Novel Apparatuses for Incorporating Natural Selection Processes into Origins-of-Life Experiments to Produce Adaptively Evolving Chemical Ecosystems
Abstract
:1. Introduction
1.1. Wet/Dry Cycles
1.2. Freeze/Thaw Cycles
1.3. Ultraviolet Light/Dark Cycles
1.4. Catalytic Surfaces
1.5. Combining Selection Processes
1.6. Thermal Vents
1.7. Introduction Summary
2. Materials and Methods
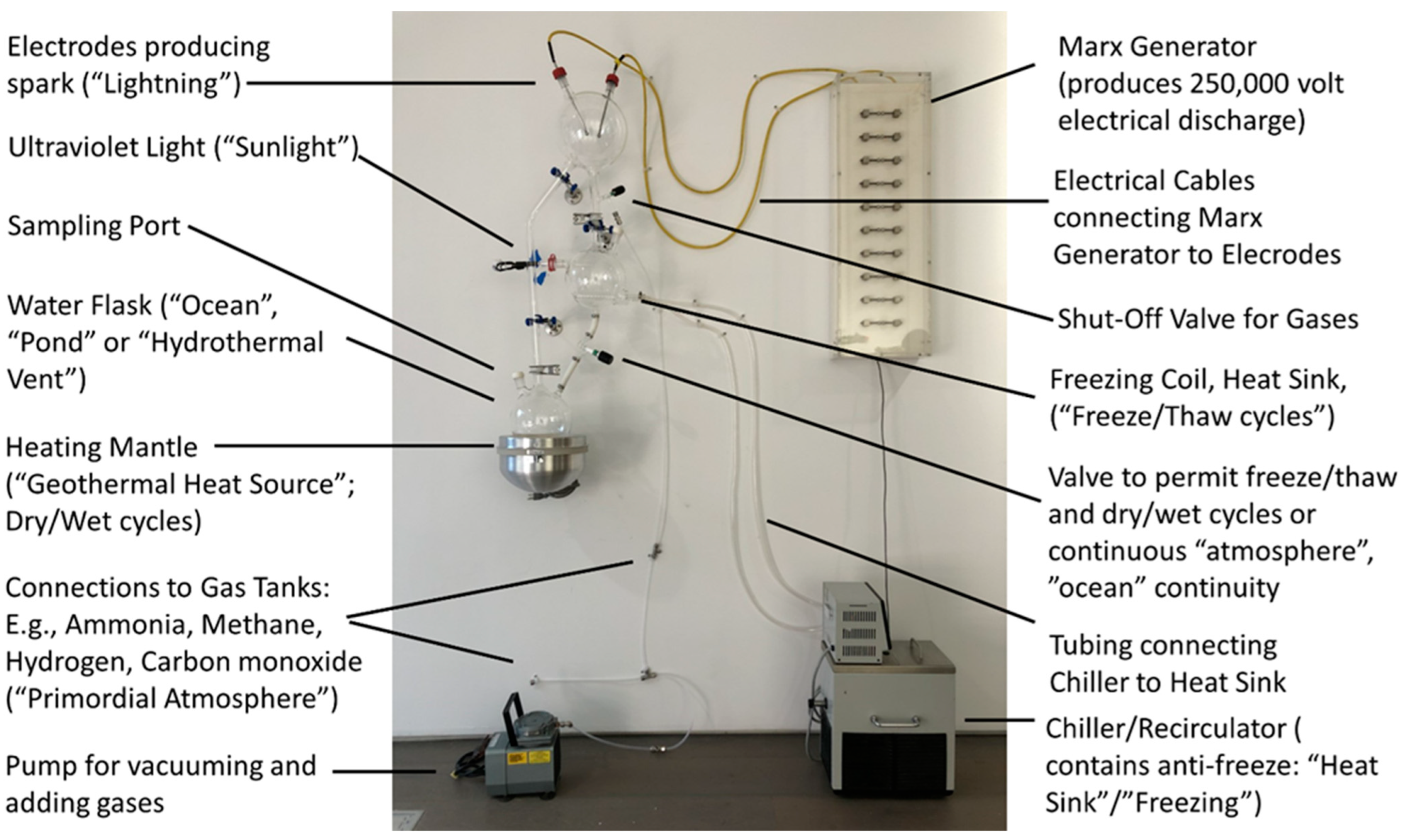
2.1. ReBioGeneSys 1.0
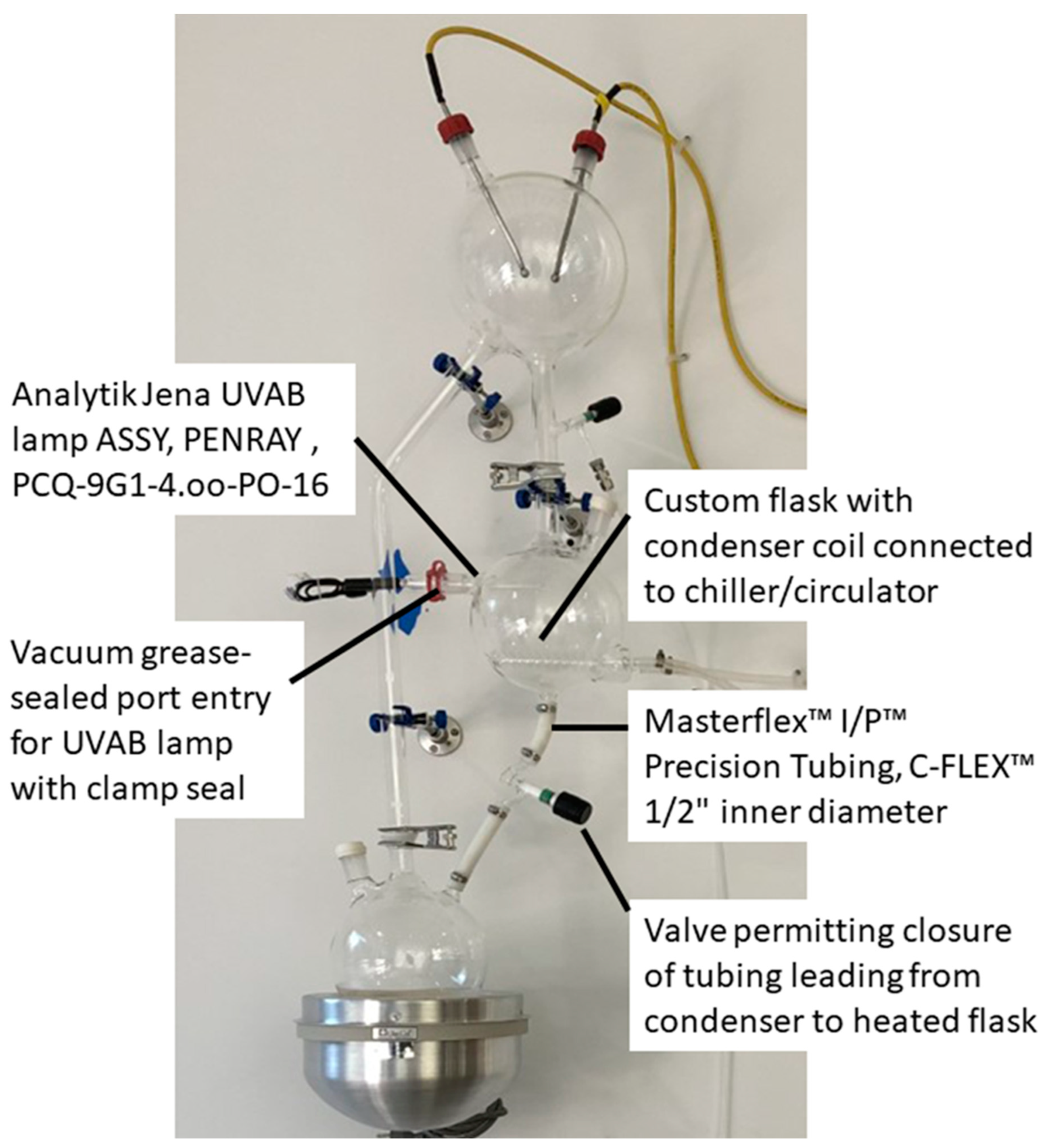
2.2. ReBioGeneSys 2.0
3. Results
3.1. Tests of the Apparatuses
3.2. Limitations of the Apparatuses
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, S.L. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef]
- Bada, J.L. New insights into prebiotic chemistry from Stanley Miller’s spark discharge experiments. Chem. Soc. Rev. 2013, 42, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, P.B.; Thompson, S.J.; Xu, J.; Russell, D.A.; Green, N.J.; Ritson, D.J.; Sutherland, J.D.; Queloz, D.P. Timescales for Prebiotic Photochemistry Under Realistic Surface Ultraviolet Conditions. Astrobiology 2021, 21, 1099–1120. [Google Scholar] [CrossRef]
- Fox, S.; Pleyer, H.L.; Strasdeit, H. An automated apparatus for the simulation of prebiotic wet-dry cycles under strictly anaerobic conditions. Int. J. Astrobiol. 2019, 18, 60–72. [Google Scholar] [CrossRef]
- Parker, E.T.; Cleaves, J.H.; Burton, A.S.; Glavin, D.P.; Dworkin, J.P.; Zhou, M.; Bada, J.L.; Fernández, F.M. Conducting Miller-Urey Experiments. J. Vis. Exp. 2014, 83, e51039. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; Menor-Salván, C.; Osuna-Esteban, S.; Veintemillas-Verdaguer, S. Prebiotic Microreactors: A Synthesis of Purines and Dihydroxy Compounds in Aqueous Aerosol. Orig. Life Evol. Biosph. 2007, 37, 123–142. [Google Scholar] [CrossRef]
- Dobson, C.M.; Ellison, G.B.; Tuck, A.F.; Vaida, V. Atmospheric aerosols as prebiotic chemical reactors. Proc. Natl. Acad. Sci. USA 2000, 97, 11864–11868. [Google Scholar] [CrossRef]
- Imai, E.-I.; Honda, H.; Hatori, K.; Brack, A.; Matsuno, K. Elongation of Oligopeptides in a Simulated Submarine Hydrothermal System. Science 1999, 283, 831–833. [Google Scholar] [CrossRef]
- Baum, D.A.; Vetsigian, K. An Experimental Framework for Generating Evolvable Chemical Systems in the Laboratory. Orig. Life Evol. Biosph. 2017, 47, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Godfrey-Smith, P. Conditions for Evolution by Natural Selection. J. Philos. 2007, 104, 489–516. Available online: https://www.petergodfreysmith.com/ConditionsNS-07-JP-web.pdf (accessed on 18 September 2022). [CrossRef]
- Dennett, D.; Sterelny, K.; Queller, D.; Godfrey-Smith, P. Darwinian Populations and Natural Selection. No Date. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiF5NDUzKH6AhV4kGoFHSJBATMQFnoECA8QAQ&url=https%3A%2F%2Fpetergodfreysmith.com%2FAgents_Acacias_PGS.pdf&usg=AOvVaw0m-0y1HUwpB-PQwNxYNZ4k (accessed on 18 September 2022).
- Vincent, L.; Berg, M.; Krismer, M.; Saghafi, S.T.; Cosby, J.; Sankari, T.; Vetsigian, K.; Cleaves, H.J., II; Baum, D.A. Chemical Ecosystem Selection on Mineral Surfaces Reveals Long-Term Dynamics Consistent with the Spontaneous Emergence of Mutual Catalysis. Life 2019, 9, 80. [Google Scholar] [CrossRef]
- Colón-Santos, S.; Cooper, G.J.T.; Cronin, L.; Cronin, L. Taming the Combinatorial Explosion of the Formose Reaction via Recursion within Mineral Environments. ChemSystemsChem 2019, 1, e1900014. [Google Scholar] [CrossRef]
- Eigen, M.; Schuster, P. The hypercycle. A principle of natural self-organization. Part A, Emergence of the hypercycle. Die Nat. 1977, 64, 541–565. [Google Scholar] [CrossRef]
- Hordijk, W.; Steel, M.; Kauffman, S.A. Molecular Diversity Required for the Formation of Autocatalytic Sets. Life 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.S.; Dillon, P.F. Molecular Complementarity I: The Complementarity Theory of the Origin and Evolution of Life. J. Theor. Biol. 1997, 188, 447–479. [Google Scholar] [CrossRef] [PubMed]
- Hunding, A.; Kepes, F.; Lancet, D.; Minsky, A.; Norris, V.; Raine, D.; Sriram, K.; Root-Bernstein, R. Compositional complementarity and prebiotic ecology in the origin of life. BioEssays 2006, 28, 399–412. [Google Scholar] [CrossRef]
- Charlat, S.; Ariew, A.; Bourrat, P.; Ruiz, M.F.; Heams, T.; Huneman, P.; Krishna, S.; Lachmann, M.; Lartillot, N.; D’Hendecourt, L.L.S.; et al. Natural Selection beyond Life? A Workshop Report. Life 2021, 11, 1051. [Google Scholar] [CrossRef]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Chemical roots of biological evolution: The origins of life as a process of development of autonomous functional systems. Open Biol. 2017, 7, 170050. [Google Scholar] [CrossRef]
- Jia, T.; Caudan, M.; Mamajanov, I. Origin of Species before Origin of Life: The Role of Speciation in Chemical Evolution. Life 2021, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Keserű, G.M.; Soós, T.; Kappe, C.O. Anthropogenic reaction parameters—The missing link between chemical intuition and the available chemical space. Chem. Soc. Rev. 2014, 43, 5387–5399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asche, S.; Cooper, G.J.T.; Keenan, G.; Mathis, C.; Cronin, L. A robotic prebiotic chemist probes long term reactions of complexifying mixtures. Nat. Commun. 2021, 12, 3547. [Google Scholar] [CrossRef] [PubMed]
- Damer, B.; Deamer, D.; Damer, B.; Deamer, D. Coupled Phases and Combinatorial Selection in Fluctuating Hydrothermal Pools: A Scenario to Guide Experimental Approaches to the Origin of Cellular Life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef] [PubMed]
- Hawker, J.R.; Oró, J. Cyanamide mediated syntheses of peptides containing histidine and hydrophobic amino acids. J. Mol. Evol. 1981, 17, 285–294. [Google Scholar] [CrossRef]
- Forsythe, J.G.; Yu, S.-S.; Mamajanov, I.; Grover, M.A.; Krishnamurthy, R.; Fernández, F.M.; Hud, N.V. Ester-Mediated Amide Bond Formation Driven by Wet-Dry Cycles: A Possible Path to Polypeptides on the Prebiotic Earth. Angew. Chem. Int. Ed. 2015, 54, 9871–9875. [Google Scholar] [CrossRef] [PubMed]
- Higgs, P.G. The effect of limited diffusion and wet–dry cycling on reversible polymerization reactions, implications for prebiotic synthesis of nucleic acids. Life 2016, 6, 24. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Haynes, J.W.; C, M.; Petrov, A.S.; Burcar, B.T.; Krishnamurthy, R.; Hud, N.V.; Leman, L.J.; Williams, L.D. Selective incorporation of proteinaceous over nonproteinaceous cationic amino acids in model prebiotic oligomerization reactions. Proc. Natl. Acad. Sci. USA 2019, 116, 16338–16346. [Google Scholar] [CrossRef]
- Becker, S.; Schneider, C.; Okamura, H.; Crisp, A.; Amatov, T.; Dejmek, M.; Carell, T. Wet-dry cycles enable the parallel origin of canonical and non-canonical nucleosides by continuous synthesis. Nat. Commun. 2018, 9, 163. [Google Scholar] [CrossRef]
- Hassenkam, T.; Deamer, D. Visualizing RNA polymers produced by hot wet-dry cycling. Sci. Rep. 2022, 12, 10098. [Google Scholar] [CrossRef]
- Damer, B.; Deamer, D. The Hot Spring Hypothesis for an Origin of Life. Astrobiology 2020, 20, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.P.; Sawant, A.A.; Rajamani, S. Spontaneous emergence of membrane-forming protoamphiphiles from a lipid–amino acid mixture under wet–dry cycles. Chem. Sci. 2021, 12, 2970–2978. [Google Scholar] [CrossRef]
- Steller, L.H.; Van Kranendonk, M.J.; Wang, A. Dehydration Enhances Prebiotic Lipid Remodeling and Vesicle Formation in Acidic Environments. ACS Central Sci. 2022, 8, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Valley, J.; Cavosie, A.; Ushikubo, T.; Reinhard, D.A.; Lawrence, D.F.; Larson, D.J.; Clifton, P.H.; Kelly, T.F.; Wilde, S.; Moser, D.E.; et al. Hadean age for a post-magma-ocean zircon confirmed by atom-probe tomography. Nat. Geosci. 2014, 7, 219–223. [Google Scholar] [CrossRef]
- Menor-Salván, C.; Marín-Yaseli, M.R. Prebiotic chemistry in eutectic solutions at the water–ice matrix. Chem. Soc. Rev. 2012, 41, 5404–5415. [Google Scholar] [CrossRef] [PubMed]
- Fraccia, T.P.; Zanchetta, G.; Rimoldi, V.; Clark, N.A.; Bellini, T. Evidence of Liquid Crystal–Assisted Abiotic Ligation of Nucleic Acids. Orig. Life Evol. Biosph. 2015, 45, 51–68. [Google Scholar] [CrossRef]
- Zhang, S.J.; Duzdevich, D.; Ding, D.; Szostak, J.W. Freeze-thaw cycles enable a prebiotically plausible and continuous pathway from nucleotide activation to nonenzymatic RNA copying. Proc. Natl. Acad. Sci. USA 2022, 119, e2116429119. [Google Scholar] [CrossRef]
- Mutschler, H.; Wochner, A.; Holliger, P. Freeze–thaw cycles as drivers of complex ribozyme assembly. Nat. Chem. 2015, 7, 502–508. [Google Scholar] [CrossRef]
- Attwater, J.; Wochner, A.; Holliger, P. In-ice evolution of RNA polymerase ribozyme activity. Nat. Chem. 2013, 5, 1011–1018. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Johnston, B.H.; Landweber, L.F.; Kazakov, S.A. Ligation activity of fragmented ribozymes in frozen solution: Implications for the RNA world. Nucleic Acids Res. 2004, 32, 2966–2974. [Google Scholar] [CrossRef]
- Qiao, H.; Hu, N.; Bai, J.; Ren, L.; Liu, Q.; Fang, L.; Wang, Z. Encapsulation of Nucleic Acids into Giant Unilamellar Vesicles by Freeze-Thaw: A Way Protocells May Form. Orig. Life Evol. Biosph. 2016, 47, 499–510. [Google Scholar] [CrossRef]
- An, Z.; Shi, C.; Li, P.; Liu, L. Stability of amino acids and related amines in human serum under different preprocessing and pre-storage conditions based on iTRAQ®-LC-MS/MS. Biol. Open 2021, 10, bio055020. [Google Scholar] [CrossRef]
- Hu, K.; Stewart, A.J.; Yuen, K.Y.; Hinrichsen, S.; Dryburgh, E.L.; Bertin, F. The effect of freeze-thaw cycles on determination of immunoreactive plasma adrenocorticotrophic hormone concentrations in horses. J. Veter- Intern. Med. 2020, 34, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Wandro, S.; Carmody, L.; Gallagher, T.; LiPuma, J.J.; Whiteson, K. Making It Last: Storage Time and Temperature Have Differential Impacts on Metabolite Profiles of Airway Samples from Cystic Fibrosis Patients. mSystems 2017, 2, e00100-17. [Google Scholar] [CrossRef] [PubMed]
- Heinz, K.A.; Glofcheski, D.J.; Lepock, J.R.; Kruuv, J. Mechanism of freeze—Thaw damage to liver alcohol dehydrogenase and protection by cryoprotectants and amino acids. Cryobiology 1990, 27, 521–538. [Google Scholar] [CrossRef]
- Cui, X.; Xu, S.; Su, W.; Sun, Z.; Yi, Z.; Ma, X.; Chen, G.; Chen, X.; Guo, B.; Li, X. Freeze–thaw cycles for biocompatible, mechanically robust scaffolds of human hair keratins. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Kellman, B.P.; Baghdassarian, H.; Pramparo, T.; Shamie, I.; Gazestani, V.; Begzati, A.; Li, S.; Nalabolu, S.; Murray, S.; Lopez, L.; et al. Multiple freeze-thaw cycles lead to a loss of consistency in poly(A)-enriched RNA sequencing. BMC Genom. 2021, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Khin, S.; Kopp, W.C. Characterization of Effect of Repeated Freeze and Thaw Cycles on Stability of Genomic DNA Using Pulsed Field Gel Electrophoresis. Biopreservation Biobanking 2012, 10, 4–11. [Google Scholar] [CrossRef]
- Ranjan, S.; Kufner, C.L.; Lozano, G.G.; Todd, Z.R.; Haseki, A.; Sasselov, D.D. UV Transmission in Natural Waters on Prebiotic Earth. Astrobiology 2022, 22, 242–262. [Google Scholar] [CrossRef]
- Ranjan, S.; Sasselov, D.D. Influence of the UV Environment on the Synthesis of Prebiotic Molecules. Astrobiology 2016, 16, 68–88. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Miller, S.L. Oceanic protection of prebiotic organic compounds from UV radiation. Proc. Natl. Acad. Sci. USA 1998, 95, 7260–7263. [Google Scholar] [CrossRef]
- Dondi, D.; Merli, D.; Pretali, L.; Fagnoni, M.; Albini, A.; Serpone, N. Prebiotic chemistry: Chemical evolution of organics on the primitive Earth under simulated prebiotic conditions. Photochem. Photobiol. Sci. 2007, 6, 1210–1217. [Google Scholar] [CrossRef]
- Ponnamperuma, C.; Mariner, R.; Sagan, C.; Ponnamperuma, R.M.C. Formation of Adenosine by Ultra-violet Irradiation of a Solution of Adenine and Ribose. Nature 1963, 198, 1199–1200. [Google Scholar] [CrossRef] [PubMed]
- Ponnamperuma, C.; Sagan, C.; Mariner, R. Synthesis of Adenosine Triphosphate Under Possible Primitive Earth Conditions. Nature 1963, 199, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Ciambecchini, U.; Crestini, C.; Costanzo, G.; Negri, R.; Di Mauro, E. One-Pot TiO2-Catalyzed Synthesis of Nucleic Bases and Acyclonucleosides from Formamide: Implications for the Origin of Life. ChemBioChem 2003, 4, 514–521. [Google Scholar] [CrossRef]
- Powner, M.; Gerland, B.; Sutherland, J.D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Ritson, D.; Sutherland, J.D. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat. Chem. 2012, 4, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Bonfio, C.; Valer, L.; Scintilla, S.; Shah, S.; Evans, D.J.; Jin, L.; Szostak, J.W.; Sasselov, D.D.; Sutherland, J.D.; Mansy, S.S. UV-light-driven prebiotic synthesis of iron–sulfur clusters. Nat. Chem. 2017, 9, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Muchowska, K.; Varma, S.J.; Moran, J. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 2019, 569, 104–107. [Google Scholar] [CrossRef]
- Ponnamperuma, C.; Peterson, E. Peptide Synthesis from Amino Acids in Aqueous Solution. Science 1965, 147, 1572–1574. [Google Scholar] [CrossRef]
- Burke, M.; Augenstein, L. A comparison of the effects of ultraviolet and ionizing radiations on trypsin activity and on its constituent amino acids. Biochem. J. 1969, 114, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kounaves, S.P. Degradation of Amino Acids on Mars by UV Irradiation in the Presence of Chloride and Oxychlorine Salts. Astrobiology 2021, 21, 793–801. [Google Scholar] [CrossRef]
- Ansari, A.S.; Khan, I.A.; Ali, R. UV degradation of arginine in the presence of hydrogen peroxide: Involvement of hydroxyl radical in the photolytic process. J. Radiat. Res. 1985, 26, 321–329. [Google Scholar] [CrossRef]
- Scappini, F.; Casadei, F.; Zamboni, R.; Monti, S.; Giorgianni, P.; Capobianco, M. Laboratory simulation of UV irradiation from the Sun on amino acids. I: Irradiation of tyrosine. Int. J. Astrobiol. 2007, 6, 123–129. [Google Scholar] [CrossRef]
- Scappini, F.; Capobianco, M.; Casadei, F.; Zamboni, R.; Giorgianni, P. Laboratory simulation of UV irradiation from the Sun on amino acids. II. Irradiation of phenylalanine and tryptophan. Int. J. Astrobiol. 2007, 6, 281–289. [Google Scholar] [CrossRef]
- Scappini, F.; Capobianco, M.; Casadei, F.; Zamboni, R. Laboratory simulation of ultraviolet irradiation from the Sun on amino acids. III. irradiation of glycine-tyrosine. Int. J. Astrobiol. 2009, 8, 63–68. [Google Scholar] [CrossRef]
- Paecht-Horowitz, M.; Berger, J.; Katchalsky, A. Prebiotic Synthesis of Polypeptides by Heterogeneous Polycondensation of Amino-acid Adenylates. Nature 1970, 228, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Lawless, J.G.; Levi, N. The role of metal ions in chemical evolution: Polymerization of alanine and glycine in a cation-exchanged clay environment. J. Mol. Evol. 1979, 13, 281–286. [Google Scholar] [CrossRef]
- Hansma, H.G. Potassium at the Origins of Life: Did Biology Emerge from Biotite in Micaceous Clay? Life 2022, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Hayatsu, R.; Studier, M.H.; Oda, A.; Fuse, K.; Anders, E. Origin of organic matter in early solar system–II. Nitrogen compounds. Geochim. Cosmochim. Acta 1968, 32, 175–190. [Google Scholar] [CrossRef]
- Liu, R.; Orgel, L.E. Polymerization on the rocks, beta-amino acids and arginine. Orig. Life Evol. Biosph. 1998, 28, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.R., Jr.; Böhler, C.; Orgel, L.E. Polymerization on the rocks, negatively-charged alpha-amino acids. Orig. Life Evol. Biosph. 1998, 28, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Jerome, C.A.; Kim, H.-J.; Mojzsis, S.J.; Benner, S.A.; Biondi, E. Catalytic Synthesis of Polyribonucleic Acid on Prebiotic Rock Glasses. Astrobiology 2022, 22, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Mizuuchi, R.; Blokhuis, A.; Vincent, L.; Nghe, P.; Lehman, N.; Baum, D. Mineral surfaces select for longer RNA molecules. Chem. Commun. 2019, 55, 2090–2093. [Google Scholar] [CrossRef]
- Campbell, T.D.; Febrian, R.; McCarthy, J.T.; Kleinschmidt, H.E.; Forsythe, J.G.; Bracher, P.J. Prebiotic condensation through wet–dry cycling regulated by deliquescence. Nat. Commun. 2019, 10, 4508. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.K.; Taylor, L.S. Role of Deliquescence Lowering in Enhancing Chemical Reactivity in Physical Mixtures. J. Phys. Chem. B 2006, 110, 10190–10196. [Google Scholar] [CrossRef]
- Shanker, U.; Bhushan, B.; Bhattacharjee, G. Kamaluddin Oligomerization of Glycine and Alanine Catalyzed by Iron Oxides: Implications for Prebiotic Chemistry. Orig. Life Evol. Biosph. 2012, 42, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Baú, J.P.T.; Carneiro, C.E.A.; da Costa, A.C.S.; Valezi, D.F.; di Mauro, E.; Pilau, E.; Zaia, D.A.M. The Effect of Goethites on the Polymerization of Glycine and Alanine Under Prebiotic Chemistry Conditions. Orig. Life Evol. Biosph. 2021, 51, 299–320. [Google Scholar] [CrossRef]
- Sandford, S.A.; Bera, P.P.; Lee, T.J.; Materese, C.K.; Nuevo, M. Photosynthesis and Photo-Stability of Nucleic Acids in Prebiotic Extraterrestrial Environments. Top. Curr. Chem. 2015, 356, 123–164. [Google Scholar] [CrossRef]
- Lee, C.-W.; Kim, J.-K.; Moon, E.-S.; Minh, Y.C.; Kang, H. Formation of glycine on ultraviolet-irradiated interstellar ice-analog films and implications for interstellar amino acids. Astrophys. J. Lett. 2009, 697, 428–435. [Google Scholar] [CrossRef]
- Lin, R.; Wang, Y.; Li, X.; Liu, Y.; Zhao, Y. pH-Dependent Adsorption of Peptides on Montmorillonite for Resisting UV Irradiation. Life 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Altair, T.; Borges, L.; Galante, D.; Varela, H. Experimental Approaches for Testing the Hypothesis of the Emergence of Life at Submarine Alkaline Vents. Life 2021, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- Sojo, V.; Herschy, B.; Whicher, A.; Camprubí, E.; Lane, N. The Origin of Life in Alkaline Hydrothermal Vents. Astrobiology 2016, 16, 181–197. [Google Scholar] [CrossRef]
- Mielke, R.E.; Russell, M.J.; Wilson, P.R.; McGlynn, S.E.; Coleman, M.; Kidd, R.; Kanik, I. Design, Fabrication, and Test of a Hydrothermal Reactor for Origin-of-Life Experiments. Astrobiology 2010, 10, 799–810. [Google Scholar] [CrossRef]
- Herschy, B.; Whicher, A.; Camprubi, E.; Watson, C.; Dartnell, L.; Ward, J.; Evans, J.R.G.; Lane, N. An Origin-of-Life Reactor to Simulate Alkaline Hydrothermal Vents. J. Mol. Evol. 2014, 79, 213–227. [Google Scholar] [CrossRef]
- White, L.M.; Shibuya, T.; Vance, S.D.; Christensen, L.E.; Bhartia, R.; Kidd, R.; Hoffmann, A.; Stucky, G.D.; Kanik, I.; Russell, M.J. Simulating Serpentinization as It Could Apply to the Emergence of Life Using the JPL Hydrothermal Reactor. Astrobiology 2020, 20, 307–326. [Google Scholar] [CrossRef]
- Brunk, C.; Marshall, C. ‘Whole Organism’, Systems Biology, and Top-Down Criteria for Evaluating Scenarios for the Origin of Life. Life 2021, 11, 690. [Google Scholar] [CrossRef]
- Stüeken, E.E.; Anderson, R.; Bowman, J.; Brazelton, W.J.; Colangelo, J.; Goldman, A.D.; Som, S.M.; Baross, J.A. Did life originate from a global chemical reactor? Geobiology 2013, 11, 101–126. [Google Scholar] [CrossRef]
- Peng, Z.; Linderoth, J.; Baum, D.A. The hierarchical organization of autocatalytic reaction networks and its relevance to the origin of life. PLoS Comput. Biol. 2022, 18, e1010498. [Google Scholar] [CrossRef]
- Root-Bernstein, R. A Modular Hierarchy-Based Theory of the Chemical Origins of Life Based on Molecular Complementarity. Accounts Chem. Res. 2012, 45, 2169–2177. [Google Scholar] [CrossRef]
- List, B.; Pojarliev, P.; Castello, C. Proline-Catalyzed Asymmetric Aldol Reactions between Ketones and α-Unsubstituted Aldehydes. Org. Lett. 2001, 3, 573–575. [Google Scholar] [CrossRef]
- Howard, T.S.; Cohen, R.D.; Nwajiobi, O.; Muneeswaran, Z.P.; Sim, Y.E.; Lahankar, N.N.; Yeh, J.T.-H.; Raj, M. Amino-Acid-Catalyzed Direct Aldol Bioconjugation. Org. Lett. 2018, 20, 5344–5347. [Google Scholar] [CrossRef]
- Breslow, R.; Cheng, Z.-L. L-amino acids catalyze the formation of an excess of D-glyceraldehyde, and thus of other D sugars, under credible prebiotic conditions. Proc. Natl. Acad. Sci. USA 2010, 107, 5723–5725. [Google Scholar] [CrossRef]
- Duschmalé, J.; Kohrt, S.; Wennemers, H. Peptide catalysis in aqueous emulsions. Chem. Commun. 2014, 50, 8109–8112. [Google Scholar] [CrossRef]
- Kosikova, T.; Philp, D. Exploring the emergence of complexity using synthetic replicators. Chem. Soc. Rev. 2017, 46, 7274–7305. [Google Scholar] [CrossRef]
- Arsène, S.; Ameta, S.; Lehman, N.; Griffiths, A.D.; Nghe, P. Coupled catabolism and anabolism in autocatalytic RNA sets. Nucleic Acids Res. 2018, 46, 9660–9666. [Google Scholar] [CrossRef]
- Hayden, E.J.; von Kiedrowski, G.; Lehman, N. Systems Chemistry on Ribozyme Self-Construction: Evidence for Anabolic Autocatalysis in a Recombination Network. Angew. Chem. Int. Ed. 2008, 47, 8424–8428. [Google Scholar] [CrossRef]
- Liu, B.; Pappas, C.G.; Ottelé, J.; Schaeffer, G.; Jurissek, C.; Pieters, P.F.; Altay, M.; Marić, I.; Stuart, M.C.A.; Otto, S. Spontaneous Emergence of Self-Replicating Molecules Containing Nucleobases and Amino Acids. J. Am. Chem. Soc. 2020, 142, 4184–4192. [Google Scholar] [CrossRef] [Green Version]

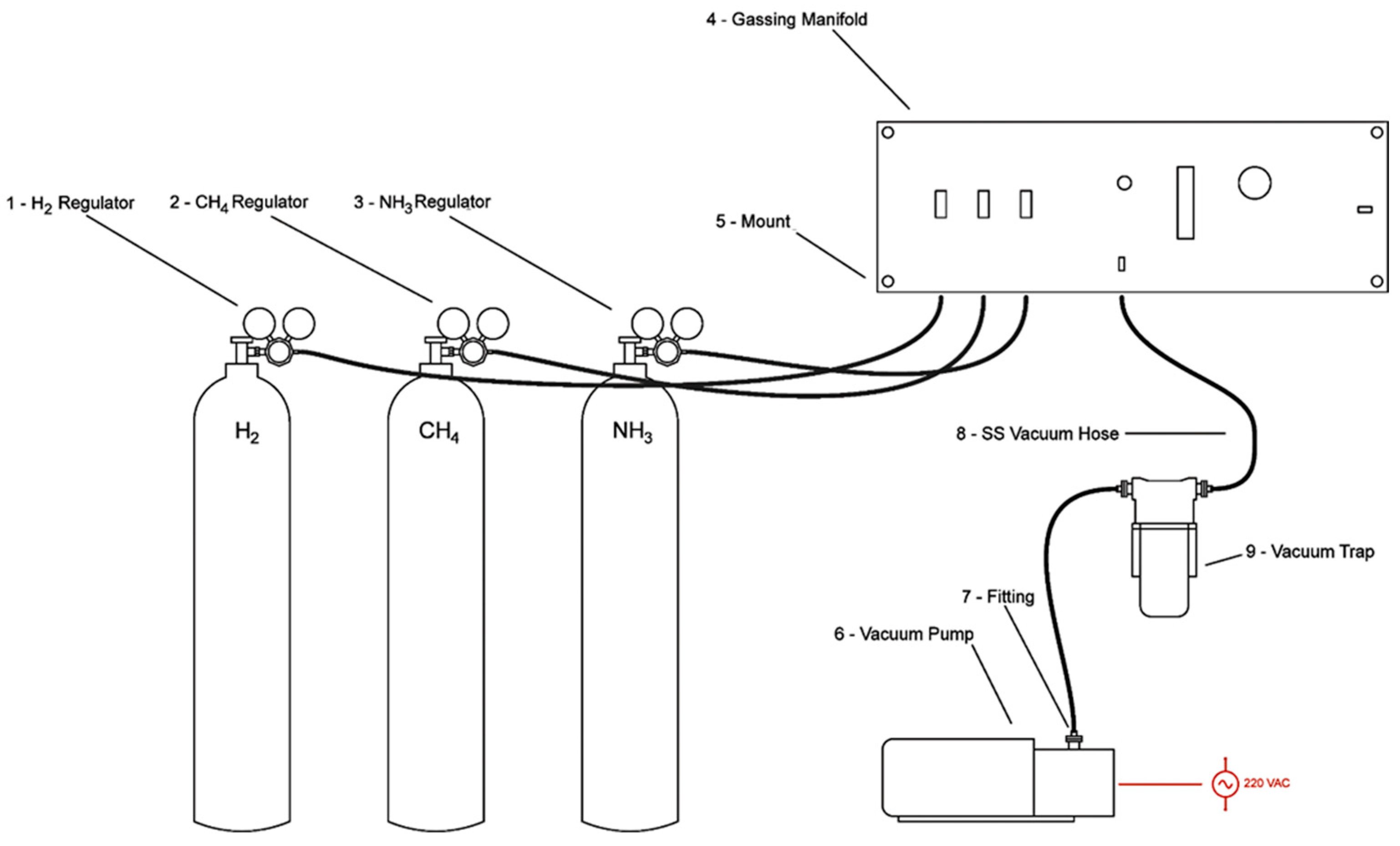
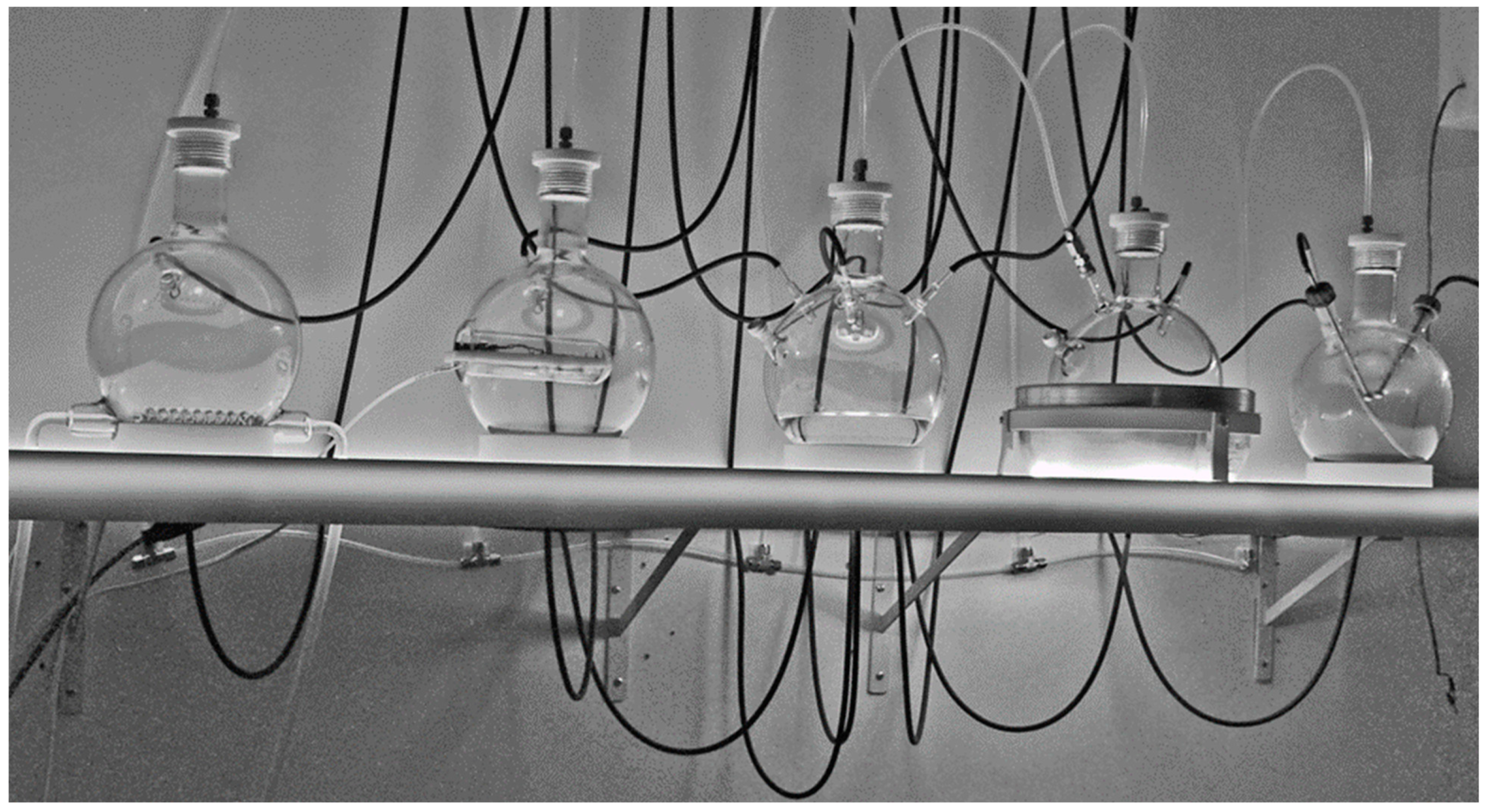

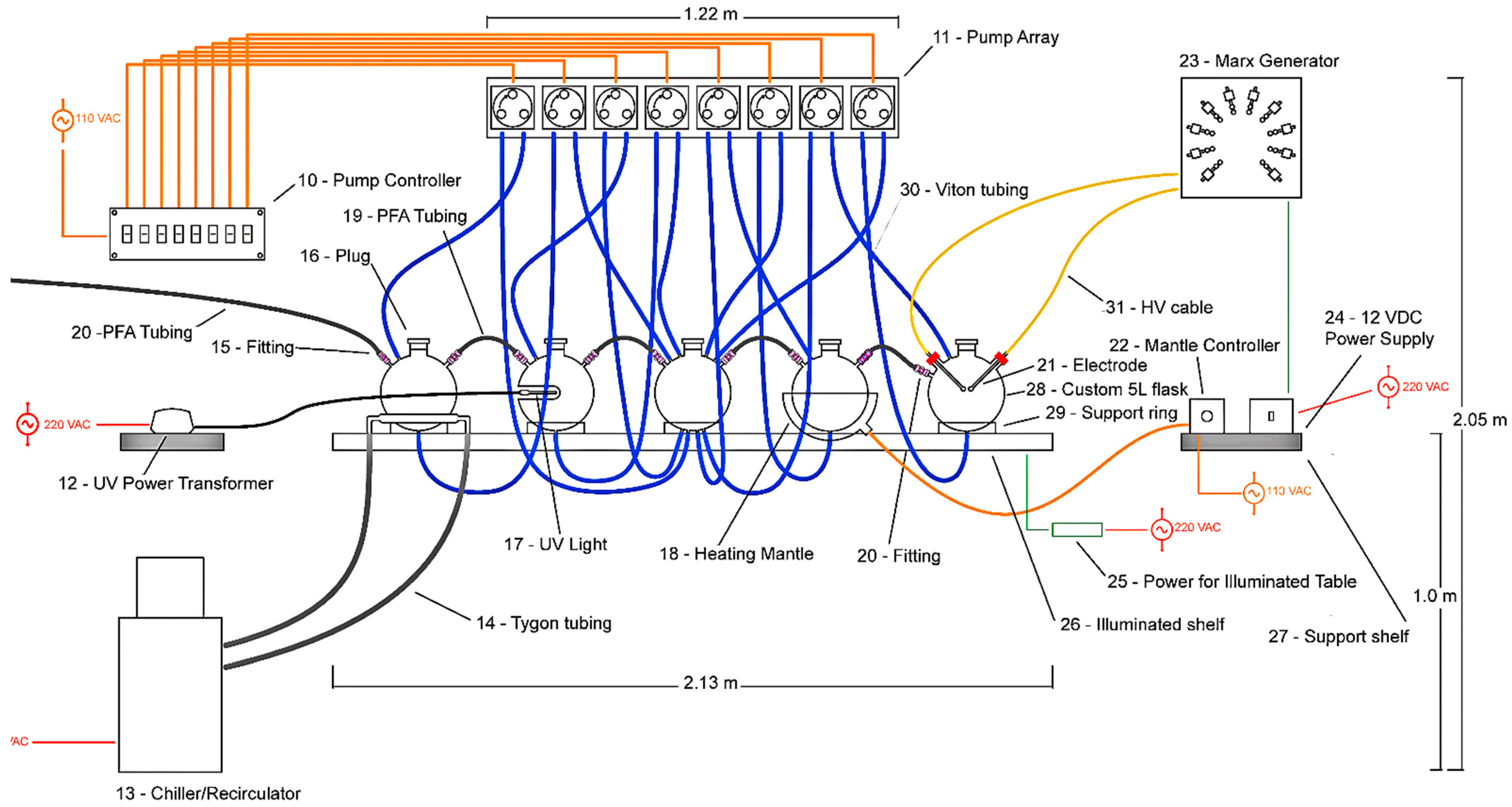
| Item Number | Part Description | Quantity |
|---|---|---|
| 1 | High pressure regulator for hydrogen | 1 |
| 2 | High pressure regulator for methane | 1 |
| 3 | Anhydrous ammonia regulator | 1 |
| 4 | Custom aluminum and stainless steel gassing manifold | 1 |
| 5 | Aluminum standoff mounts for the gassing manifold | 4 |
| 6 | Adixen (Pfeiffer) Chemical Pascal 2005C1 3.5CFM vacuum | 1 |
| 7 | Stainless steel KF-25 to ¼” compression fitting, centering ring and clamp | 3 |
| 8 | Stainless steel braided low pressure vacuum hose | 2 |
| 9 | Visitrap water and ammonia vacuum trap | 1 |
| 10 | Custom switching controller for peristaltic pump array | 1 |
| 11 | Custom peristaltic pump array made of Delrin | 1 |
| 12 | Ultraviolet light power transformer | 1 |
| 13 | Cole Parmer recirculating heater/chiller | 1 |
| 14 | ½” OD Tygon tubing | 2 |
| 15 | ¼” stainless steel Swagelok Ultra Torr to compression fitting: SS-4-UT-6-400 | 1 |
| 16 | 50 mm PFA threaded plug | 5 |
| 17 | Analytic Jena UV(AB) light | 1 |
| 18 | Eating mantle for 5 L round bottom flask | 1 |
| 19 | 3/8” PFA tubing | 4 |
| 20 | 3/8” stainless steel Swagelok Ultra Torr to compression fitting: SS-6-UT-6-600 | 8 |
| 21 | Custom stainless steel electrodes with round ball caps | 2 |
| 22 | Heating mantle controller | 1 |
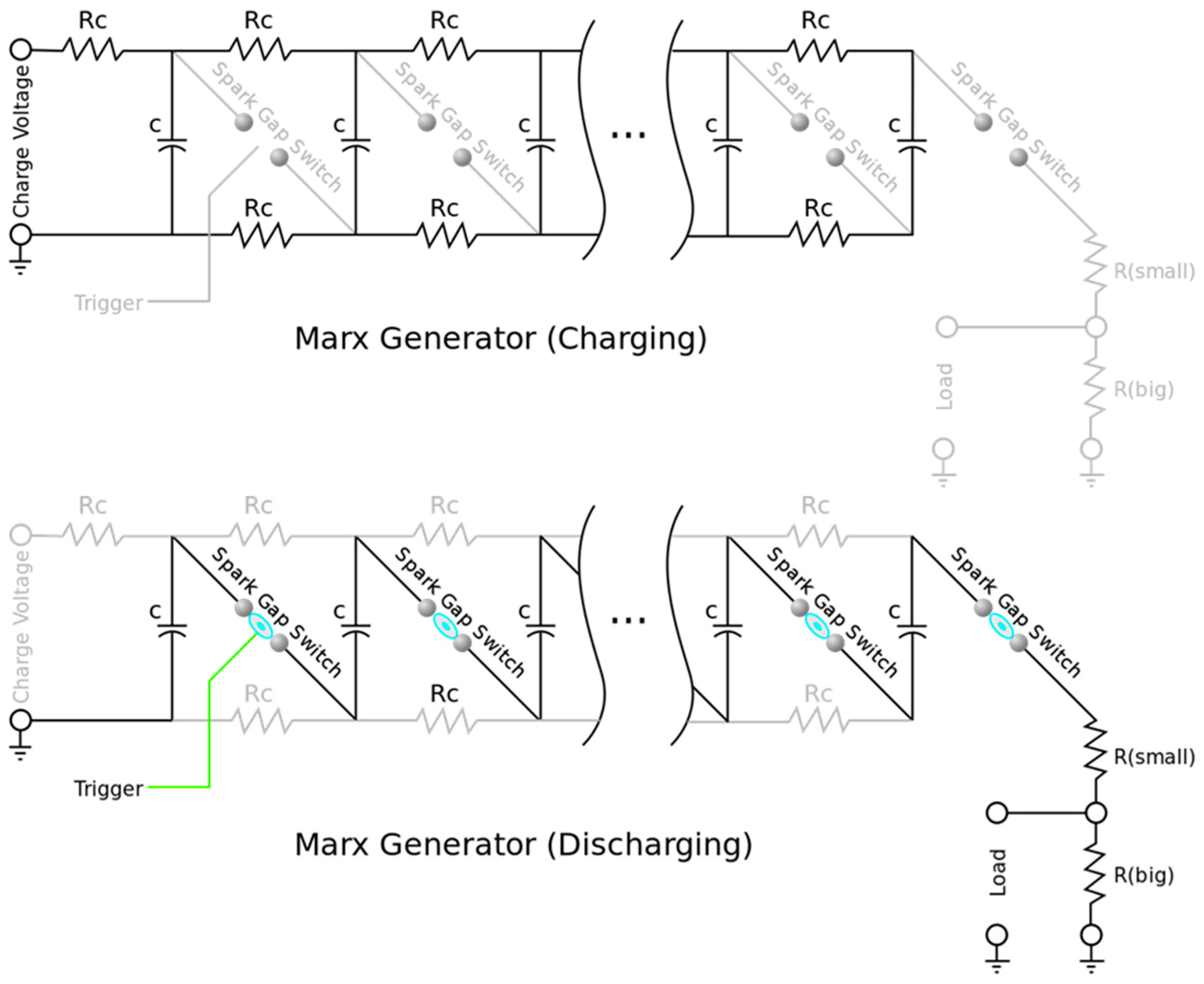
| 23 | Custom Marx generator, HV electronics, stainless steel and Delrin | 1 |
| 24 | 12 VDC variable power transformer for Marx generator | 1 |
| 25 | Power for illuminated shelf | 1 |
| 26 | Custom made Corian shelf with LED illumination | 1 |
| 27 | Corian support shelf made to match main Corian shelf | 1 |
| 28 | 5L custom borosilicate glass round bottom flask, modified to accept input and output tubing, threaded plugs, UV light, chiller condenser, heater, or electrodes | 5 |
| 29 | Support ring | 4 |
| 30 | Masterflex FDA Viton tubing L/S #96412-25 | 16 |
| 31 | Heavy insulated high voltage cable | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Root-Bernstein, R.; Brown, A.W. Novel Apparatuses for Incorporating Natural Selection Processes into Origins-of-Life Experiments to Produce Adaptively Evolving Chemical Ecosystems. Life 2022, 12, 1508. https://doi.org/10.3390/life12101508
Root-Bernstein R, Brown AW. Novel Apparatuses for Incorporating Natural Selection Processes into Origins-of-Life Experiments to Produce Adaptively Evolving Chemical Ecosystems. Life. 2022; 12(10):1508. https://doi.org/10.3390/life12101508
Chicago/Turabian StyleRoot-Bernstein, Robert, and Adam W. Brown. 2022. "Novel Apparatuses for Incorporating Natural Selection Processes into Origins-of-Life Experiments to Produce Adaptively Evolving Chemical Ecosystems" Life 12, no. 10: 1508. https://doi.org/10.3390/life12101508
APA StyleRoot-Bernstein, R., & Brown, A. W. (2022). Novel Apparatuses for Incorporating Natural Selection Processes into Origins-of-Life Experiments to Produce Adaptively Evolving Chemical Ecosystems. Life, 12(10), 1508. https://doi.org/10.3390/life12101508








