Resistance of Bacteria toward 475 nm Blue Light Exposure and the Possible Role of the SOS Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Chemicals
2.2. Culture Conditions
2.3. Light Source
2.4. Blue Light Treatment
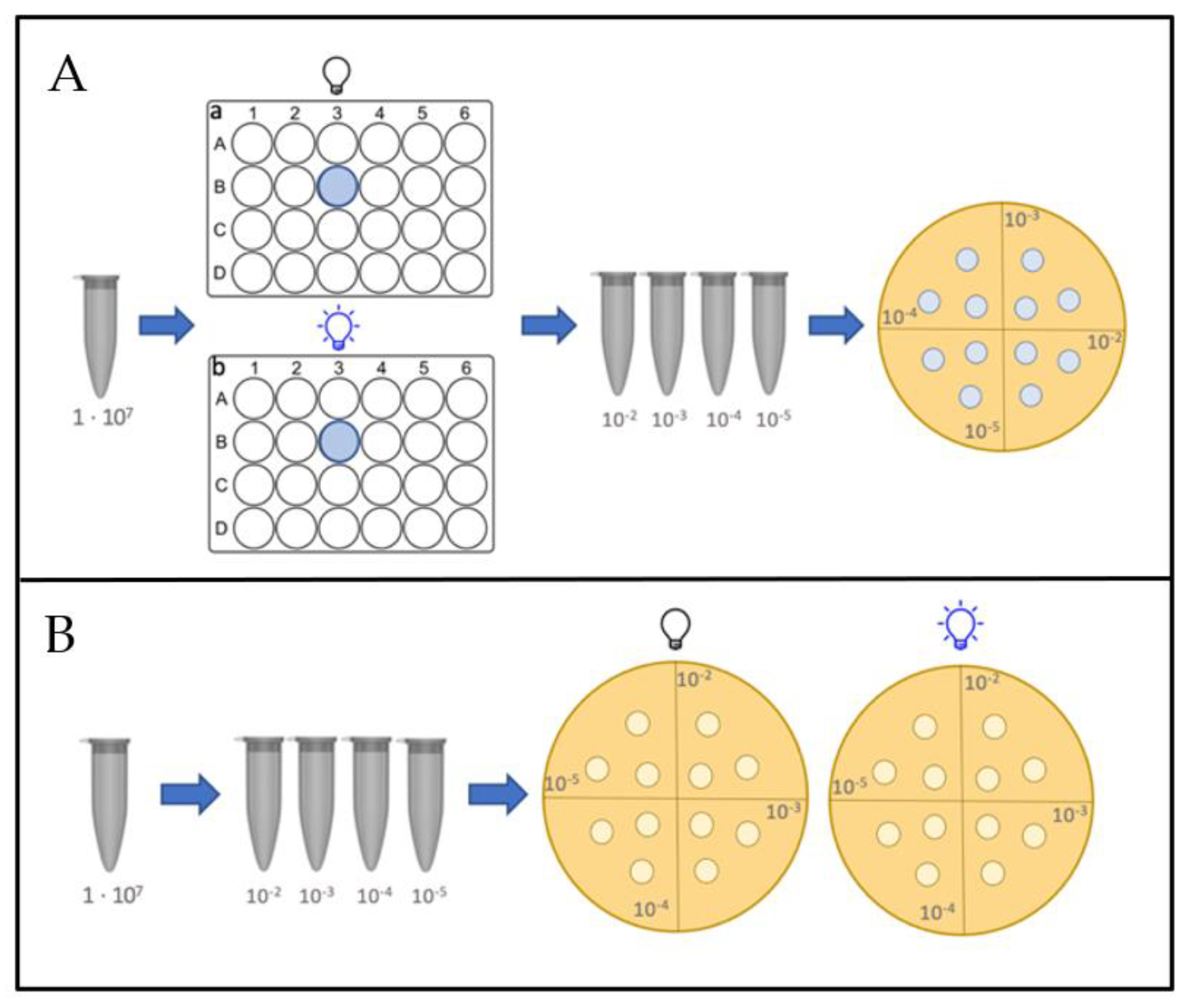
2.4.1. Planktonic Assays
2.4.2. Assays on Agar Plates
2.5. Testing the Role of RecA Inhibitors for Protection against 475 nm Blue Light
2.6. Insertion of a Plasmid Carrying the Gene RecA into RecA Negative (RecA−) Bacterial Strains and Subsequent Blue Light Exposure
2.7. Statistical Analysis and Calculation of Logarithmic Reduction Values
3. Results
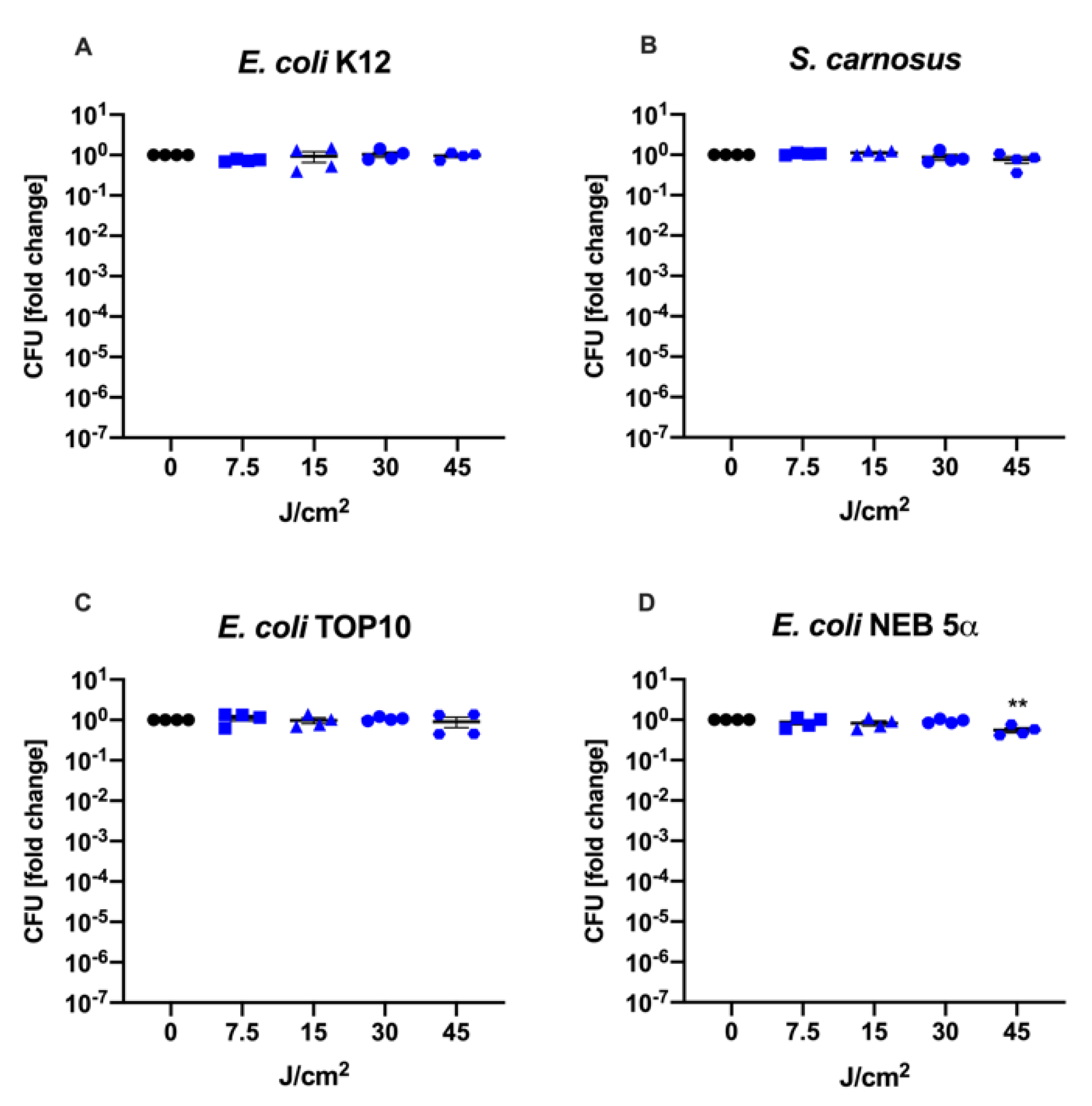
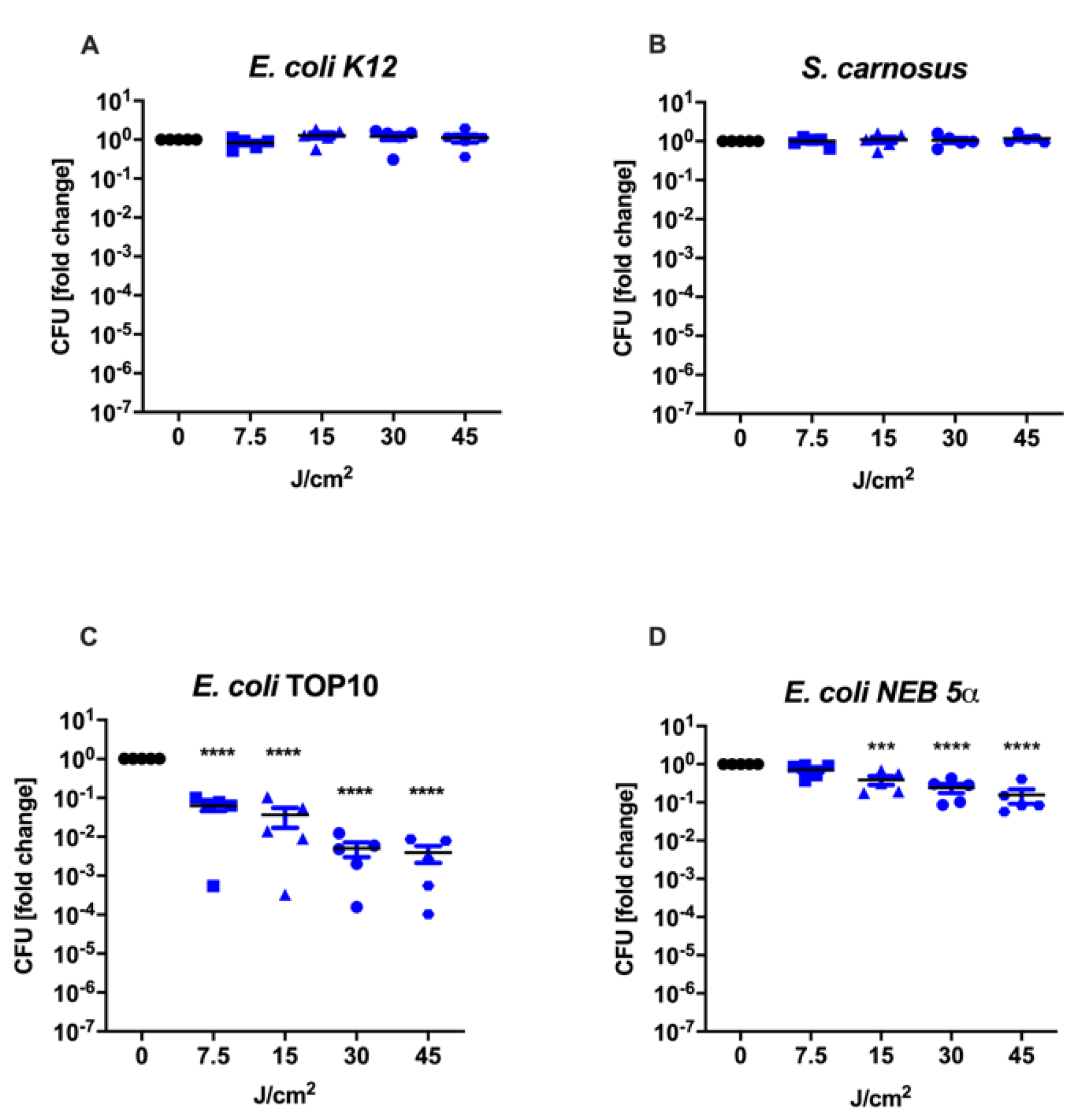
3.1. Culture Conditions during aBL Treatment Determine Therapy Outcome
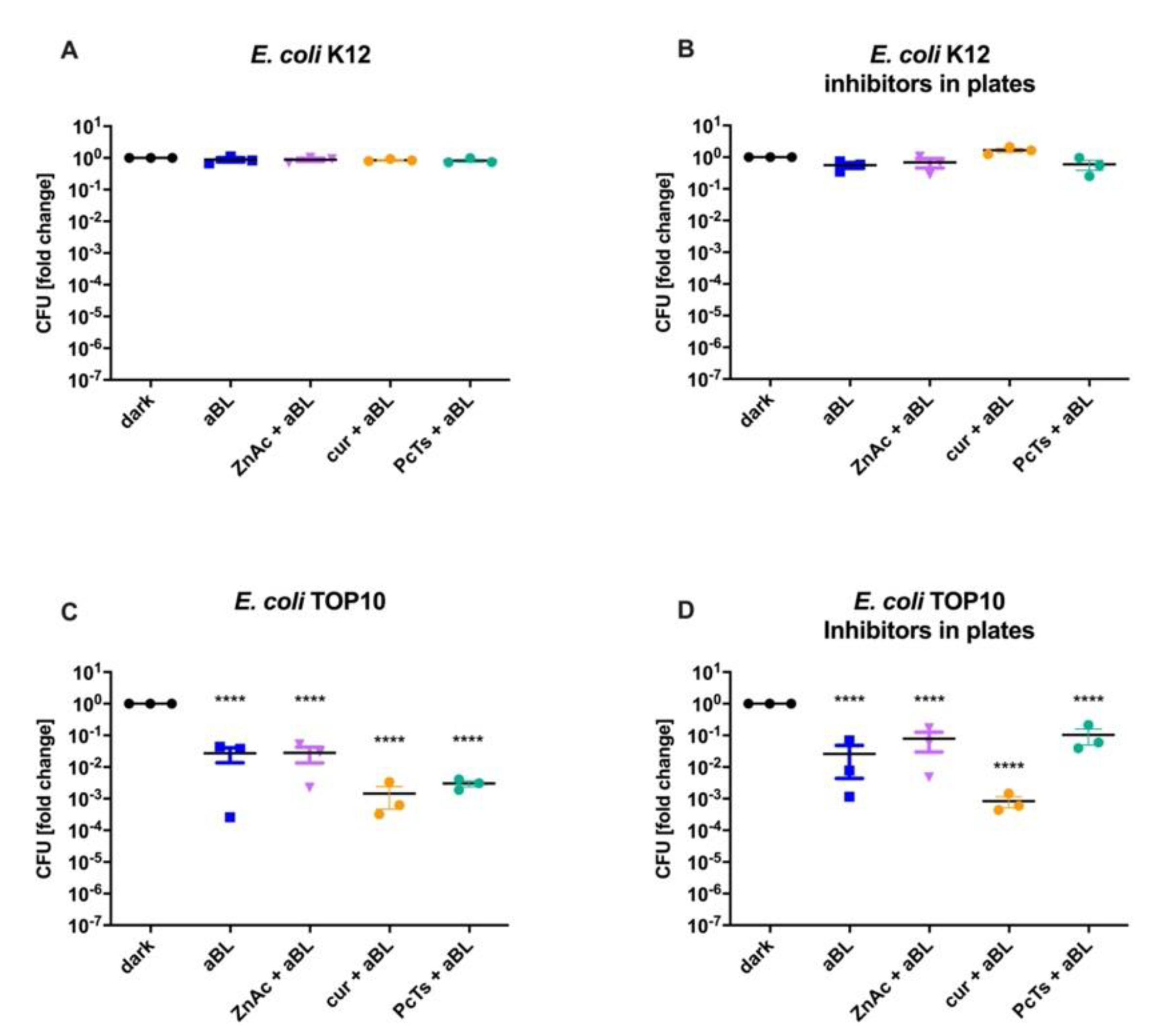
3.2. Resistance toward 475 nm Blue Light Was Not Affected by RecA Inhibitors (ZnAc, Curcumin or Fe(III)-PcTs)
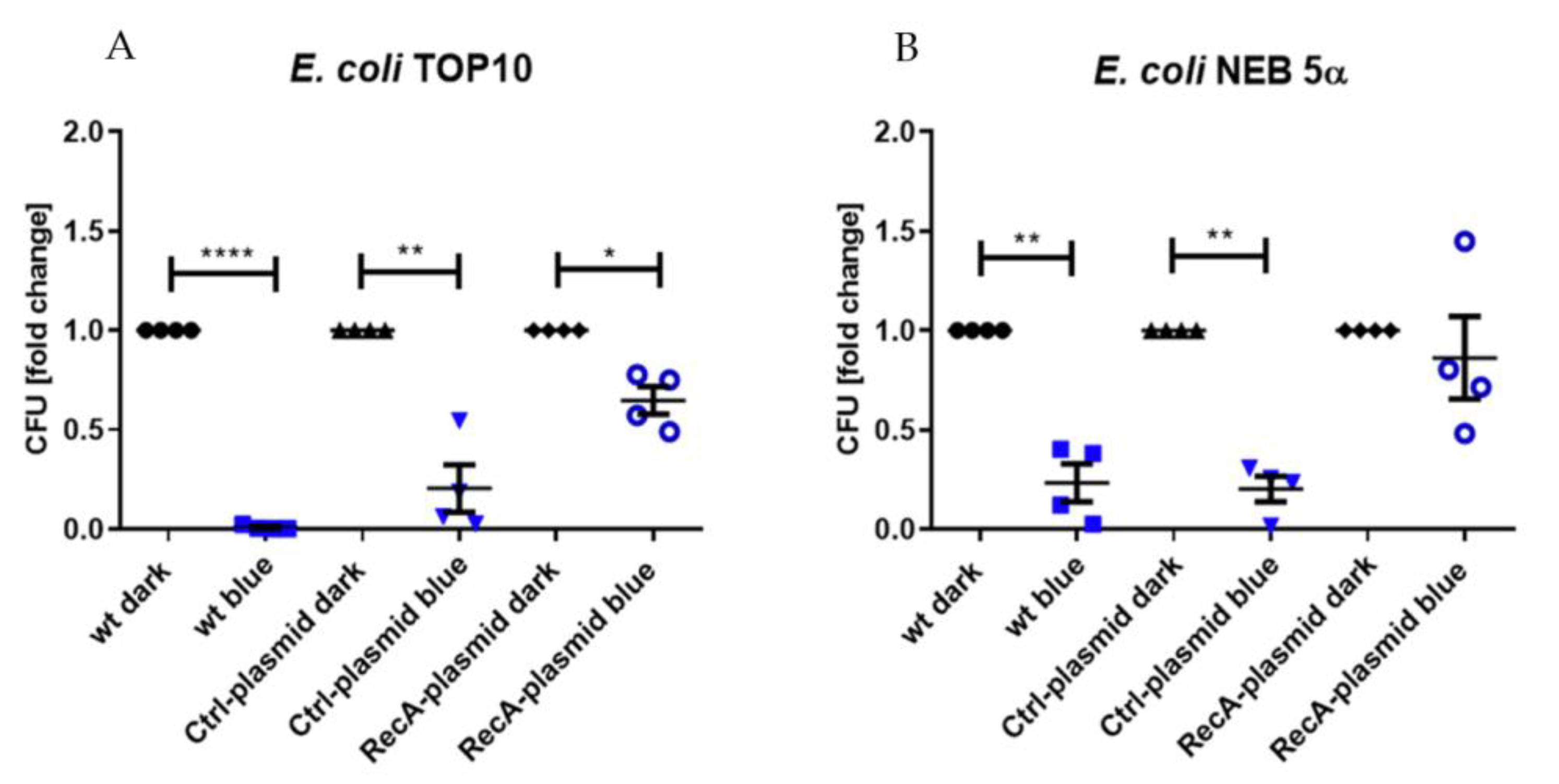
3.3. Prevention of aBL Toxicity by RecA Overexpression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control. Antibiotic Resistance Threats in the United States. 2019. Available online: www.cdc.gov/DrugResistance/Biggest-Threats.html (accessed on 3 August 2022).
- Ito, A.; Ito, T. Absorption spectra of deoxyribose, ribosephosphate, atp and dna by direct transmission measurements in the vacuum-uv (150—190 nm) and far-uv (190—260 nm) regions using synchrotron radiation as a light source. Photochem. Photobiol. 1986, 44, 355–358. [Google Scholar] [CrossRef]
- Kemmink, J.; Boelens, R.; Koning, T.M.G.; Kaptein, R.; Marel, G.A.; Boom, J.H. Conformational changes in the oligonucleotide duples d(GCGTTGCG). d (CGCAACGC) induced by formation of a cis-syn thymine dimer. A two-dimensional NMR study. Eur. J. Biochem. 1987, 162, 37–43. [Google Scholar] [CrossRef]
- Rycyna, R.E.; Alderfer, J.L. UV irradiation of nucleic acids: Formation, purification and solution conformational analysis of the ‘6-4 lesion’ of dTpdT. Nucleic Acids Res. 1985, 13, 5949–5963. [Google Scholar] [CrossRef]
- Mouret, S.; Philippe, C.; Gracia-Chantegrel, J.; Banyasz, A.; Karpati, S.; Markovitsi, D.; Douki, T. UVA-induced cyclobutane pyrimidine dimers in DNA: A direct photochemical mechanism? Org. Biomol. Chem. 2010, 8, 1706–1711. [Google Scholar] [CrossRef]
- Rideout, W.M.; Coetzee, G.A.; Olumi, A.F.; Jones, P.A. 5-Methylcytosine as an endogenous mutagen in the human LDL receptor and p53 genes. Science 1990, 249, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P.; Besaratinia, A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem. Photobiol. Sci. 2011, 11, 90–97. [Google Scholar] [CrossRef]
- Madson, J.G.; Lynch, D.T.; Tinkum, K.L.; Putta, S.K.; Hansen, L.A. Erbb2 regulates inflammation and proliferation in the skin after ultraviolet irradiation. Am. J. Pathol. 2006, 169, 1402–1414. [Google Scholar] [CrossRef]
- Berhane, T.; Halliday, G.M.; Cooke, B.; Barnetson, R.S.C. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br. J. Dermatol. 2002, 146, 810–815. [Google Scholar] [CrossRef]
- Fisher, M.S.; Kripke, M.L. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc. Natl. Acad. Sci. USA 1977, 74, 1688–1692. [Google Scholar] [CrossRef]
- Donawho, C.K.; Kripke, M.L. Evidence That the Local Effect of Ultraviolet Radiation on the Growth of Murine Melanomas Is Immunologically Mediated. Cancer Res. 1991, 51, 4176–4181. [Google Scholar]
- Cavanagh, L.L.; Halliday, G.M. Dendritic Epidermal T Cells in Ultraviolet-irradiated Skin Enhance Skin Tumor Growth by Inhibiting CD4+ T-Cell-mediated Immunity. Cancer Res. 1996, 56, 2607–2615. [Google Scholar] [PubMed]
- Bernstein, E.F.; Brown, D.B.; Urbach, F.; Forbes, D.; Monaco, M.D.; Wu, M.; Katchman, S.D.; Uitto, J. Ultraviolet radiation activates the human elastin promoter in transgenic mice: A novel in vivo and in vitro model of cutaneous photoaging. J. Investig. Dermatol. 1995, 105, 269–273. [Google Scholar] [CrossRef]
- Moloney, S.J.; Edmonds, S.H.; Giddens, L.D.; Learn, D.B. The Hairless Mouse Model of Photoaging: Evaluation of the Relationship between Dermal Elastin, Collagen, Skin Thickness and Wrinkles. Photochem. Photobiol. 1992, 56, 505–511. [Google Scholar] [CrossRef]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.p.; Halperin, A.J.; Pontén, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Conner-Kerr, T.A.; Sullivan, P.; Gaillard, J.-M.; Franklin, M.E.; Jones, R.M. The effects of ultraviolet radiation on antibiotic-resistant bacteria in vitro. Ostomy Wound Manag. 1998, 44, 50–56. [Google Scholar]
- Rao, B.K.; Kumar, P.; Rao, S.; Gurung, B. Bactericidal Effect of Ultraviolet C (UVC), Direct and Filtered Through Transparent Plastic, on Gram-Positive Cocci: An in vitro Study. Ostomy Wound Manag. 2011, 57, 46. [Google Scholar]
- Bak, J.; Ladefoged, S.D.; Tvede, M.; Begovic, T.; Gregersen, A. Dose requirements for UVC disinfection of catheter biofilms. Biofouling 2009, 25, 289–296. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Williams, D.; Hollosi, S.; Yens, D.; Enwemeka, S.K. Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg. Med. 2008, 40, 734–737. [Google Scholar] [CrossRef]
- Ashkenazi, H.; Malik, Z.; Harth, Y.; Nitzan, Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 2003, 35, 17–24. [Google Scholar] [CrossRef]
- Murdoch, L.E.; Maclean, M.; Endarko, E.; MacGregor, S.J.; Anderson, J.G. Bactericidal Effects of 405 nm Light Exposure Demonstrated by Inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium Species in Liquid Suspensions and on Exposed Surfaces. Sci. World J. 2012, 2012, 137805. [Google Scholar] [CrossRef]
- Ganz, R.A.; Veveiros, B.S.J.; Ahmad, A.; Ahmadi, A.; Khalil, A.; Tolkoff, M.S.J.; Nishioka, N.S.; Hamblin, M.R. Helicobacter pylori in patients can be killed by visible light. Lasers Surg. Med. 2005, 36, 260–265. [Google Scholar] [CrossRef]
- Halstead, F.D.; Thwaite, J.E.; Burt, R.; Laws, T.R.; Raguse, M.; Moeller, R.; Webber, M.A.; Oppenheima, B.A. Antibacterial activity of blue light against nosocomial wound pathogens growing planktonically and as mature biofilms. Appl. Environ. Microbiol. 2016, 82, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Dungel, P.; Mittermayr, R.; Haindl, S.; Osipov, A.; Wagner, C.; Redl, H.; Kozlov, A.V. Illumination with blue light reactivates respiratory activity of mitochondria inhibited by nitric oxide, but not by glycerol trinitrate. Arch. Biochem. Biophys. 2008, 471, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Dungel, P.; Hartinger, J.; Chaudary, S.; Slezak, P.; Hofmann, A.; Hausner, T.; Strassl, M.; Wintner, E.; Redl, H.; Mittermayr, R. Low level light therapy by LED of different wavelength induces angiogenesis and improves ischemic wound healing. Lasers Surg. Med. 2014, 46, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Eichler, M.; Lavi, R.; Shainberg, A.; Lubart, R. Flavins are source of visible-light-induced free radical formation in cells. Lasers Surg. Med. 2005, 37, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Dungel, P.; Hartinger, J.; Chaudary, S.; Slezak, P.; Hofmann, A.; Hausner, T.; Strassl, M.; Wintner, E.; Redl, H.; Mittermayr, R. Phototargeting oral black-pigmented bacteria. Antimicrob. Agents Chemother. 2005, 49, 1391–1396. [Google Scholar]
- McDonald, R.; MacGregor, S.J.; Anderson, J.G.; Maclean, M.; Grant, M.H. Effect of 405-nm high-intensity narrow-spectrum light on fibroblast-populated collagen lattices: An in vitro model of wound healing. J. Biomed. Opt. 2011, 16, 048003. [Google Scholar] [CrossRef] [PubMed]
- Kleinpenning, M.M.; Smits, T.; Frunt, M.H.A.; van Erp, P.E.J.; van de Kerkhof, P.C.M.; Gerritsen, R.M.J.P. Clinical and histological effects of blue light on normal skin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 16–21. [Google Scholar] [CrossRef]
- Costa, R.M.A.; Chiganças, V.; Galhardo, R.D.S.; Carvalho, H.; Menck, C.F.M. The eukaryotic nucleotide excision repair pathway. Biochimie 2003, 85, 1083–1099. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.E.; Escobar, J.F.; Bair, K.L.; Sutton, M.D.; Crane, J.K. Zinc blocks SOS-induced antibiotic resistance via inhibition of RecA in Escherichia coli. PLoS ONE 2017, 12, e0178303. [Google Scholar] [CrossRef]
- Alam, M.K.; Alhhazmi, A.; Decoteau, J.F.; Luo, Y.; Geyer, C.R. RecA Inhibitors Potentiate Antibiotic Activity and Block Evolution of Antibiotic Resistance. Cell Chem. Biol. 2016, 23, 381–391. [Google Scholar] [CrossRef]
- Bellio, P.; Brisdelli, F.; Perilli, M.; Sabatini, A.; Bottoni, C.; Segatore, B.; Setacci, D.; Amicosante, G.; Celenza, G. Curcumin inhibits the SOS response induced by levofloxacin in Escherichia coli. Phytomedicine 2014, 21, 430–434. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Guffey, J.S.; Payne, W.; Jones, T.; Martin, K. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomed. Laser Surg. 2013, 31, 179–182. [Google Scholar] [CrossRef]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Pieranski, M.; Woziwodzka, A.; Bielawski, K.P.; Grinholc, M. Development of Staphylococcus aureus tolerance to antimicrobial photodynamic inactivation and antimicrobial blue light upon sub-lethal treatment. Sci. Rep. 2019, 9, 1–18. [Google Scholar]
- Alcántara-Díaz, D.; Breña-Valle, M.; Serment-Guerrero, J. Divergent adaptation of Escherichia coli to cyclic ultraviolet light exposures. Mutagenesis 2004, 19, 349–354. [Google Scholar] [CrossRef]
- Adamskaya, N.; Dungel, P.; Mittermayr, R.; Hartinger, J.; Feichtinger, G.; Wassermann, K.; Redl, H.; van Griensven, M. Light therapy by blue LED improves wound healing in an excision model in rats. Injury 2011, 42, 917–921. [Google Scholar] [CrossRef]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef]
- Opländer, C.; Hidding, S.; Werners, F.B.; Born, M.; Pallua, N.; Suschek, C.V. Effects of blue light irradiation on human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2011, 103, 118–125. [Google Scholar] [CrossRef]
- Krassovka, J.M.; Suschek, C.V.; Prost, M.; Grotheer, V.; Schiefer, J.L.; Demir, E.; Fuchs, P.C.; Windolf, J.; Stürmer, E.K.; Opländer, C. The impact of non-toxic blue light (453 nm) on cellular antioxidative capacity, TGF-β1 signaling, and myofibrogenesis of human skin fibroblasts. J. Photochem. Photobiol. B. 2020, 209, 111952. [Google Scholar] [CrossRef]
- Fyrestam, J.; Bjurshammar, N.; Paulsson, E.; Mansouri, N.; Johannsen, A.; Östman, C. Influence of culture conditions on porphyrin production in Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis. Photodiagnosis Photodyn. Ther. 2017, 17, 115–123. [Google Scholar] [CrossRef]
- Rieble, S.; Ormerod, J.G.; Beale, S.I. Transformation of glutamate to delta-aminolevulinic acid by soluble extracts of Chlorobium vibrioforme. J. Bacteriol. 1989, 171, 3782–3787. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, H.; Pavletich, N.P. Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures. Nature 2008, 453, 489–494. [Google Scholar] [CrossRef]
- Friedman, N.; Vardi, S.; Ronen, M.; Alon, U.; Stavans, J. Precise temporal modulation in the response of the SOS DNA repair network in individual bacteria. PLoS Biol. 2005, 3, 1261–1268. [Google Scholar] [CrossRef]
- Little, J.W. Mechanism of specific LexA cleavage: Autodigestion and the role of RecA coprotease. Biochimie 1991, 73, 411–421. [Google Scholar] [CrossRef]
- Horii, T.; Ogawa, T.; Nakatani, T.; Hase, T.; Matsubara, H.; Ogawa, H. Regulation of SOS functions: Purification of E. coli LexA protein and determination of its specific site cleaved by the RecA protein. Cell 1981, 27, 515–522. [Google Scholar] [CrossRef]
- Lewis, L.K.; Harlow, G.R.; Gregg-Jolly, L.A.; Mount, D.W. Identification of high affinity binding sites for LexA which define new DNA damage-inducible genes in Escherichia coli. J. Mol. Biol. 1994, 241, 507–523. [Google Scholar] [CrossRef]
- Cohen, S.E.; Foti, J.J.; Simmons, L.A.; Walker, G.C. The SOS Regulatory Network. EcoSal Plus 2008, 3. [Google Scholar] [CrossRef]
- Sassanfar, M.; Roberts, J.W. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J. Mol. Biol. 1990, 212, 79–96. [Google Scholar] [CrossRef]
- Maiques, E.; Ubeda, C.; Campoy, S.; Salvador, N.; Lasa, I.; Novick, R.P.; Barbe, J.; Penades, J.R. β-lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in Staphylococcus aureus. J. Bacteriol. 2006, 188, 2726–2729. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Thomsen, L.E.; Gaggero, C.; Mosseri, R.; Ingmer, H.; Cohen, S.N. SOS response induction by β-lactams and bacterial defense against antibiotic lethality. Science 2004, 305, 1629–1631. [Google Scholar] [CrossRef]
- Aksenov, S.V. Induction of the SOS Response in Ultraviolet-Irradiated Escherichia coli Analyzed by Dynamics of LexA, RecA and SulA Proteins. J. Biol. Phys. 1999, 25, 263–277. [Google Scholar] [CrossRef]
- Rodríguez-Rosado, A.I.; Valencia, E.Y.; Rodríguez-Rojas, A.; Costas, C.; Galhardo, R.S.; Blázquez, J.; Rodríguez-Beltrán, J. Reactive oxygen species are major contributors to SOS-mediated mutagenesis induced by fluoroquinolones. BioRxiv 2018. [Google Scholar]
- Campoy, S.; Hervàs, A.; Busquets, N.; Erill, I.; Teixidó, L.; Barbé, J. Induction of the SOS response by bacteriophage lytic development in Salmonella enterica. Virology 2016, 351, 360–367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, M.; Shen, X.; Frank, E.G.; O’Donnell, M.; Woodgate, R.; Goodman, M.F. UmuD’2C is an error-prone DNA polymerase, Escherichia coli pol V. Proc. Natl. Acad. Sci. USA 1999, 96, 8919–8924. [Google Scholar] [CrossRef]
- Beaber, J.W.; Hochhut, B.; Waldor, M.K. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 2004, 427, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Sterer, N.; Jeffet, U.; Dadoun, A.; Greenstein, R.B.N.; Kohavi, D. Zinc enhances the phototoxic effect of blue light against malodour-producing bacteria in an experimental oral biofilm. J. Med. Microbiol. 2014, 63, 1071–1075. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oda, Y. Inhibitory effect of curcumin on SOS functions induced by UV irradiation. Mutat. Res. Lett. 1995, 348, 67–73. [Google Scholar] [CrossRef]
- Nautiyal, A.; Patil, K.N.; Muniyappa, K. Suramin is a potent and selective inhibitor of Mycobacterium tuberculosis RecA protein and the SOS response: RecA as a potential target for antibacterial drug discovery. J. Antimicrob. Chemother. 2014, 69, 1834–1843. [Google Scholar] [CrossRef]
- Kogut, M.; Harris, M. Effects of Streptomycin in Bacterial Cultures Growing at Different Rates; Interaction with Bacterial Ribosomes in vivo. Eur. J. Biochem. 1969, 9, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Cozens, R.M.; Tuomanen, E.; Tosch, W.; Zak, O.; Suter, J.; Tomasz, A. Evaluation of the bactericidal activity of β-lactam antibiotics on slowly growing bacteria cultured in the chemostat. Antimicrob. Agents Chemother. 1986, 29, 797–802. [Google Scholar] [CrossRef]
- Eng, R.H.K.; Padberg, F.T.; Smith, S.M.; Tan, E.N.; Cherubin, C.E. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob. Agents Chemother. 1991, 35, 1824–1828. [Google Scholar] [CrossRef]

| Bacterial Strain | Functional Copy of the Gene recA |
|---|---|
| E. coli K12 | + |
| E. coli TOP10 | − |
| E. coli NEB 5α | − |
| S. carnosus | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metzger, M.; Hacobian, A.; Karner, L.; Krausgruber, L.; Grillari, J.; Dungel, P. Resistance of Bacteria toward 475 nm Blue Light Exposure and the Possible Role of the SOS Response. Life 2022, 12, 1499. https://doi.org/10.3390/life12101499
Metzger M, Hacobian A, Karner L, Krausgruber L, Grillari J, Dungel P. Resistance of Bacteria toward 475 nm Blue Light Exposure and the Possible Role of the SOS Response. Life. 2022; 12(10):1499. https://doi.org/10.3390/life12101499
Chicago/Turabian StyleMetzger, Magdalena, Ara Hacobian, Lisa Karner, Leonie Krausgruber, Johannes Grillari, and Peter Dungel. 2022. "Resistance of Bacteria toward 475 nm Blue Light Exposure and the Possible Role of the SOS Response" Life 12, no. 10: 1499. https://doi.org/10.3390/life12101499
APA StyleMetzger, M., Hacobian, A., Karner, L., Krausgruber, L., Grillari, J., & Dungel, P. (2022). Resistance of Bacteria toward 475 nm Blue Light Exposure and the Possible Role of the SOS Response. Life, 12(10), 1499. https://doi.org/10.3390/life12101499






