr. Opin. Organ Transplant. 2017, 22, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-H.; Kim, S.R.; Han, D.H.; Yu, H.T.; Han, Y.; Kim, J.H.; Kim, S.H.; Lee, C.J.; Min, B.-H.; Kim, D.-H.; et al. Senescent T Cells Predict the Development of Hyperglycemia in Humans. Diabetes 2018, 68, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, R.P.; Mehta, A.K.; Perez, S.D.; Winterberg, P.; Cheeseman, J.; Johnson, B.; Kwun, J.; Monday, S.; Stempora, L.; Warshaw, B.; et al. Premature T Cell Senescence in Pediatric CKD. J. Am. Soc. Nephrol. 2016, 28, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shao, Q.; Peng, G. Exhaustion and senescence: Two crucial dysfunctional states of T cells in the tumor microenvironment. Cell. Mol. Immunol. 2019, 17, 27–35. [Google Scholar] [CrossRef]
- Ovadya, Y.; Landsberger, T.; Leins, H.; Vadai, E.; Gal, H.; Biran, A.; Yosef, R.; Sagiv, A.; Agrawal, A.; Shapira, A.; et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 2018, 9, 5435. [Google Scholar] [CrossRef] [Green Version]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, K.; Nagano, M.; Salazar, G.; Yamashita, T.; Tsuboi, I.; Mishima, H.; Matsushita, S.; Sato, F.; Yamagata, K.; Ohneda, O. The Role of CCL5 in the Ability of Adipose Tissue-Derived Mesenchymal Stem Cells to Support Repair of Ischemic Regions. Stem Cells Dev. 2014, 23, 488–501. [Google Scholar] [CrossRef] [Green Version]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The CCL5/CCR5 Axis in Cancer Progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Azenshtein, E.; Luboshits, G.; Shina, S.; Neumark, E.; Shahbazian, D.; Weil, M.; Wigler, N.; Keydar, I.; Ben-Baruch, A. The CC chemokine RANTES in breast carcinoma progression: Regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002, 62, 1093–1102. [Google Scholar]
- Mrowietz, U.; Schwenk, U.; Maune, S.; Bartels, J.; Küpper, M.; Fichtner, I.; Schröder, J.-M.; Schadendorf, D. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. Br. J. Cancer 1999, 79, 1025–1031. [Google Scholar] [CrossRef]
- An, G.; Wu, F.; Huang, S.; Feng, L.; Bai, J.; Gu, S.; Zhao, X. Effects of CCL5 on the biological behavior of breast cancer and the mechanisms of its interaction with tumor-associated macrophages. Oncol. Rep. 2019, 42, 2499–2511. [Google Scholar] [CrossRef]
- Cambien, B.; Richard-Fiardo, P.; Karimdjee, B.F.; Martini, V.; Ferrua, B.; Pitard, B.; Schmid-Antomarchi, H.; Schmid-Alliana, A. CCL5 Neutralization Restricts Cancer Growth and Potentiates the Targeting of PDGFRβ in Colorectal Carcinoma. PLoS ONE 2011, 6, e28842. [Google Scholar] [CrossRef]
- Wertel, I. Relationship between RANTES and dendritic cells in ovarian cancer patients. Front. Biosci. 2011, E3, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Walens, A.; DiMarco, A.V.; Lupo, R.; Kroger, B.; Damrauer, J.S.; Alvarez, J.V. CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Xie, R.; Xiang, T.; Zhao, Z.; Lin, S.; Liang, Z.; Chen, Z.; Zhu, B. Autocrine CCL5 Signaling Promotes Invasion and Migration of CD133+Ovarian Cancer Stem-Like Cells via NF-κB-Mediated MMP-9 Upregulation. Stem Cells 2012, 30, 2309–2319. [Google Scholar] [CrossRef]
- Kato, T.; Fujita, Y.; Nakane, K.; Mizutani, K.; Terazawa, R.; Ehara, H.; Kanimoto, Y.; Kojima, T.; Nozawa, Y.; Deguchi, T.; et al. CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3 prostate cancer cells by increasing secretion of MMPs 2/9 and by activating ERK and Rac signaling. Cytokine 2013, 64, 251–257. [Google Scholar] [CrossRef]
- Aldinucci, D.; Lorenzon, D.; Cattaruzza, L.; Pinto, A.; Gloghini, A.; Carbone, A.; Colombatti, A. Expression of CCR5 receptors on Reed–Sternberg cells and Hodgkin lymphoma cell lines: Involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int. J. Cancer 2007, 122, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wan, S.; Sun, L.; Hu, J.; Fang, D.; Zhao, R.; Yuan, S.; Zhang, L. Chemokine C-C motif receptor 5 and C-C motif ligand 5 promote cancer cell migration under hypoxia. Cancer Sci. 2012, 103, 904–912. [Google Scholar] [CrossRef]
- Swamydas, M.; Ricci, K.; Rego, S.L.; Dréau, D. Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor cell invasion and the activation of matrix metalloproteinases. Cell Adhes. Migr. 2013, 7, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Callejero, L.; Pérez-Martínez, L.; Rubio-Mediavilla, S.; Oteo, J.A.; Martínez, A.; Blanco, J.-R. Maraviroc, a CCR5 Antagonist, Prevents Development of Hepatocellular Carcinoma in a Mouse Model. PLoS ONE 2013, 8, e53992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Arnatt, C.K.; Zhang, F.; Wang, J.; Haney, K.M.; Fang, X. The potential role of anibamine, a natural product CCR5 antagonist, and its analogues as leads toward development of anti-ovarian cancer agents. Bioorganic Med. Chem. Lett. 2012, 22, 5093–5097. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Velázquez, M.; Jiao, X.; De La Fuente-Granada, M.; Pestell, T.G.; Ertel, A.; Lisanti, M.; Pestell, R.G. CCR5 Antagonist Blocks Metastasis of Basal Breast Cancer Cells. Cancer Res. 2012, 72, 3839–3850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasly, M.; Doronzo, G.; Cappello, P.; Valdembri, D.; Arese, M.; Mitola, S.; Moore, P.; Alessandri, G.; Giovarelli, M.; Bussolino, F. CCL16 activates an angiogenic program in vascular endothelial cells. Blood 2004, 103, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Zhang, X.; Tang, J.; Zhang, Z.; Du, R.; Luo, D.; Liu, X.; Xia, Y.; Li, Y.; Wang, S.; et al. CCL16 maintains stem cell-like properties in breast cancer by activating CCR2/GSK3β/β-catenin/OCT4 axis. Theranostics 2021, 11, 2297–2317. [Google Scholar] [CrossRef]
- Hwang, J.; Son, K.-N.; Kim, C.W.; Ko, J.; Na, D.S.; Kwon, B.S.; Gho, Y.S.; Kim, J. Human CC chemokine CCL23, a ligand for CCR1, induces endothelial cell migration and promotes angiogenesis. Cytokine 2005, 30, 254–263. [Google Scholar] [CrossRef]
- Son, K.-N.; Hwang, J.; Kwon, B.S.; Kim, J. Human CC chemokine CCL23 enhances expression of matrix metalloproteinase-2 and invasion of vascular endothelial cells. Biochem. Biophys. Res. Commun. 2006, 340, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Han, K.Y.; Kim, C.W.; Lee, T.H.; Son, Y.; Kim, J. CCL23 up-regulates expression of KDR/Flk-1 and potentiates VEGF-induced proliferation and migration of human endothelial cells. Biochem. Biophys. Res. Commun. 2009, 382, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Hara, I.; Horikawa, T.; Fujii, M.; Kurimoto, M.; Kamidono, S.; Ichihashi, M. Antitumor Effects on Mouse Melanoma Elicited by Local Secretion of Interleukin-12 and Their Enhancement by Treatment with Interleukin-18. Cancer Investig. 2000, 18, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Farnebo, J.; Kurimoto, M.; Cao, Y. Interleukin-18 acts as an angiogenesis and tumor suppressor. FASEB J. 1999, 13, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, Z.; Sarkar, S.H.; Li, Y.; Banerjee, S.; Saliganan, A.; Kim, H.-R.C.; Cher, M.L.; Sarkar, F.H. Platelet-Derived Growth Factor-D Overexpression Contributes to Epithelial-Mesenchymal Transition of PC3 Prostate Cancer Cells. Stem Cells 2008, 26, 1425–1435. [Google Scholar] [CrossRef] [Green Version]
- Ustach, C.V.; Taube, M.E.; Hurst, N.J.; Bhagat, S.; Bonfil, R.D.; Cher, M.L.; Schuger, L.; Kim, H.-R.C. A potential oncogenic activity of platelet-derived growth factor d in prostate cancer progression. Cancer Res. 2004, 64, 1722–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Fredriksson, L.; Li, X.; Eriksson, U. PDGF-D is a potent transforming and angiogenic growth factor. Oncogene 2003, 22, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Tong, R.; Cochran, D.; Jain, R.K. Blocking Platelet-Derived Growth Factor-D/Platelet-Derived Growth Factor Receptor β Signaling Inhibits Human Renal Cell Carcinoma Progression in an Orthotopic Mouse Model. Cancer Res. 2005, 65, 5711–5719. [Google Scholar] [CrossRef] [Green Version]
- Carlow, D.A.; Gold, M.; Ziltener, H.J. Lymphocytes in the Peritoneum Home to the Omentum and Are Activated by Resident Dendritic Cells. J. Immunol. 2009, 183, 1155–1165. [Google Scholar] [CrossRef] [Green Version]
- Arend, R.; Martinez, A.; Szul, T.; Birrer, M.J. Biomarkers in ovarian cancer: To be or not to be. Cancer 2019, 125, 4563–4572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, C.M.; Zhang, B.; Palmer, A.; Ogrodnik, M.; Pirtskhalava, T.; Thalji, N.M.; Hagler, M.; Jurk, D.; Smith, L.A.; Casaclang-Verzosa, G.; et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016, 15, 973–977. [Google Scholar] [CrossRef]
- Hickson, L.; Prata, L.L.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.-J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nat. Cell Biol. 2020, 583, 127–132. [Google Scholar] [CrossRef]
- Sears, J.D.; Waldron, K.J.; Wei, J.; Chang, C. Targeting metabolism to reverse T-cell exhaustion in chronic viral infections. Immunology 2020, 162, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Urpilainen, E.; Puistola, U.; Boussios, S.; Karihtala, P. Metformin and ovarian cancer: The evidence. Ann. Transl. Med. 2020, 8, 1711. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yang, J.; Wu, X.; Zhang, G.; Li, T.; Wang, X.; Zhang, H.; Wang, C.-C.; Liu, G.-H.; Wang, L. Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell 2018, 17, e12765. [Google Scholar] [CrossRef]
- Alard, E.; Butnariu, A.-B.; Grillo, M.; Kirkham, C.; Zinovkin, D.A.; Newnham, L.; Macciochi, J.; Pranjol, Z.I. Advances in Anti-Cancer Immunotherapy: Car-T Cell, Checkpoint Inhibitors, Dendritic Cell Vaccines, and Oncolytic Viruses, and Emerging Cellular and Molecular Targets. Cancers 2020, 12, 1826. [Google Scholar] [CrossRef]
| T Cell SASP | Cell Proliferation | Cell Migration | Tumour Invasion | Angiogenesis | Immune Suppression | Ref. |
|---|---|---|---|---|---|---|
| CCL5 | ↑ | ↑ | ↑ | ↑ | ↑ | [75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90] |
| CCL16 | n.d. | ↑ | n.d. | ↑ | n.d. | [91,92] |
| CCL23 | n.d. | ↑ | n.d. | ↑ | ↑ | [93,94,95] |
| IL-18 | ↓ | n.d. | n.d. | ↓ | n.d. | [96,97] |
| PDGF-D | ↑ | n.d. | ↑ | ↑ | n.d. | [98,99,100,101] |
| TNF-α | ↑ | n.d. | ↑ | ↑ | n.d. | [78,79,81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broadway, R.; Patel, N.M.; Hillier, L.E.; El-Briri, A.; Korneva, Y.S.; Zinovkin, D.A.; Pranjol, M.Z.I. Potential Role of Diabetes Mellitus-Associated T Cell Senescence in Epithelial Ovarian Cancer Omental Metastasis. Life 2021, 11, 788. https://doi.org/10.3390/life11080788
Broadway R, Patel NM, Hillier LE, El-Briri A, Korneva YS, Zinovkin DA, Pranjol MZI. Potential Role of Diabetes Mellitus-Associated T Cell Senescence in Epithelial Ovarian Cancer Omental Metastasis. Life. 2021; 11(8):788. https://doi.org/10.3390/life11080788
Chicago/Turabian StyleBroadway, Rhianne, Nikita M. Patel, Lucy E. Hillier, Amal El-Briri, Yulia S. Korneva, Dmitry A. Zinovkin, and Md Zahidul I. Pranjol. 2021. "Potential Role of Diabetes Mellitus-Associated T Cell Senescence in Epithelial Ovarian Cancer Omental Metastasis" Life 11, no. 8: 788. https://doi.org/10.3390/life11080788
APA StyleBroadway, R., Patel, N. M., Hillier, L. E., El-Briri, A., Korneva, Y. S., Zinovkin, D. A., & Pranjol, M. Z. I. (2021). Potential Role of Diabetes Mellitus-Associated T Cell Senescence in Epithelial Ovarian Cancer Omental Metastasis. Life, 11(8), 788. https://doi.org/10.3390/life11080788








