Regulation of LL-37 in Bone and Periodontium Regeneration
Abstract
1. Introduction
1.1. Cathelicidin
1.2. LL-37
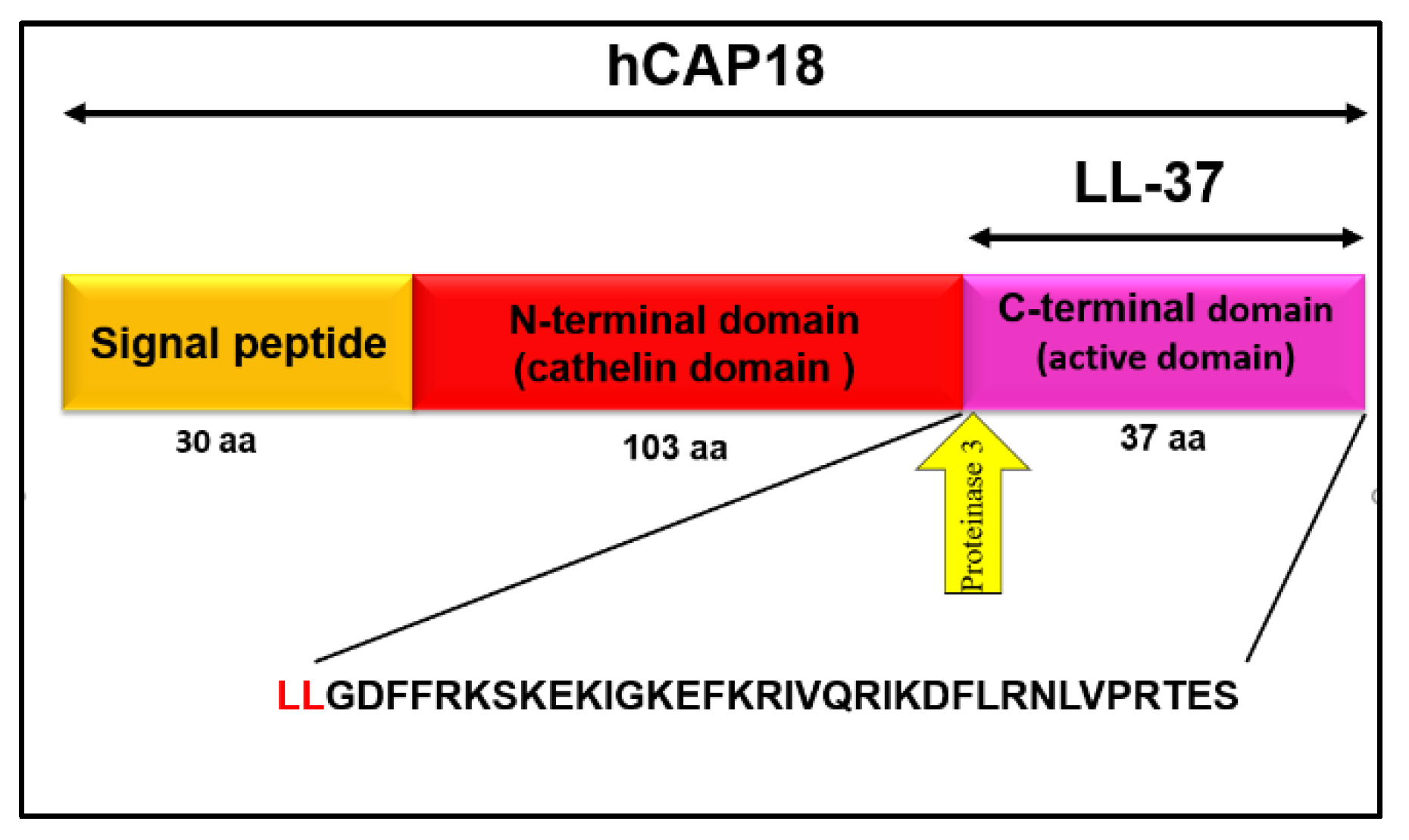
1.2.1. Structure of LL-37
1.2.2. Expression and Function
2. Effects of LL-37 on Bone Cells
2.1. Osteoclasts
2.2. Osteoblasts
3. Role of LL-37 in the Oral Cavity
3.1. Dental Caries
3.2. Dental Pulp
3.3. Periodontium
4. Regeneration
4.1. Role of LL-37 in Bone Regeneration
4.2. Role of LL-37 in Periodontium Regeneration
5. Signaling Pathways of LL-37 Regulation
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Wang, G. Apd: The Antimicrobial Peptide Database. Nucleic Acids Res. 2004, 32, D590–D592. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Selsted, M.E.; Szklarek, D.; Harwig, S.S.; Daher, K.; Bainton, D.F.; Lehrer, R.I. Defensins. Natural Peptide Antibiotics of Human Neutrophils. J. Clin. Investig. 1985, 76, 1427–1435. [Google Scholar] [CrossRef]
- Brown, K.L.; Hancock, R.E. Cationic Host Defense (Antimicrobial) Peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef]
- Guani-Guerra, E.; Santos-Mendoza, T.; Lugo-Reyes, S.O.; Teran, L.M. Antimicrobial Peptides: General Overview and Clinical Implications in Human Health and Disease. Clin. Immunol. 2010, 135, 1–11. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial Peptides of Innate Immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Selsted, M.E.; Harwig, S.S.; Ganz, T.; Schilling, J.W.; Lehrer, R.I. Primary Structures of Three Human Neutrophil Defensins. J. Clin. Investig. 1985, 76, 1436–1439. [Google Scholar] [CrossRef]
- De Smet, K.; Contreras, R. Human Antimicrobial Peptides: Defensins, Cathelicidins and Histatins. Biotechnol. Lett. 2005, 27, 1337–1347. [Google Scholar] [CrossRef]
- Larrick, J.W.; Lee, J.; Ma, S.; Li, X.; Francke, U.; Wright, S.C.; Balint, R.F. Structural, Functional Analysis and Localization of the Human Cap18 Gene. FEBS Lett. 1996, 398, 74–80. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in Tissue Engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Murakami, M.; Lopez-Garcia, B.; Braff, M.; Dorschner, R.A.; Gallo, R.L. Postsecretory Processing Generates Multiple Cathelicidins for Enhanced Topical Antimicrobial Defense. J. Immunol. 2004, 172, 3070–3077. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Huang, L.C.; Romanowski, E.G.; Yates, K.A.; Proske, R.J.; McDermott, A.M. Human Cathelicidin (Ll-37), a Multifunctional Peptide, Is Expressed by Ocular Surface Epithelia and Has Potent Antibacterial and Antiviral Activity. Curr. Eye Res. 2005, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Thennarasu, S.; Tan, A.; Penumatchu, R.; Shelburne, C.E.; Heyl, D.L.; Ramamoorthy, A. Antimicrobial and Membrane Disrupting Activities of a Peptide Derived from the Human Cathelicidin Antimicrobial Peptide Ll37. Biophys. J. 2010, 98, 248–257. [Google Scholar] [CrossRef]
- Koczulla, R.; von Degenfeld, G.; Kupatt, C.; Krotz, F.; Zahler, S.; Gloe, T.; Issbrucker, K.; Unterberger, P.; Zaiou, M.; Lebherz, C.; et al. An Angiogenic Role for the Human Peptide Antibiotic Ll-37/Hcap-18. J. Clin. Investig. 2003, 111, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Gennaro, R.; Romeo, D. Cathelicidins: A Novel Protein Family with a Common Proregion and a Variable C-Terminal Antimicrobial Domain. FEBS Lett. 1995, 374, 1–5. [Google Scholar] [CrossRef]
- Zanetti, M.; Litteri, L.; Griffiths, G.; Gennaro, R.; Romeo, D. Stimulus-Induced Maturation of Probactenecins, Precursors of Neutrophil Antimicrobial Polypeptides. J. Immunol. 1991, 146, 4295–4300. [Google Scholar]
- Scocchi, M.; Skerlavaj, B.; Romeo, D.; Gennaro, R. Proteolytic Cleavage by Neutrophil Elastase Converts Inactive Storage Proforms to Antibacterial Bactenecins. Eur. J. Biochem. 1992, 209, 589–595. [Google Scholar] [CrossRef]
- Panyutich, A.; Shi, J.; Boutz, P.L.; Zhao, C.; Ganz, T. Porcine Polymorphonuclear Leukocytes Generate Extracellular Microbicidal Activity by Elastase-Mediated Activation of Secreted Proprotegrins. Infect. Immun. 1997, 65, 978–985. [Google Scholar] [CrossRef]
- Sorensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Tjabringa, G.S.; Hiemstra, P.S.; Borregaard, N. Human Cathelicidin, Hcap-18, Is Processed to the Antimicrobial Peptide Ll-37 by Extracellular Cleavage with Proteinase 3. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef]
- Romeo, D.; Skerlavaj, B.; Bolognesi, M.; Gennaro, R. Structure and Bactericidal Activity of an Antibiotic Dodecapeptide Purified from Bovine Neutrophils. J. Biol. Chem. 1988, 263, 9573–9575. [Google Scholar] [CrossRef]
- Gennaro, R.; Skerlavaj, B.; Romeo, D. Purification, Composition, and Activity of Two Bactenecins, Antibacterial Peptides of Bovine Neutrophils. Infect. Immun. 1989, 57, 3142–3146. [Google Scholar] [CrossRef] [PubMed]
- Kopitar, M.; Ritonja, A.; Popovic, T.; Gabrijelcic, D.; Krizaj, I.; Turk, V. A New Type of Low-Molecular Mass Cysteine Proteinase Inhibitor from Pig Leukocytes. Biol. Chem. Hoppe Seyler 1989, 370, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; del Sal, G.; Storici, P.; Schneider, C.; Romeo, D. The Cdna of the Neutrophil Antibiotic Bac5 Predicts a Pro-Sequence Homologous to a Cysteine Proteinase Inhibitor That Is Common to Other Neutrophil Antibiotics. J. Biol. Chem. 1993, 268, 522–526. [Google Scholar] [CrossRef]
- Weistroffer, P.L. Cathelicidins: A History and Current Knowledge with Experimental Data on the Antimicrobial and Cytotoxic Activities of Smap29 and Congeners. Master’s Thesis, University of Iowa, Iowa, IA, USA, 2007. [Google Scholar]
- Raison, R.L.; dos Remedios, N.J. The Hagfish Immune System; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Uzzell, T.; Stolzenberg, E.D.; Shinnar, A.E.; Zasloff, M. Hagfish Intestinal Antimicrobial Peptides Are Ancient Cathelicidins. Peptides 2003, 24, 1655–1667. [Google Scholar] [CrossRef]
- Tomasinsig, L.; Zanetti, M. The Cathelicidins—tructure, Function and Evolution. Curr. Protein Pept. Sci. 2005, 6, 23–34. [Google Scholar] [CrossRef]
- Howell, M.D.; Jones, J.F.; Kisich, K.O.; Streib, J.E.; Gallo, R.L.; Leung, D.Y. Selective Killing of Vaccinia Virus by Ll-37: Implications for Eczema Vaccinatum. J. Immunol. 2004, 172, 1763–1767. [Google Scholar] [CrossRef]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral Activity and Increased Host Defense against Influenza Infection Elicited by the Human Cathelicidin Ll-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef]
- Sousa, F.H.; Casanova, V.; Findlay, F.; Stevens, C.; Svoboda, P.; Pohl, J.; Proudfoot, L.; Barlow, P.G. Cathelicidins Display Conserved Direct Antiviral Activity Towards Rhinovirus. Peptides 2017, 95, 76–83. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B.; Legowska, A.; Rolka, K.; Hui, M.; Cho, C.H. Antifungal action of human cathelicidin fragment (LL13–37) on Candida albicans. Peptides 2011, 32, 1996–2002. [Google Scholar] [CrossRef]
- Ordonez, S.R.; Amarullah, I.H.; Wubbolts, R.W.; Veldhuizen, E.J.A.; Haagsman, H.P. Fungicidal Mechanisms of Cathelicidins LL-37 and CATH-2 Revealed by Live-Cell Imaging. Antimicrob. Agents Chemother. 2014, 58, 2240–2248. [Google Scholar] [CrossRef]
- Rico-Mata, R.; De Leon-Rodriguez, L.M.; Avila, E.E. Effect of antimicrobial peptides derived from human cathelicidin LL-37 on Entamoeba histolytica trophozoites. Exp. Parasitol. 2013, 133, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Andrault, P.-M.; Samsonov, S.A.; Weber, G.; Coquet, L.; Nazmi, K.; Bolscher, J.G.M.; Lalmanach, A.-C.; Jouenne, T.; Brömme, D.; Pisabarro, M.T.; et al. Antimicrobial Peptide LL-37 Is Both a Substrate of Cathepsins S and K and a Selective Inhibitor of Cathepsin L. Biochemistry 2015, 54, 2785–2798. [Google Scholar] [CrossRef] [PubMed]
- Larrick, J.W.; Hirata, M.; Balint, R.F.; Lee, J.; Zhong, J.; Wright, S.C. Human Cap18: A Novel Antimicrobial Lipopolysaccharide-Binding Protein. Infect. Immun. 1995, 63, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, G.H.; Agerberth, B.; Odeberg, J.; Bergman, T.; Olsson, B.; Salcedo, R. The Human Gene FALL39 and Processing of the Cathelin Precursor to the Antibacterial Peptide LL-37 in Granulocytes. JBIC J. Biol. Inorg. Chem. 1996, 238, 325–332. [Google Scholar] [CrossRef]
- Porcelli, F.; Verardi, R.; Shi, L.; Henzler-Wildman, K.A.; Ramamoorthy, A.; Veglia, G. Nmr Structure of the Cathelicidin-Derived Human Antimicrobial Peptide Ll-37 in Dodecylphosphocholine Micelles. Biochemistry 2008, 47, 5565–5572. [Google Scholar] [CrossRef]
- Wang, G. Structures of Human Host Defense Cathelicidin Ll-37 and Its Smallest Antimicrobial Peptide Kr-12 in Lipid Micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef]
- Braff, M.H.; Hawkins, M.A.; di Nardo, A.; Lopez-Garcia, B.; Howell, M.D.; Wong, C.; Lin, K.; Streib, J.E.; Dorschner, R.; Leung, D.Y.; et al. Structure-Function Relationships among Human Cathelicidin Peptides: Dissociation of Antimicrobial Properties from Host Immunostimulatory Activities. J. Immunol. 2005, 174, 4271–4278. [Google Scholar] [CrossRef]
- Oren, Z.; Lerman, J.C.; Gudmundsson, G.H.; Agerberth, B.; Shai, Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: Relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 1999, 341, 501–513. [Google Scholar] [CrossRef]
- Johansson, J.; Gudmundsson, G.H.; Rottenberg, M.E.; Berndt, K.D.; Agerberth, B. Conformation-dependent Antibacterial Activity of the Naturally Occurring Human Peptide LL-37. J. Biol. Chem. 1998, 273, 3718–3724. [Google Scholar] [CrossRef]
- Wang, G. Human Antimicrobial Peptides and Proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef]
- Durr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. Ll-37, the Only Human Member of the Cathelicidin Family of Antimicrobial Peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [PubMed]
- Dorschner, R.A.; Lin, K.H.; Murakami, M.; Gallo, R.L. Neonatal Skin in Mice and Humans Expresses Increased Levels of Antimicrobial Peptides: Innate Immunity During Development of the Adaptive Response. Pediatr. Res. 2003, 53, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Marchini, G.; Lindow, S.; Brismar, H.; Stabi, B.; Berggren, V.; Ulfgren, A.-K.; Lonne-Rahm, S.; Agerberth, B.; Gudmundsson, G. The newborn infant is protected by an innate antimicrobial barrier: Peptide antibiotics are present in the skin and vernix caseosa. Br. J. Dermatol. 2002, 147, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Eckmann, L.; Leopard, J.D.; Varki, N.; Kagnoff, M.F. Cell Differentiation Is a Key Determinant of Cathelicidin Ll-37/Human Cationic Antimicrobial Protein 18 Expression by Human Colon Epithelium. Infect. Immun. 2002, 70, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Gallo, R.L. Innate Immune Defense System of the Skin. Vet. Dermatol. 2013, 24, 32-e9. [Google Scholar]
- Schauber, J.; Gallo, R.L. Antimicrobial peptides and the skin immune defense system. J. Allergy Clin. Immunol. 2009, 124, R13–R18. [Google Scholar] [CrossRef]
- Sorensen, O.E.; Arnljots, K.; Cowland, J.B.; Bainton, D.F.; Borregaard, N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 1997, 90, 2796–2803. [Google Scholar] [CrossRef]
- Büchau, A.S.; Morizane, S.; Trowbridge, J.; Schauber, J.; Kotol, P.; Bui, J.D.; Gallo, R.L. The Host Defense Peptide Cathelicidin Is Required for NK Cell-Mediated Suppression of Tumor Growth. J. Immunol. 2009, 184, 369–378. [Google Scholar]
- Sigurdardottir, S.L.; Thorleifsdottir, R.H.; Guzman, A.M.; Gudmundsson, G.H.; Valdimarsson, H.; Johnston, A. The Anti-Microbial Peptide Ll-37 Modulates Immune Responses in the Palatine Tonsils Where It Is Exclusively Expressed by Neutrophils and a Subset of Dendritic Cells. Clin. Immunol. 2012, 142, 139–149. [Google Scholar]
- Sonawane, A.; Santos, J.C.; Mishra, B.B.; Jena, P.; Progida, C.; Sorensen, O.E.; Gallo, R.; Appelberg, R.; Griffiths, G. Cathelicidin Is Involved in the Intracellular Killing of Mycobacteria in Macrophages. Cell. Microbiol. 2011, 13, 1601–1617. [Google Scholar]
- Rivas-Santiago, B.; Hernandez-Pando, R.; Carranza, C.; Juarez, E.; Contreras, J.L.; Aguilar-Leon, D.; Torres, M.; Sada, E. Expression of Cathelicidin Ll-37 During Mycobacterium Tuberculosis Infection in Human Alveolar Macrophages, Monocytes, Neutrophils, and Epithelial Cells. Infect. Immun. 2008, 76, 935–941. [Google Scholar]
- Agerberth, B.; Charo, J.; Werr, J.; Olsson, B.; Idali, F.; Lindbom, L.; Kiessling, R.; Jornvall, H.; Wigzell, H.; Gudmundsson, G.H. The Human Antimicrobial and Chemotactic Peptides Ll-37 and Alpha-Defensins Are Expressed by Specific Lymphocyte and Monocyte Populations. Blood 2000, 96, 3086–3093. [Google Scholar]
- Di Nardo, A.; Vitiello, A.; Gallo, R.L. Cutting Edge: Mast Cell Antimicrobial Activity Is Mediated by Expression of Cathelicidin Antimicrobial Peptide. J. Immunol. 2003, 170, 2274–2278. [Google Scholar]
- Coffelt, S.B.; Marini, F.C.; Watson, K.; Zwezdaryk, K.J.; Dembinski, J.L.; LaMarca, H.L.; Tomchuck, S.L.; zu Bentrup, K.H.; Danka, E.S.; Henkle, S.L.; et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3806–3811. [Google Scholar] [PubMed]
- Wu, W.; Kim, C.H.; Liu, R.; Kucia, M.; Marlicz, W.; Greco, N.; Ratajczak, J.; Laughlin, M.J.; Ratajczak, M.Z. The Bone Marrow-Expressed Antimicrobial Cationic Peptide Ll-37 Enhances the Responsiveness of Hematopoietic Stem Progenitor Cells to an Sdf-1 Gradient and Accelerates Their Engraftment after Transplantation. Leukemia 2012, 26, 736–745. [Google Scholar] [PubMed]
- Armogida, S.A.; Yannaras, N.M.; Melton, A.L.; Srivastava, M.D. Identification and quantification of innate immune system mediators in human breast milk. Allergy Asthma Proc. 2004, 25, 297–304. [Google Scholar] [PubMed]
- Murakami, M.; Dorschner, R.A.; Stern, L.J.; Lin, K.H.; Gallo, R.L. Expression and Secretion of Cathelicidin Antimicrobial Peptides in Murine Mammary Glands and Human Milk. Pediatr. Res. 2005, 57, 10–15. [Google Scholar] [PubMed]
- Murakami, M.; Ohtake, T.; Dorschner, R.A.; Schittek, B.; Garbe, C.; Gallo, R.L. Cathelicidin Anti-Microbial Peptide Expression in Sweat, an Innate Defense System for the Skin. J. Investig. Dermatol. 2002, 119, 1090–1095. [Google Scholar] [PubMed]
- Murakami, M.; Ohtake, T.; Dorschner, R.; Gallo, R. Cathelicidin Antimicrobial Peptides are Expressed in Salivary Glands and Saliva. J. Dent. Res. 2002, 81, 845–850. [Google Scholar]
- Woo, J.-S.; Jeong, J.Y.; Hwang, Y.J.; Chae, S.W.; Hwang, S.J.; Lee, H.-M. Expression of Cathelicidin in Human Salivary Glands. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 211–214. [Google Scholar] [PubMed]
- Andersson, E.; Sorensen, O.E.; Frohm, B.; Borregaard, N.; Egesten, A.; Malm, J. Isolation of human cationic antimicrobial protein-18 from seminal plasma and its association with prostasomes. Hum. Reprod. 2002, 17, 2529–2534. [Google Scholar]
- Turner, J.; Cho, Y.; Dinh, N.-N.; Waring, A.J.; Lehrer, R.I. Activities of LL-37, a Cathelin-Associated Antimicrobial Peptide of Human Neutrophils. Antimicrob. Agents Chemother. 1998, 42, 2206–2214. [Google Scholar] [PubMed]
- Wildman, K.A.H.; Lee, D.-K.; Ramamoorthy, A. Mechanism of Lipid Bilayer Disruption by the Human Antimicrobial Peptide, LL-37. Biochemistry 2003, 42, 6545–6558. [Google Scholar]
- Kim, H.J.; Cho, D.H.; Lee, K.J.; Cho, C.S.; Bang, S.I.; Cho, B.K.; Park, H.J. LL-37 suppresses sodium nitroprusside-induced apoptosis of systemic sclerosis dermal fibroblasts. Exp. Dermatol. 2011, 20, 843–845. [Google Scholar] [PubMed]
- Rosenfeld, Y.; Papo, N.; Shai, Y. Endotoxin (Lipopolysaccharide) Neutralization by Innate Immunity Host-Defense Peptides. Peptide Properties and Plausible Modes of Action. J. Biol. Chem. 2006, 281, 1636–1643. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar]
- Barlow, P.; Li, Y.; Wilkinson, T.; Bowdish, D.; Lau, Y.E.; Cosseau, C.; Haslett, C.; Simpson, J.; Hancock, R.; Davidson, D.J. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J. Leukoc. Biol. 2006, 80, 509–520. [Google Scholar]
- Chamorro, C.I.; Weber, G.; Grönberg, A.; Pivarcsi, A.; Ståhle, M. The Human Antimicrobial Peptide LL-37 Suppresses Apoptosis in Keratinocytes. J. Investig. Dermatol. 2009, 129, 937–944. [Google Scholar] [PubMed]
- Nagaoka, I.; Tamura, H.; Hirata, M. An Antimicrobial Cathelicidin Peptide, Human Cap18/Ll-37, Suppresses Neutrophil Apoptosis Via the Activation of Formyl-Peptide Receptor-Like 1 and P2x7. J. Immunol. 2006, 176, 3044–3052. [Google Scholar]
- Zasloff, M. Antimicrobial Peptides and Suppression of Apoptosis in Human Skin. J. Investig. Dermatol. 2009, 129, 824–826. [Google Scholar]
- Barlow, P.; Beaumont, P.E.; Cosseau, C.; Mackellar, A.; Wilkinson, T.; Hancock, R.; Haslett, C.; Govan, J.R.W.; Simpson, J.; Davidson, D.J. The Human Cathelicidin LL-37 Preferentially Promotes Apoptosis of Infected Airway Epithelium. Am. J. Respir. Cell Mol. Biol. 2010, 43, 692–702. [Google Scholar]
- Ciornei, C.D.; Tapper, H.; Bjartell, A.; Sternby, N.H.; Bodelsson, M. Human Antimicrobial Peptide Ll-37 Is Present in Atherosclerotic Plaques and Induces Death of Vascular Smooth Muscle Cells: A Laboratory Study. BMC Cardiovasc. Disord. 2006, 6, 49. [Google Scholar]
- Jönsson, D.; Nilsson, B.-O. The antimicrobial peptide LL-37 is anti-inflammatory and proapoptotic in human periodontal ligament cells. J. Periodontal Res. 2011, 47, 330–335. [Google Scholar]
- Zhang, Z.; Cherryholmes, G.; Shively, J.E. Neutrophil secondary necrosis is induced by LL-37 derived from cathelicidin. J. Leukoc. Biol. 2008, 84, 780–788. [Google Scholar] [PubMed]
- Mader, J.S.; Ewen, C.; Hancock, R.E.; Bleackley, R.C. The Human Cathelicidin, LL-37, Induces Granzyme-mediated Apoptosis in Regulatory T Cells. J. Immunother. 2011, 34, 229–235. [Google Scholar] [CrossRef]
- Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [PubMed]
- Nijnik, A.; Hancock, R.E.W. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr. Opin. Hematol. 2009, 16, 41–47. [Google Scholar]
- Kahlenberg, J.M.; Kaplan, M.J. Little Peptide, Big Effects: The Role of LL-37 in Inflammation and Autoimmune Disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [PubMed]
- Van der Does, A.M.; Beekhuizen, H.; Ravensbergen, B.; Vos, T.; Ottenhoff, T.H.M.; van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; Nibbering, P.H. LL-37 Directs Macrophage Differentiation toward Macrophages with a Proinflammatory Signature. J. Immunol. 2010, 185, 1442–1449. [Google Scholar]
- Yu, J.; Mookherjee, N.; Wee, K.; Bowdish, D.M.; Pistolic, J.; Li, Y.; Rehaume, L.; Hancock, R.E. Host Defense Peptide Ll-37, in Synergy with Inflammatory Mediator Il-1beta, Augments Immune Responses by Multiple Pathways. J. Immunol. 2007, 179, 7684–7691. [Google Scholar]
- Niyonsaba, F.; Iwabuchi, K.; Someya, A.; Hirata, M.; Matsuda, H.; Ogawa, H.; Nagaoka, I. A Cathelicidin Family of Human Antibacterial Peptide Ll-37 Induces Mast Cell Chemotaxis. Immunology 2002, 106, 20–26. [Google Scholar]
- Yang, D.; Chen, Q.; Schmidt, A.P.; Anderson, G.M.; Wang, J.M.; Wooters, J.; Oppenheim, J.J.; Chertov, O. Ll-37, the Neutrophil Granule- and Epithelial Cell-Derived Cathelicidin, Utilizes Formyl Peptide Receptor-Like 1 (Fprl1) as a Receptor to Chemoattract Human Peripheral Blood Neutrophils, Monocytes, and T Cells. J. Exp. Med. 2000, 192, 1069–1074. [Google Scholar]
- Niyonsaba, F.; Someya, A.; Hirata, M.; Ogawa, H.; Nagaoka, I. Evaluation of the Effects of Peptide Antibiotics Human Beta-Defensins-1/-2 and Ll-37 on Histamine Release and Prostaglandin D(2) Production from Mast Cells. Eur. J. Immunol. 2001, 31, 1066–1075. [Google Scholar]
- Chen, X.; Takai, T.; Xie, Y.; Niyonsaba, F.; Okumura, K.; Ogawa, H. Human Antimicrobial Peptide Ll-37 Modulates Proinflammatory Responses Induced by Cytokine Milieus and Double-Stranded Rna in Human Keratinocytes. Biochem. Biophys. Res. Commun. 2013, 433, 532–537. [Google Scholar]
- Brown, K.; Poon, G.F.T.; Birkenhead, D.; Pena, O.M.; Falsafi, R.; Dahlgren, C.; Karlsson, A.; Bylund, J.; Hancock, R.; Johnson, P. Host Defense Peptide LL-37 Selectively Reduces Proinflammatory Macrophage Responses. J. Immunol. 2011, 186, 5497–5505. [Google Scholar] [PubMed]
- Di Nardo, A.; Braff, M.H.; Taylor, K.R.; Na, C.; Granstein, R.D.; McInturff, J.E.; Krutzik, S.; Modlin, R.L.; Gallo, R.L. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J. Immunol. 2007, 178, 1829–1834. [Google Scholar]
- Kittaka, M.; Shiba, H.; Kajiya, M.; Fujita, T.; Iwata, T.; Rathvisal, K.; Ouhara, K.; Takeda, K.; Fujita, T.; Komatsuzawa, H.; et al. The antimicrobial peptide LL37 promotes bone regeneration in a rat calvarial bone defect. Peptides 2013, 46, 136–142. [Google Scholar]
- Pfosser, A.; El-Aouni, C.; Pfisterer, I.; Dietz, M.; Globisch, F.; Stachel, G.; Trenkwalder, T.; Pinkenburg, O.; Horstkotte, J.; Hinkel, R.; et al. Nf Kappab Activation in Embryonic Endothelial Progenitor Cells Enhances Neovascularization Via Psgl-1 Mediated Recruitment: Novel Role for Ll37. Stem Cells 2010, 28, 376–385. [Google Scholar]
- Salvado, M.D.; di Gennaro, A.; Lindbom, L.; Agerberth, B.; Haeggstrom, J.Z. Cathelicidin Ll-37 Induces Angiogenesis Via Pge2-Ep3 Signaling in Endothelial Cells, in Vivo Inhibition by Aspirin. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1965–1972. [Google Scholar] [CrossRef]
- Tokumaru, S.; Sayama, K.; Shirakata, Y.; Komatsuzawa, H.; Ouhara, K.; Hanakawa, Y.; Yahata, Y.; Dai, X.; Tohyama, M.; Nagai, H.; et al. Induction of Keratinocyte Migration Via Transactivation of the Epidermal Growth Factor Receptor by the Antimicrobial Peptide Ll-37. J. Immunol. 2005, 175, 4662–4668. [Google Scholar] [PubMed]
- Carretero, M.; Escámez, M.J.; García, M.; Duarte, B.; Holguín, A.; Retamosa, L.; Jorcano, J.L.; del Río, M.; Larcher, F. In vitro and In vivo Wound Healing-Promoting Activities of Human Cathelicidin LL-37. J. Investig. Dermatol. 2008, 128, 223–236. [Google Scholar] [PubMed]
- Otte, J.-M.; Zdebik, A.-E.; Brand, S.; Chromik, A.M.; Strauss, S.; Schmitz, F.; Steinstraesser, L.; Schmidt, W.E. Effects of the cathelicidin LL-37 on intestinal epithelial barrier integrity. Regul. Pept. 2009, 156, 104–117. [Google Scholar]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardão, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.-W.; Matthay, M.A. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part from Secretion of the Antimicrobial Peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [PubMed]
- Liu, Z.; Yuan, X.; Liu, M.; Fernandes, G.; Zhang, Y.; Yang, S.; Ionita, C.N. Antimicrobial Peptide Combined with BMP2-Modified Mesenchymal Stem Cells Promotes Calvarial Repair in an Osteolytic Model. Mol. Ther. 2018, 26, 199–207. [Google Scholar] [PubMed]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar]
- Udagawa, N.; Takahashi, N.; Akatsu, T.; Tanaka, H.; Sasaki, T.; Nishihara, T.; Koga, T.; Martin, T.J.; Suda, T. Origin of Osteoclasts: Mature Monocytes and Macrophages Are Capable of Differentiating into Osteoclasts under a Suitable Microenvironment Prepared by Bone Marrow-Derived Stromal Cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7260–7264. [Google Scholar]
- Supanchart, C.; Thawanaphong, S.; Makeudom, A.; Bolscher, J.G.; Nazmi, K.; Kornak, U.; Krisanaprakornkit, S. The Antimicrobial Peptide, Ll-37, Inhibits in Vitro Osteoclastogenesis. J. Dent. Res. 2012, 91, 1071–1077. [Google Scholar]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.-I.; et al. Induction and Activation of the Transcription Factor NFATc1 (NFAT2) Integrate RANKL Signaling in Terminal Differentiation of Osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar]
- Supanchart, C.; Makeudom, A.; Bolscher, J.G.M.; Krisanaprakornkit, S. Tlr9 Involvement in the Inhibition of Osteoclast Formation by Ll-37. In Proceedings of the IADR Asia/Pacific Region (APR) Regional Meeting and Co-Annual Scientific Meeting of IADR Divisions, Bangkok, Thailand, 21–23 August 2013. [Google Scholar]
- Horibe, K.; Nakamichi, Y.; Uehara, S.; Nakamura, M.; Koide, M.; Kobayashi, Y.; Takahashi, N.; Udagawa, N. Roles of cathelicidin-related antimicrobial peptide in murine osteoclastogenesis. Immunology 2013, 140, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Pellegatti, P.; Falzoni, S.; Donvito, G.; Lemaire, I.; Di Virgilio, F. P2X7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J. 2011, 25, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shively, J.E. Generation of Novel Bone Forming Cells (Monoosteophils) from the Cathelicidin-Derived Peptide LL-37 Treated Monocytes. PLoS ONE 2010, 5, e13985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shively, J.E. Acceleration of Bone Repair in Nod/Scid Mice by Human Monoosteophils, Novel Ll-37-Activated Monocytes. PLoS ONE 2013, 8, e67649. [Google Scholar]
- Yu, X.; Quan, J.; Long, W.; Chen, H.; Wang, R.; Guo, J.; Lin, X.; Mai, S. LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp. Cell Res. 2018, 372, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Al Baadani, M.A.; He, H.; Cai, L.; Wu, Z.; Yao, L.; Wu, X.; Wu, S.; Chen, M.; Zhang, H.; et al. Antibacterial and osteogenesis performances of LL37-loaded titania nanopores in vitro and in vivo. Int. J. Nanomed. 2019, 14, 3043–3054. [Google Scholar] [CrossRef]
- Jin, M.; Zhu, J.; Meng, Z.; Jiang, X.; Chen, Z.; Xu, J.; Gao, H.; Zhu, J.; Wu, F. Tio(2)Nanotubes-Mos(2)/Pda-Ll-37 Exhibits Efficient Anti-Bacterial Activity and Facilitates New Bone Formation under near-Infrared Laser Irradiation. Biomed. Mater. 2022, 17, 045025. [Google Scholar] [CrossRef]
- Cheng, Q.; Zeng, K.; Kang, Q.; Qian, W.; Zhang, W.; Gan, Q.; Xia, W. The Antimicrobial Peptide LL-37 Promotes Migration and Odonto/Osteogenic Differentiation of Stem Cells from the Apical Papilla through the Akt/Wnt/β-catenin Signaling Pathway. J. Endod. 2020, 46, 964–972. [Google Scholar] [CrossRef]
- Tokajuk, J.; Deptuła, P.; Piktel, E.; Daniluk, T.; Chmielewska, S.; Wollny, T.; Wolak, P.; Fiedoruk, K.; Bucki, R. Cathelicidin LL-37 in Health and Diseases of the Oral Cavity. Biomedicines 2022, 10, 1086. [Google Scholar] [CrossRef]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef]
- Hicks, J.; Garcia-Godoy, F.; Flaitz, C. Biological factors in dental caries: Role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J. Clin. Pediatr. Dent. 2004, 28, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Slade, H.D. Biology, Immunology, and Cariogenicity of Streptococcus Mutans. Microbiol. Rev. 1980, 44, 331–384. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Altman, H.; Steinberg, D.; Porat, Y.; Mor, A.; Fridman, D.; Friedman, M.; Bachrach, G. In vitro assessment of antimicrobial peptides as potential agents against several oral bacteria. J. Antimicrob. Chemother. 2006, 58, 198–201. [Google Scholar] [PubMed]
- Tao, R.; Jurevic, R.J.; Coulton, K.K.; Tsutsui, M.T.; Roberts, M.C.; Kimball, J.R.; Wells, N.; Berndt, J.; Dale, B.A. Salivary Antimicrobial Peptide Expression and Dental Caries Experience in Children. Antimicrob. Agents Chemother. 2005, 49, 3883–3888. [Google Scholar] [CrossRef]
- Dale, B.A.; Tao, R.; Kimball, J.R.; Jurevic, R.J. Oral Antimicrobial Peptides and Biological Control of Caries. BMC Oral Health 2006, 6 (Suppl. S1), S13. [Google Scholar]
- Phattarataratip, E.; Olson, B.; Broffitt, B.; Qian, F.; Brogden, K.A.; Drake, D.R.; Levy, S.M.; Banas, J.A. Streptococcus mutans strains recovered from caries-active or caries-free individuals differ in sensitivity to host antimicrobial peptides. Mol. Oral Microbiol. 2011, 26, 187–199. [Google Scholar] [CrossRef]
- Davidopoulou, S.; Diza, E.; Menexes, G.; Kalfas, S. Salivary concentration of the antimicrobial peptide LL-37 in children. Arch. Oral Biol. 2012, 57, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-J.; Zhang, B.; Feng, X.-S.; Ren, H.-X.; Xue-Song, F. RETRACTED ARTICLE: Human cathelicidin LL-37 enhance the antibiofilm effect of EGCG on Streptococcus mutans. BMC Oral Health 2016, 16, 101. [Google Scholar]
- Nakashima, M.; Akamine, A. The Application of Tissue Engineering to Regeneration of Pulp and Dentin in Endodontics. J. Endod. 2005, 31, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.-L.; Liewehr, F.R. Relationships between Caries Bacteria, Host Responses, and Clinical Signs and Symptoms of Pulpitis. J. Endod. 2007, 33, 213–219. [Google Scholar] [CrossRef]
- Graves, D.T.; Oates, T.; Garlet, G.P. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J. Oral Microbiol. 2011, 3, 5304. [Google Scholar] [CrossRef]
- Nireeksha; Varma, S.; Damdoum, M.; Alsaegh, M.; Hegde, M.; Kumari, S.; Ramamurthy, S.; Narayanan, J.; Imran, E.; Shabbir, J.; et al. Immunomodulatory Expression of Cathelicidins Peptides in Pulp Inflammation and Regeneration: An Update. Curr. Issues Mol. Biol. 2021, 43, 116–126. [Google Scholar] [CrossRef]
- Kajiya, M.; Shiba, H.; Komatsuzawa, H.; Ouhara, K.; Fujita, T.; Takeda, K.; Uchida, Y.; Mizuno, N.; Kawaguchi, H.; Kurihara, H. The Antimicrobial Peptide Ll37 Induces the Migration of Human Pulp Cells: A Possible Adjunct for Regenerative Endodontics. J. Endod. 2010, 36, 1009–1013. [Google Scholar] [CrossRef]
- Khung, R.; Shiba, H.; Kajiya, M.; Kittaka, M.; Ouhara, K.; Takeda, K.; Mizuno, N.; Fujita, T.; Komatsuzawa, H.; Kurihara, H. LL37 induces VEGF expression in dental pulp cells through ERK signalling. Int. Endod. J. 2014, 48, 673–679. [Google Scholar] [CrossRef]
- Tran-Hung, L.; Mathieu, S.; About, I. Role of human pulp fibroblasts in angiogenesis. J. Dent. Res. 2006, 85, 819–823. [Google Scholar] [CrossRef]
- Milhan, N.V.M.; de Barros, P.P.; Zutin, E.A.D.L.; de Oliveira, F.E.; Camargo, C.H.R.; Camargo, S.E.A. The Antimicrobial Peptide LL-37 as a Possible Adjunct for the Proliferation and Differentiation of Dental Pulp Stem Cells. J. Endod. 2017, 43, 2048–2053. [Google Scholar] [CrossRef]
- Palumbo, A. The Anatomy and Physiology of the Healthy Periodontium; InTech: London, UK, 2011. [Google Scholar]
- Loesche, W.J.; Grossman, N.S. Periodontal Disease as a Specific, Albeit Chronic, Infection: Diagnosis and Treatment. Clin. Microbiol. Rev. 2001, 14, 727–752. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Periodontal Microbial Ecology. Periodontol. 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Slots, J. Selection of antimicrobial agents in periodontal therapy. J. Periodontal Res. 2002, 37, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Alonso, B.; Leon, R.; Roldan, S.; Sanz, M. Antimicrobial Therapy in Periodontitis: The Use of Systemic Antimicrobials against the Subgingival Biofilm. J. Clin. Periodontol. 2008, 35, 45–66. [Google Scholar] [CrossRef]
- Gorr, S.-U. Antimicrobial peptides of the oral cavity. Periodontology 2000 2009, 51, 152–180. [Google Scholar] [CrossRef]
- Gursoy, U.K.; Könönen, E. Understanding the roles of gingival beta-defensins. J. Oral Microbiol. 2012, 4, 15127. [Google Scholar] [CrossRef]
- Tanaka, D.; Miyasaki, K.T.; Lehrer, R.I. Sensitivity of Actinobacillus actinomycetemcomitans and Capnocytophaga spp. to the bactericidal action of LL-37: A cathelicidin found in human leukocytes and epithelium. Oral Microbiol. Immunol. 2000, 15, 226–231. [Google Scholar] [CrossRef]
- Zambon, J.J.; Christersson, L.A.; Slots, J. Actinobacillus actinomycetemcomitans in Human Periodontal Disease: Prevalence in Patient Groups and Distribution of Biotypes and Serotypes Within Families. J. Periodontol. 1983, 54, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Genco, R.J. Black-Pigmented Bacteroides Species, Capnocytophaga Species, and Actinobacillus Actinomycetemcomitans in Human Periodontal Disease: Virulence Factors in Colonization, Survival, and Tissue Destruction. J. Dent. Res. 1984, 63, 412–421. [Google Scholar] [CrossRef]
- Sol, A.; Ginesin, O.; Chaushu, S.; Karra, L.; Coppenhagen-Glazer, S.; Ginsburg, I.; Bachrach, G. LL-37 Opsonizes and Inhibits Biofilm Formation of Aggregatibacter actinomycetemcomitans at Subbactericidal Concentrations. Infect. Immun. 2013, 81, 3577–3585. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas Gingivalis: An Overview of Periodontopathic Pathogen Below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Walters, S.M.; Dubey, V.S.; Jeffrey, N.R.; Dixon, D.R. Antibiotic-Induced Porphyromonas Gingivalis Lps Release and Inhibition of Lps-Stimulated Cytokines by Antimicrobial Peptides. Peptides 2010, 31, 1649–1653. [Google Scholar] [CrossRef]
- Putsep, K.; Carlsson, G.; Boman, H.G.; Andersson, M. Deficiency of Antibacterial Peptides in Patients with Morbus Kostmann: An Observation Study. Lancet 2002, 360, 1144–1149. [Google Scholar] [CrossRef]
- Carlsson, G.; Wahlin, Y.-B.; Johansson, A.; Olsson, A.; Eriksson, T.; Claesson, R.; Hänström, L.; Henter, J.-I. Periodontal Disease in Patients from the Original Kostmann Family with Severe Congenital Neutropenia. J. Periodontol. 2006, 77, 744–751. [Google Scholar] [CrossRef]
- Hart, T.C.; Hart, P.S.; Michalec, M.D.; Zhang, Y.; Firatli, E.; Van Dyke, T.E.; Stabholz, A.; Zlorogorski, A.; Shapira, L.; Soskolne, W.A. Haim-Munk syndrome and Papillon-Lefevre syndrome are allelic mutations in cathepsin C. J. Med. Genet. 2000, 37, 88–94. [Google Scholar] [CrossRef]
- De Haar, S.F.; Hiemstra, P.S.; van Steenbergen, M.T.J.M.; Everts, V.; Beertsen, W. Role of Polymorphonuclear Leukocyte-Derived Serine Proteinases in Defense against Actinobacillus actinomycetemcomitans. Infect. Immun. 2006, 74, 5284–5291. [Google Scholar] [CrossRef]
- McCrudden, M.T.C.; Orr, D.F.; Yu, Y.; Coulter, W.A.; Manning, G.; Irwin, C.R.; Lundy, F.T. LL-37 in periodontal health and disease and its susceptibility to degradation by proteinases present in gingival crevicular fluid. J. Clin. Periodontol. 2013, 40, 933–941. [Google Scholar] [CrossRef]
- Koziel, J.; Karim, A.Y.; Przybyszewska, K.; Ksiazek, M.; Rapala-Kozik, M.; Nguyen, K.-A.; Potempa, J. Proteolytic Inactivation of LL-37 by Karilysin, a Novel Virulence Mechanism of Tannerella forsythia. J. Innate Immun. 2010, 2, 288–293. [Google Scholar] [CrossRef]
- Gutner, M.; Chaushu, S.; Balter, D.; Bachrach, G. Saliva Enables the Antimicrobial Activity of LL-37 in the Presence of Proteases of Porphyromonas gingivalis. Infect. Immun. 2009, 77, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B. Mechanisms involved in regulation of periodontal ligament cell production of pro-inflammatory cytokines: Implications in periodontitis. J. Periodontal Res. 2020, 56, 249–255. [Google Scholar] [CrossRef]
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.; Hancock, R.E. The Human Antimicrobial Peptide Ll-37 Is a Multifunctional Modulator of Innate Immune Responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef]
- Aidoukovitch, A.; Anders, E.; Dahl, S.; Nebel, D.; Svensson, D.; Nilsson, B.O. The Host Defense Peptide Ll-37 Is Internalized by Human Periodontal Ligament Cells and Prevents Lps-Induced Mcp-1 Production. J. Periodontal Res. 2019, 54, 662–670. [Google Scholar] [CrossRef]
- Bedran, T.B.; Mayer, M.; Spolidorio, D.M.P.; Grenier, D. Synergistic Anti-Inflammatory Activity of the Antimicrobial Peptides Human Beta-Defensin-3 (hBD-3) and Cathelicidin (LL-37) in a Three-Dimensional Co-Culture Model of Gingival Epithelial Cells and Fibroblasts. PLoS ONE 2014, 9, e106766. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Coutinho, O.; Reis, R.L. Bone Tissue Engineering: State of the Art and Future Trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Bashutski, J.D.; Wang, H.-L. Periodontal and Endodontic Regeneration. J. Endod. 2009, 35, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.; Cooper, M.S. Bone loss in inflammatory disorders. J. Endocrinol. 2009, 201, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of Osteogenesis-Angiogenesis Coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef]
- Street, J.; Bao, M.; DeGuzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Yuan, Q.; Sun, J.; Zhuang, A.; Bi, X. Cathelicidin LL37 Promotes Osteogenic Differentiation in vitro and Bone Regeneration in vivo. Front. Bioeng. Biotechnol. 2021, 9, 638494. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Nie, B.; Du, Z.; Long, T.; Yue, B. The antimicrobial peptide KR-12 promotes the osteogenic differentiation of human bone marrow stem cells by stimulating BMP/SMAD signaling. RSC Adv. 2018, 8, 15547–15557. [Google Scholar] [CrossRef]
- Kittaka, M.; Shiba, H.; Kajiya, M.; Ouhara, K.; Takeda, K.; Kanbara, K.; Fujita, T.; Kawaguchi, H.; Komatsuzawa, H.; Kurihara, H. Antimicrobial peptide LL37 promotes vascular endothelial growth factor-A expression in human periodontal ligament cells. J. Periodontal Res. 2012, 48, 228–234. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Yang, Y.; Jiang, Z.; Deng, Y.; Song, S.; Yang, W.; Chen, Z.G. Bioinspired, Biocompatible and Peptide-Decorated Silk Fibroin Coatings for Enhanced Osteogenesis of Bioinert Implant. J. Biomater. Sci. Polym. Ed. 2018, 29, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, M.R.; Restrepo, C.; Maltenfort, M.G.; Purtill, J.J.; Parvizi, J. Risk Factors for Surgical Site Infection Following Total Joint Arthroplasty. J. Bone Jt. Surg. 2014, 96, e158. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Godson, C.; Guiry, P.J.; Agerberth, B.; Haeggstrom, J.Z. Leukotriene B4/Antimicrobial Peptide Ll-37 Proinflammatory Circuits Are Mediated by Blt1 and Fpr2/Alx and Are Counterregulated by Lipoxin A4 and Resolvin E1. FASEB J. 2011, 25, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Verjans, E.T.; Zels, S.; Luyten, W.; Landuyt, B.; Schoofs, L. Molecular Mechanisms of Ll-37-Induced Receptor Activation: An Overview. Peptides 2016, 85, 16–26. [Google Scholar] [CrossRef]
- Gaudreault, E.; Gosselin, J. Leukotriene B4 Induces Release of Antimicrobial Peptides in Lungs of Virally Infected Mice. J. Immunol. 2008, 180, 6211–6221. [Google Scholar] [CrossRef] [PubMed]
- Iaccio, A.; Cattaneo, F.; Mauro, M.; Ammendola, R. FPRL1-mediated induction of superoxide in LL-37-stimulated IMR90 human fibroblast. Arch. Biochem. Biophys. 2009, 481, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Tjabringa, G.S.; Aarbiou, J.; Ninaber, D.K.; Drijfhout, J.W.; Sorensen, O.E.; Borregaard, N.; Rabe, K.F.; Hiemstra, P. The Antimicrobial Peptide LL-37 Activates Innate Immunity at the Airway Epithelial Surface by Transactivation of the Epidermal Growth Factor Receptor. J. Immunol. 2003, 171, 6690–6696. [Google Scholar] [CrossRef] [PubMed]
- Girnita, A.; Zheng, H.; Grönberg, A.; Girnita, L.; Ståhle, M. Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor. Oncogene 2011, 31, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Brown, K.L.; Bowdish, D.M.; Doria, S.; Falsafi, R.; Hokamp, K.; Roche, F.M.; Mu, R.; Doho, G.H.; Pistolic, J.; et al. Modulation of the Tlr-Mediated Inflammatory Response by the Endogenous Human Host Defense Peptide Ll-37. J. Immunol. 2006, 176, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA–antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009, 206, 1983–1994. [Google Scholar] [CrossRef]
- Elssner, A.; Duncan, M.; Gavrilin, M.; Wewers, M.D. A Novel P2X7 Receptor Activator, the Human Cathelicidin-Derived Peptide LL37, Induces IL-1β Processing and Release. J. Immunol. 2004, 172, 4987–4994. [Google Scholar] [CrossRef]
- Horibe, K.; Hosoya, A.; Hiraga, T.; Nakamura, H. Expression and localization of CRAMP in rat tooth germ and during reparative dentin formation. Clin. Oral Investig. 2018, 22, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Craske, M.; Severino, P.; De Lima, T.M.; Labhart, P.; Chammas, R.; Velasco, I.T.; Machado, M.C.C.; Egan, B.; Nakaya, H.I.; et al. Antimicrobial peptide LL-37 participates in the transcriptional regulation of melanoma cells. J. Cancer 2016, 7, 2341–2345. [Google Scholar] [CrossRef] [PubMed]
- Nagant, C.; Pitts, B.; Nazmi, K.; Vandenbranden, M.; Bolscher, J.G.; Stewart, P.S.; Dehaye, J.-P. Identification of Peptides Derived from the Human Antimicrobial Peptide LL-37 Active against Biofilms Formed by Pseudomonas aeruginosa Using a Library of Truncated Fragments. Antimicrob. Agents Chemother. 2012, 56, 5698–5708. [Google Scholar] [CrossRef]
- Lozeau, L.D.; Rolle, M.; Camesano, T.A. A QCM-D study of the concentration- and time-dependent interactions of human LL37 with model mammalian lipid bilayers. Colloids Surf. B Biointerfaces 2018, 167, 229–238. [Google Scholar] [CrossRef]


| Authors | Results | Reference Number |
|---|---|---|
| Kittaka et al. (2013) | LL37 enhances angiogenesis and recruits stem cells to promote bone regeneration in rat calvarial defects. | [87] |
| Liu et al. (2018) | LL37 increases the migration, proliferation, and osteogenic differentiation of MSCs in addition to inhibiting osteoclast differentiation. LL-37 combined with BMP2 dramatically promotes MSCs- mediated angiogenesis and bone regeneration in LPS-induced mouse calvarial osteolytic bone defects. | [95] |
| Li et al. (2021) | Application of LL-37 accompanied with PSeD gel and hADSCs significantly accelerates the process of bone regeneration through enhancing osteogenic differentiation and reducing inflammation in rat calvarial bone defect. | [160] |
| Li et al. (2018) | KR-12 (the smallest fragment of LL-37) stimulates the osteogenic differentiation of hBMMSCs via the activation of the BMP/SMAD pathway signaling pathway | [161] |
| Kittaka et al. (2013) | LL-37 contributes to periodontal regeneration through upregulating VEGF-A expression resulting in the activation of ERK and NF-kB signaling cascades in HPL cells and inducing angiogenesis. | [162] |
| Shen et al. (2019) | LL-37-loaded NP structure significantly improves cell adhesion, spreading, and osteogenic differentiation. Implantation of LL37-loaded NP structures into infected and uninfected rat femurs significantly improves bone formation. | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinipardaz, Z.; Zhong, J.M.; Yang, S. Regulation of LL-37 in Bone and Periodontium Regeneration. Life 2022, 12, 1533. https://doi.org/10.3390/life12101533
Chinipardaz Z, Zhong JM, Yang S. Regulation of LL-37 in Bone and Periodontium Regeneration. Life. 2022; 12(10):1533. https://doi.org/10.3390/life12101533
Chicago/Turabian StyleChinipardaz, Zahra, Jessica M. Zhong, and Shuying Yang. 2022. "Regulation of LL-37 in Bone and Periodontium Regeneration" Life 12, no. 10: 1533. https://doi.org/10.3390/life12101533
APA StyleChinipardaz, Z., Zhong, J. M., & Yang, S. (2022). Regulation of LL-37 in Bone and Periodontium Regeneration. Life, 12(10), 1533. https://doi.org/10.3390/life12101533






