2.1. Case 1
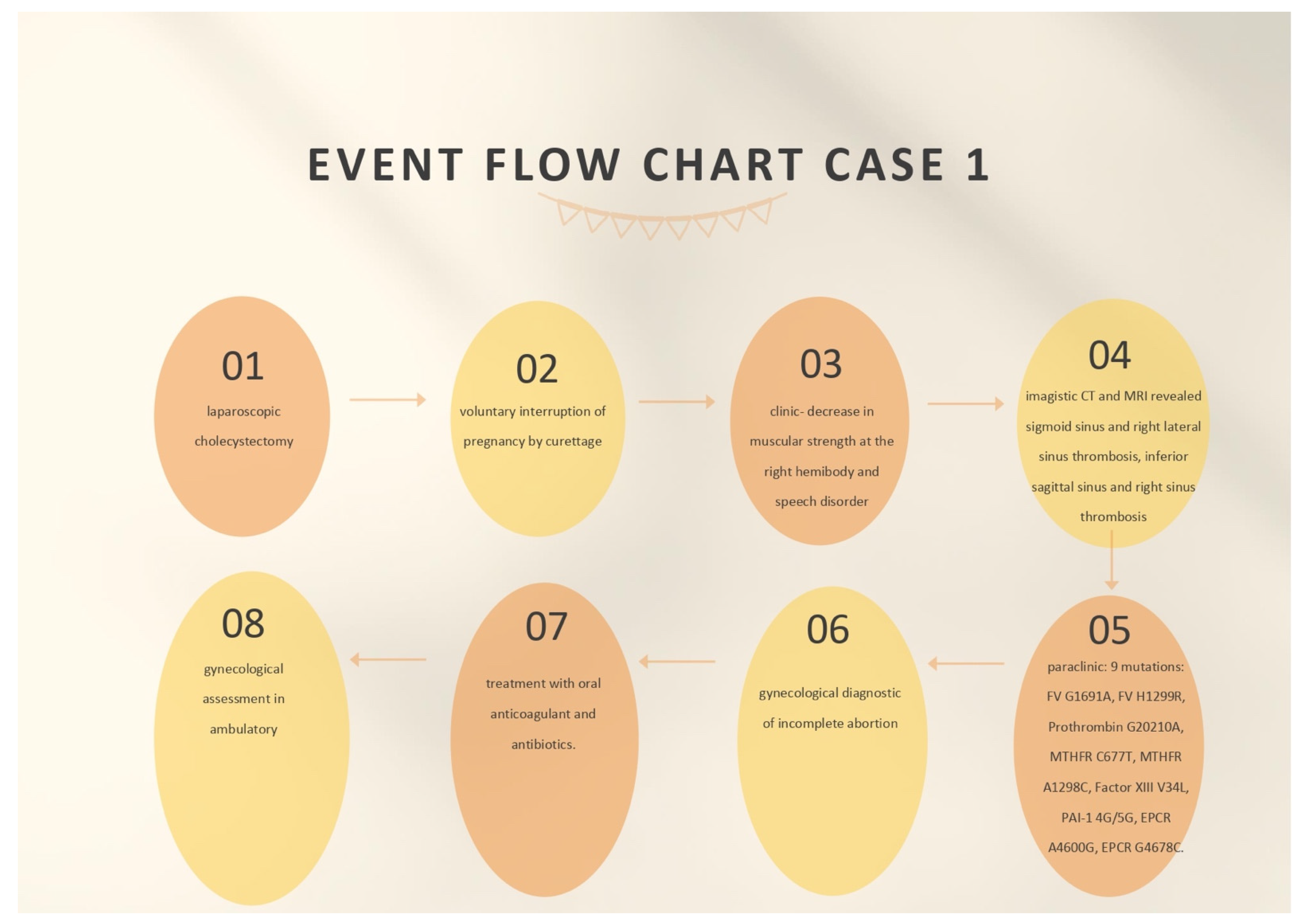
The first case was a female patient aged 40, known to be affected by laparoscopic cholecystectomy and abortion by curettage in ambulatory surgery. The patient was ad-mitted to the neurology department for a period of 11 days due to a decrease in muscular strength in the right hemibody and a speech disorder that onset during the same day.
At the emergency ward, the patient underwent a native and contrast-enhanced CT scan, which highlighted the following: the right temporal surface was most probably compatible, in terms of imaging, with encephalitis; a cranial cerebral MRI examination and a pulmonary radiography (X-ray), which did not reveal pleuro-pulmonary evolutive lesions, were recommended.
When admitted to the neurology department, during the neurological objective examination, the patient was conscious, barely cooperative, had expressive aphasia, had no stiffness in the back of the head, had normal ocular motricity, and had no nystagmus; further, the patient presented with right hemiparesis 3/5 equally distributed, and a cutaneous plantar reflex with bilateral flexion.
On the first day of hospitalization, the patient underwent a cerebral native and contrast-enhanced MRI and angiography, and a CT venography, which highlighted sigmoid sinus and right lateral sinus thrombosis, and inferior sagittal sinus and right sinus thrombosis, associated with right temporal cortical and subcortical subacute hemorrhage, supratentorial recent subacute synchronous lacunar infarct, (cytotoxic and vasogenic) thalamic–lenticular–caudal oedema, and supratentorial non-specific demyelinating lesions.
On the first day of hospitalization, biological material was collected in order to create the hereditary thrombosis profile, which was transmitted to the Clinical Service of Patho-logical Anatomy and tested to identify the mutations associated with cardiovascular disease and thrombophilia.
The following genotypes were identified: heterozygous for V factor mutation H1299R (R2) and heterozygous for mutation 4G of PAI-1. Moreover, the A1/A1 haplotype of EPCR was identified.
On the second day of admission, the patient underwent native thoracic CT and angiography for pulmonary arteries, without any images suggesting pulmonary thromboembolism.
On the same day (the second day of admission), the patient underwent a gynecological exam. The diagnosis of incomplete abortion was confirmed, and the patient was recommended to perform beta-human chorionic gonadotropin (hCG) after 48 h. The patient was recommended to take antibiotics (2 g intravenous ampicillin every 12 h and 80 g intravenous gentamicin every 12 h), and a nonsteroidal anti-inflammatory drug (75 mg diclofenac, one 0.2 mg tablet every 12 h) and to undergo reevaluation according to the results of the tests. Uterine curettage was recommended to be performed after neurological rebalancing of the patient.
During admission, on the fifth day of hospitalization, the patient underwent a cardiac assessment. She also had a blood pressure of 105/60 mmHg, a heart rate of 64 beats per minute, a normal electrocardiogram (ECG) with sinus rhythm, and angiography of the pulmonary arteries that infirmed pulmonary thromboembolism.
On the seventh day of admission, a gynecological reassessment was requested for the result of beta-hCG testing, and the investigation indicated a decrease in its value. Following a consultation, the patient was advised to continue therapy with antibiotics and to repeat the beta-hCG test after 7 days for a reassessment of the results.
On the ninth day, the gynecological reassessment indicated a paraclinical decrease in beta-hCG and the patient was recommended to repeat this test on a weekly basis until negative results were obtained and to have a gynecological assessment in ambulatory after 1 week. A hematological assessment was also requested on the ninth day of admission, which confirmed that the mutations detected in the hereditary thrombophilia profile fell into the low-risk class. It was recommended that the screening test for thrombophilia be intensified for C and S proteins, AT III, the dosage of serum homocysteine, and lupus anticoagulant in the ambulatory. The details of treating the patient and the findings can be seen in
Figure 1.
During her admission, the patient received brain depletive medication (20% mannitol, 250 mL every 12 h, for 5 days), hydro-electrolytic rebalancing (500 mL of normal saline solution every 12 h), heparin with a small molecular weight (0.4 mL enoxaparin sodium, one ampoule per day during the entire period of admission), cortico-steroid anti-inflammatory drugs (one ampoule of intravenous dexamethasone every 12 h for the first 2 days of admission), benzodiazepine (one ampoule of diazepam in 10 mL of a normal intravenous saline solution, given slowly in case of convulsive spasm), antibiotics (2 g intravenous ampicillin every 12 h, 80 mg intravenous gentamicin every 12 h), a non-steroidal anti-inflammatory (one ampoule of ketoprofen per day), a gastric protector (one 40 mg pantoprazole tablet per day), a probiotic enhancer (one 250 mg enterolum tablet twice a day, 2 h after the antibiotic), and an oral anticoagulant (4 mg acenocoumarol) as follows: 1/4 tablet on the seventh day of admission; 1/2 tablet per day on days 8 and 9; 3/4 tablet per day on days 10 and 11, with international normalized ratio (INR) control performed on a daily basis, in order to adjust the dose according to the INR. The patient recovered well.
At discharge, the patient continued the treatment with the oral anticoagulant (4 mg acenocoumarol as follows: 3/4 tablet alternating with 1 tablet per day, according to the INR dosage every 7, 14, and 21 days, then, on a monthly basis), to which a pain-killer was added (one tablet metamizole, if necessary), along with a brain trophic (one 400 mg Actovegin tablet, three times per day) and a dietary supplement with alpha-lipoic acid and a complex of vitamins.
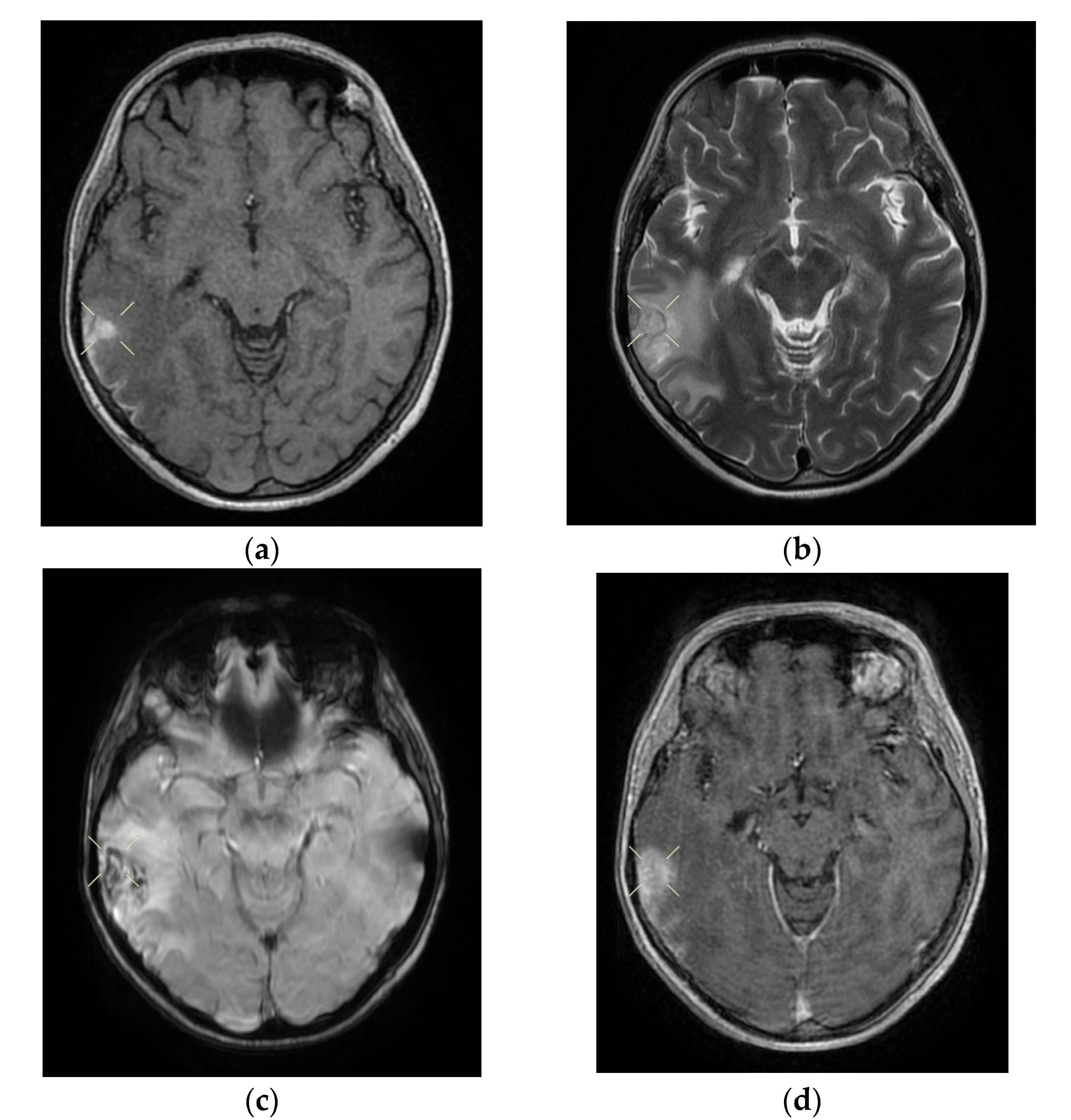
Upon discharge, the neurological examination indicated that the patient was conscious, cooperative, oriented in time and space, without stiffness in the back of the head, no nystagmus, with normal ocular motricity, denial of diplopia, possible deglutition for liquid and solid food, normal speech, no movement deficits, no sensitivity or coordination disorders, and a cutaneous plantar reflex with bilateral flexion. The images of the MRI can be seen below in
Figure 2.
2.2. Case 2
The second case we present was a 25-year-old female patient, with no pathological personal history, in the sixth day of puerperium after caesarian delivery, who came to the Emergency Unit of the Sf. Apostol Andrei Clinical Hospital in Constanta. The patient presented with cephalalgia, paresthesia of the right hemibody, a decrease in muscular strength in the right upper limb, and tonic–clonic convulsive spasms.
Following medical history, clinical examination and clinical and paraclinical investigations, it was decided that the patient should be admitted to the obstetrics and gynecology department, with the following diagnostics: 6-day puerperium after caesarian delivery, a mild form of secondary anemia and convulsion spasms to be investigated. She was oriented in time and space, was afebrile, had normally colored skin, had post-caesarian operation soft plaque in the process of healing (sutures removed), had a weak muscular system, was hypokinetic in the right upper and lower limbs, had a soft abdomen, and had a contracted uterus, had serosanguinous leaking, had supple breasts, was lactating, had a movement deficit in the right upper limb, with a lingual trauma mark.
The native cerebral CT scan highlighted a normality, with no detectable cranio-cerebral pathological modifications. The patient also underwent a native thorax CT and angiography for the pulmonary arteries, which did not indicate images suggestive of pulmonary thromboembolism.
A neurological consultation was requested when she was admitted to the gynecology department. When consulted, the patient was conscious, cooperative, oriented in time and space, had symmetrical pupils, was reactive on the median line, with a bitten tongue, showed right hemiparesis (upper limb 3/5 and lower limb 4/5) and had a bilaterally traced plantar cutaneous reflex. The diagnosis of tonic–clonic generalized convulsive spasm with tongue biting, and a post-critical status was given.
A cerebral and angiography MRI with venous time and reevaluation of the result was recommended, along with treatment with antiepileptic drugs (200 mg carbamazepine, 1/2 tablet per day; one intravenous ampoule of phenytoin 250 mg/5 mL, diluted in case of convulsive spasm), and a platelet antiaggregatory agent (one 75 mg aspenter tablet per day). Another neurological examination was requested on the same day, as the patient presented involuntary movements of the right lower limb. She received treatment with antiepileptic drugs (the dose was increased to one 200 mg carbamazepine tablet three times per day, plus one ampoule of intravenous phenytoin 250 mg/5 mL in 20 mL of normal saline solution), a cerebral depletive (20% mannitol, one 250 mL ampoule twice a day), a corticosteroid anti-inflammatory (one dexamethasone ampoule twice a day), a gastric protector (one 40 mg pantoprazole tablet per day), and heparin with a small molecular weight (one 0.6 mL enoxaparin sodium ampoule per day). The use of the steroid anti-inflammatory drugs in cerebral venous thrombosis was the choice of the treating physician.
On the second day of hospitalization, the patient underwent a native and contrast-enhanced cerebral MRI and angiography with arterial and venous sequence. The examination was suggestive of the left parietal cortical vein associated with ipsilateral superior parietal subcortical venous infarction and demyelinating lesions organized in the crown radiated and parietal on the right side, most probably with an ischemic vascular sublayer. This was the reason why the patient was transferred to the neurology department on the same day.
When admitted to the neurology department, on the second day of hospitalization, the patient was objectively examined and presented a good general health: a Glasgow Coma Scale (GCS) of 15 points, afebrile, conscious, cooperative, oriented in time and space, no deficit of the cranial nerves, a tongue with a traumatic trace, right hemiparesis 4/5 (predominantly brachial), right tactile hypesthesia; she denied any lost pregnancies, thrombophilia, or eclampsia.
On the third day of admission, biological material was drawn for the purpose of examining the hereditary thromboliphilia profile and was sent to the Clinical Service of Pathological Anatomy to be tested to identify mutations associated with cardiovascular disease and thrombophilia.
The test indicated the following genetic variants: heterozygous double genotype for the
C677T and
A1298C alleles in the
MTHFR gene, mutant homozygous for the
V34L allele in the
factor XIII gene, wild type homozygous genotype
5G/5G of the
PAI-1 gene and haplotype
A2/A2 (H2/H2) of
EPCR. The findings about and treatment of the patient can be seen in
Figure 3.
During admission to the neurology department, the patient was treated with a cerebral depletive (20% mannitol, 250 mL every 12 h for 5 days), hydro-electrolytic rebalancing (500 mL normal saline solution two times a day), heparin with low molecular weight (one 0.6 mL enoxaparin sodium ampoule every 12 h), painkillers (one metamizole ampoule every 12 h), antiepileptic medicines (one 200 mg carbamazepine tablet three times a day), a corticosteroid anti-inflammatory (dexamethasone, 1/2 ampoule every 12 h), benzodiazepine (one ampoule of intravenous diazepam slowly in 10 mL of normal saline solution, in case of convulsive spasm), a gastric protector (one 40 mg pantoprazole tablet per day), an oral anticoagulant (4 mg acenocoumarol, 1/2 tablet, according to the INR dosage; the patient was administered this medicine on the seventh day of admission). She recovered well.
When discharged, the patient continued to take oral anticoagulants (4 mg acenocoumarol, 1/2 tablet per day, according to the INR dosage on days 7 and 14, and on a monthly basis thereafter), antiepileptic medicines (200 mg carbamazepine, 1/2 tablet three times a day), and painkillers (one metamizole tablet if necessary).
When discharged, the patient was conscious, cooperative, oriented in time and space, without stiffness in the back of the head, had no nystagmus, showed normal ocular motricity, denied diplopia, had possible deglutition for liquid and solid food, displayed symmetric facies, had no movement deficits, had no superficial tactile sensitivity or coordination disorders and showed a cutaneous plantar reflex with bilateral flexion. The images of the MRI can be seen below in
Figure 4.