Seaweed-Derived Phenolic Compounds in Growth Promotion and Stress Alleviation in Plants
Abstract
:Simple Summary
Abstract
1. Introduction
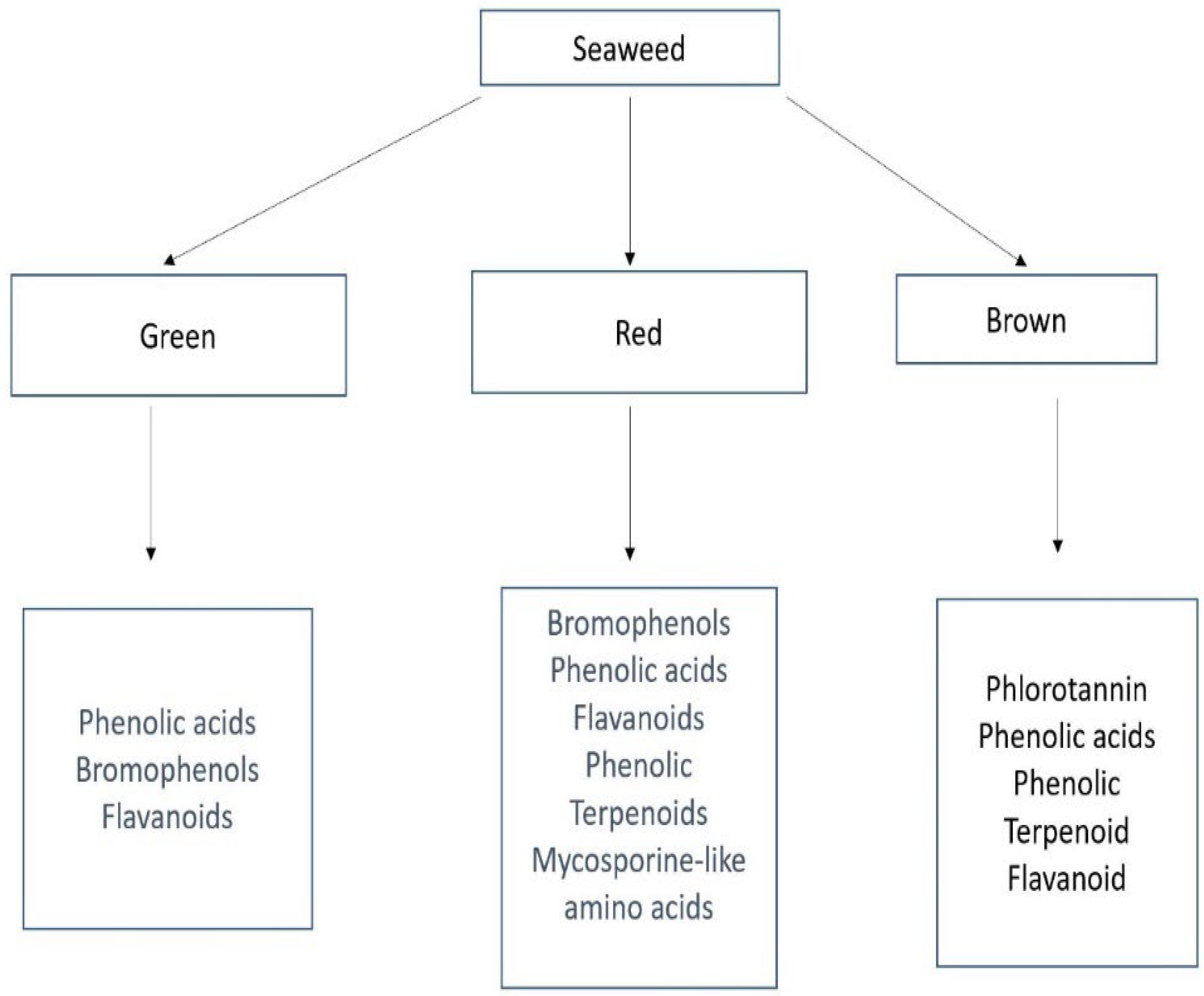
2. Description and Classification of Seaweed
3. Phenolic Compounds as an Important Bioactive Compound in Seaweed
Extraction of Phenolic Compounds from Seaweed
4. Application of Phenolic Compounds in Agriculture
4.1. Role of Seaweed-Derived Phenolic Compound in Promoting Plant Growth
4.2. Phenolic Compounds and Abiotic Stress Intervention in Plants
4.2.1. Drought
4.2.2. Salinity
4.2.3. Extreme Temperature
4.2.4. Heavy Metal
4.3. Phenolic Compounds and Biotic Stress Intervention in Plants
4.3.1. Phenolic Compounds and Fungal Diseases
4.3.2. Phenolic Compounds and Bacteria Diseases
4.3.3. Phenolic Compounds Used to Control Viral Diseases
4.3.4. Phenolic Compounds Used against Herbivore and Insect Attack
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pati, M.P.; Sharma, S.; Nayak, L.; Panda, C.R. Uses of seaweed and its application to human welfare: A review. Int. J. Pharm. Pharm. Sci. 2016, 8, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Xunmeng, L.; Wang, K.; Zhang, S.; Feng, M. Distribution and Flora of Seaweed Beds in the Coastal Waters of China. Sustainability 2021, 13, 3009. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. The rise of seaweed gastronomy: Phycogastronomy. Bot. Mar. 2019, 62, 195–209. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M. An overview to the health benefits of seaweeds consumption. Mar. Drugs 2021, 19, 341. [Google Scholar] [CrossRef]
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of algae in cosmetics: An overview. Int. J. Innov. Res. Sci. Eng. Technol 2018, 7, 1269. [Google Scholar]
- Stoyneva-Gärtner, M.; Uzunov, B.; Gärtner, G. Enigmatic microalgae from aeroterrestrial and extreme habitats in cosmetics: The potential of the untapped natural sources. Cosmetics 2020, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.; Cotas, J. Historical use of seaweed as an agricultural fertilizer in the European Atlantic area. In Seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–22. [Google Scholar]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 41–106. [Google Scholar]
- Stirk, W.A.; Rengasamy, K.R.; Kulkarni, M.G.; van Staden, J. Plant biostimulants from seaweed: An overview. In The Chemical Biology of Plant Biostimulants; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 31–55. [Google Scholar]
- Al, M.A.; Akhtar, A.; Rahman, M.F.; Kamal, A.H.M.; Karim, N.U.; Hassan, M.L. Habitat structure and diversity patterns of seaweeds in the coastal waters of Saint Martin’s Island, Bay of Bengal, Bangladesh. Reg. Stud. Mar. Sci. 2020, 33, 100959. [Google Scholar]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a source of natural antioxidants: Therapeutic activity and food applications. J. Food Qual. 2021, 2021, 5753391. [Google Scholar] [CrossRef]
- Koche, D.; Shirsat, R.; Kawale, M. An overerview of major classes of phytochemicals: Their types and role in disease prevention. Hislopia J. 2016, 9, 976–2124. [Google Scholar]
- Cotas, L.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Goncalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary anti-aging polyphenols and potential mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Alba, J.D.M.; Hernández, S.I.M.; López, G.C.V.; Flores, M.A. Anti-inflammatory effect of caffeic acid in an experimental model of pulpitis in guinea pigs. Rev. Asoc. Dent. Mex. 2016, 73, 250–254. [Google Scholar]
- Gao, Q.; Li, Y.; Li, Y.; Zhang, Z.; Liang, Y. Antioxidant and prooxidant activities of phenolic acids commonly existed in vegetables and their relationship with structures. Food Sci. Technol. 2022, 42, e07622. [Google Scholar] [CrossRef]
- Rosa, L.d.S.; Jordão, N.A.; Soares, N.d.C.P.; Mesquita, J.F.d.; Monteiro, M.; Teodoro, A.J. Pharmacokinetic, antiproliferative and apoptotic effects of phenolic acids in human colon adenocarcinoma cells using in vitro and in silico approaches. Molecules 2018, 23, 2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birošová, L.; Mikulášová, M.; Vaverková, Š. Antimutagenic effect of phenolic acids. Biomed. Pap. 2005, 149, 489–491. [Google Scholar] [CrossRef] [Green Version]
- Samar, J.; Butt, G.Y.; Shah, A.A.; Shah, A.N.; Ali, S.; Jan, B.L.; Abdelsalam, N.R.; Hussaan, M. Phycochemical and Biological Activities From Different Extracts of Padina antillarum (Kützing) Piccone. Front. Plant Sci. 2022, 13, 929368. [Google Scholar] [CrossRef]
- Soumya, K.; James, J.; Archana, T.; Dhanya, A.; Shahid, A.; Sudheesh, S. Cytotoxic and antigenotoxic properties of phenolic compound isolated from the fruit of Terminalia chebula on HeLa cell. Beni Suef Univ. J. Basic Appl. Sci. 2019, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Alvarado-Martinez, Z.; Bravo, P.; Kennedy, N.-F.; Krishna, M.; Hussain, S.; Young, A.C.; Biswas, D. Antimicrobial and antivirulence impacts of phenolics on Salmonella enterica serovar Typhimurium. Antibiotics 2020, 9, 668. [Google Scholar] [CrossRef]
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. Environmental impact on seaweed phenolic production and activity: An important step for compound exploitation. Mar. Drugs 2021, 19, 245. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef] [PubMed]
- Gall, E.A.; Lelchat, F.; Hupel, M.; Jégou, C.; Stiger-Pouvreau, V. Extraction and purification of phlorotannins from brown algae. In Natural Products from Marine Algae; Springer: Berlin/Heidelberg, Germany, 2015; pp. 131–143. [Google Scholar]
- Shoubaky, G.A.E.; Abdel-Daim, M.M.; Mansour, M.H.; Salem, E.A. Isolation and identification of a flavone apigenin from marine red alga Acanthophora spicifera with antinociceptive and anti-Inflammatory activities. J. Exp. Neurosci. 2016, 10, JEN-S25096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agregan, R.; Munekata, P.E.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Krishnamoorthy, P.; Dhamotharan, R. Isolation, identification of bromophenol compound and antibacterial activity of Kappaphycus sp. Int. J. Pharm. Biol. Sci. 2012, 3, 173–186. [Google Scholar]
- Sumayya, S.; Murugan, K. Antioxidant potentialities of marine red algae Gracillaria dura: A search. Pharma Innov. J. 2019, 8, 1157–1161. [Google Scholar]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, A.; Sharma, J. Stress physiology in plants. In Reforms in Agriculture and Rural Development Under COVID-19 Pandemic; Society of Human Resources and Innovation: Agra, India, 2020; pp. 175–186. [Google Scholar]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar]
- Anami, B.S.; Malvade, N.N.; Palaiah, S. Classification of yield affecting biotic and abiotic paddy crop stresses using field images. Inf. Processing Agric. 2020, 7, 272–285. [Google Scholar] [CrossRef]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The threat of the combined effect of biotic and abiotic stress factors in forestry under a changing climate. Front. Plant Sci. 2020, 11, 601009. [Google Scholar] [CrossRef]
- Nkomo, M.A. The Role of p-Coumaric Acid on Physiological and Biochemical Response of Chia Seedling Under Salt Stress. Ph.D. Thesis, University of the Western Cape, Cape Town, South Africa, 2020. [Google Scholar]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Zhao, B.; He, D.; Wang, L. Advances in Fusarium drug resistance research. J. Glob. Antimicrob. Resist. 2021, 24, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Jeong, G.-J.; Khan, M.S.A.; Tabassum, N.; Kim, Y.-M. Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms. Mar. Drugs 2022, 20, 384. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, R.; Pandey, R. Exogenous application of rutin and gallic acid regulate antioxidants and alleviate reactive oxygen generation in Oryza sativa L. Physiol. Mol. Biol. Plants 2017, 23, 301–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.A. Exogenous Caffeic Acid Alters Molecular Responses in Salvia hispanica L. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2016. [Google Scholar]
- Nguyen, T.Q. Application of Exogenous Phenolics for Drought Tolerance Improvement in Rice (Oryza sativa L.). Master’s Thesis, Hiroshima University, Hiroshima, Japan, 2018. [Google Scholar]
- Kumar, Y.; Singhal, S.; Tarafdar, A.; Pharande, A.; Ganesan, M.; Badgujar, P.C. Ultrasound assisted extraction of selected edible macroalgae: Effect on antioxidant activity and quantitative assessment of polyphenols by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Algal Res. 2020, 52, 102114. [Google Scholar] [CrossRef]
- Fabrowska, J.; Ibañez, E.; Łęska, B.; Herrero, M. Supercritical fluid extraction as a tool to valorize underexploited freshwater green algae. Algal Res. 2016, 19, 237–245. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Gomes, L.; Monteiro, P.; Cotas, J.; Gonçalves, A.M.; Fernandes, C.; Gonçalves, T.; Pereira, L. Seaweeds’ pigments and phenolic compounds with antimicrobial potential. Biomol. Concepts 2022, 13, 89–102. [Google Scholar] [CrossRef]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef]
- Neoh, Y.Y.; Matanjun, P.; Lee, J.S. Effects of Various Drying Processes on Malaysian Brown Seaweed, Sargassum polycystum Pertaining to Antioxidants Content and Activity. Trans. Sci. Technol. 2021, 8, 25–37. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Yuen, A.K.; Zhang, R.; Wright, J.T.; Taylor, R.B.; Maschmeyer, T.; de Nys, R. A comparative assessment of microwave assisted (MAE) and conventional solid-liquid (SLE) techniques for the extraction of phloroglucinol from brown seaweed. Algal Res. 2017, 23, 28–36. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Muñoz, M.J.G. Ultrasound-assisted extraction of bioactive carbohydrates. In Water Extraction of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 317–331. [Google Scholar]
- Michalak, I.; Dmytryk, A.; Wieczorek, P.P.; Rój, E.; Łęska, B.; Górka, B.; Messyasz, B.; Lipok, J.; Mikulewicz, M.; Wilk, R. Supercritical algal extracts: A source of biologically active compounds from nature. J. Chem. 2015, 2015, 597140. [Google Scholar] [CrossRef] [Green Version]
- Cikoš, A.-M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-López, C.; Rojas, R.; Sánchez-Alejo, E.J.; Niño-Medina, G.; Martínez-Ávila, G.C. Phenolic compounds recovery from grape fruit and by-products: An overview of extraction methods. In Grape And Wine Biotechnology; InTech: Vienna, Austria, 2016; pp. 103–123. [Google Scholar]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging technologies for the extraction of marine phenolics: Opportunities and challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef]
- Zaid, A.; Mohammad, F.; Wani, S.H.; Siddique, K.M. Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicol. Environ. Saf. 2019, 180, 575–587. [Google Scholar] [CrossRef]
- Saidi, I.; Guesmi, F.; Kharbech, O.; Hfaiedh, N.; Djebali, W. Gallic acid improves the antioxidant ability against cadmium toxicity: Impact on leaf lipid composition of sunflower (Helianthus annuus) seedlings. Ecotoxicol. Environ. Saf. 2021, 210, 111906. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Kulkarni, M.G.; Stirk, W.A.; Van Staden, J. Eckol-a new plant growth stimulant from the brown seaweed Ecklonia maxima. J. Appl. Phycol. 2015, 27, 581–587. [Google Scholar] [CrossRef]
- Ali, A.S.; Elozeiri, A.A. Metabolic processes during seed germination. In Advances in Seed Biology; InTech: Vienna, Austria, 2017; pp. 141–166. [Google Scholar]
- Joshi, R. Role of enzymes in seed germination. Int. J. Creat. Res. Thoughts 2018, 6, 1481–1485. [Google Scholar]
- Aremu, A.O.; Masondo, N.A.; Rengasamy, K.R.; Amoo, S.O.; Gruz, J.; Bíba, O.; Šubrtová, M.; Pěnčík, A.; Novák, O.; Doležal, K. Physiological role of phenolic biostimulants isolated from brown seaweed Ecklonia maxima on plant growth and development. Planta 2015, 241, 1313–1324. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Kulkarni, M.G.; Pendota, S.C.; Van Staden, J. Enhancing growth, phytochemical constituents and aphid resistance capacity in cabbage with foliar application of eckol—A biologically active phenolic molecule from brown seaweed. New Biotechnol. 2016, 33, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Briand, X.; Salamagne, S.C. Use of Phlorotannins as a Stimulant for Mychorrhizal and Rhizobial Symbioses. US Patent Application 15/754,741, 13 September 2018. [Google Scholar]
- Kulkarni, M.G.; Rengasamy, K.R.; Pendota, S.C.; Gruz, J.; Plačková, L.; Novák, O.; Doležal, K.; Van Staden, J. Bioactive molecules derived from smoke and seaweed Ecklonia maxima showing phytohormone-like activity in Spinacia oleracea L. New Biotechnol. 2019, 48, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tak, Y.; Potkule, J.; Choyal, P.; Tomar, M.; Meena, N.L.; Kaur, C. Phenolics as plant protective companion against abiotic stress. In Plant Phenolics in Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 277–308. [Google Scholar]
- Ficke, A.; Cowger, C.; Bergstrom, G.; Brodal, G. Understanding yield loss and pathogen biology to improve disease management: Septoria nodorum blotch-a case study in wheat. Plant Dis. 2018, 102, 696–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Zahid Nabi, S.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant drought stress tolerance: Understanding its physiological, biochemical and molecular mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925. [Google Scholar] [CrossRef]
- Sun, W.-J.; Nie, Y.-X.; Gao, Y.; Dai, A.-H.; Bai, J.-G. Exogenous cinnamic acid regulates antioxidant enzyme activity and reduces lipid peroxidation in drought-stressed cucumber leaves. Acta Physiol. Plant. 2012, 34, 641–655. [Google Scholar] [CrossRef]
- Quan, N.T.; Xuan, T.D. Foliar application of vanillic and p-hydroxybenzoic acids enhanced drought tolerance and formation of phytoalexin momilactones in rice. Arch. Agron. Soil Sci. 2018, 64, 1831–1846. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; Farag, M.A.; Kontominas, M.; Shakour, Z.T.; Ramadan, A.R. Nanoencapsulated Extract of a Red Seaweed (Rhodophyta) Species as a Promising Source of Natural Antioxidants. ACS Omega 2022, 7, 6539–6548. [Google Scholar] [CrossRef]
- Sumayya, S.; Lubaina, A.; Murugan, K. Phytochemical, HPLC and FTIR Analysis of Methanolic Extract from Gracilaria dura (C Agardh) J Agardh. J. Drug Deliv. Ther. 2020, 10, 114–118. [Google Scholar] [CrossRef]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef]
- Babich, O.; Sukhikh, S.; Larina, V.; Kalashnikova, O.; Kashirskikh, E.; Prosekov, A.; Noskova, S.; Ivanova, S.; Fendri, I.; Smaoui, S. Algae: Study of Edible and Biologically Active Fractions, Their Properties and Applications. Plants 2022, 11, 780. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Kucukoduk, M. Upregulation of antioxidant enzymes by exogenous gallic acid contributes to the amelioration in Oryza sativa roots exposed to salt and osmotic stress. Environ. Sci. Pollut. Res. 2015, 22, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Ofori, S.A.; Cobbina, S.J.; Obiri, S. Climate change, land, water, and food security: Perspectives From Sub-Saharan Africa. Front. Sustain. Food Syst. 2021, 5, 680924. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Cheng, Z.-Y.; Sun, L.; Wang, X.-J.; Sun, R.; An, Y.-Q.; An, B.-L.; Zhu, M.-X.; Zhao, C.-F.; Bai, J.-G. Ferulic acid pretreatment alleviates heat stress in blueberry seedlings by inducing antioxidant enzymes, proline, and soluble sugars. Biol. Plant. 2018, 62, 534–542. [Google Scholar] [CrossRef]
- Zhizhong, Z.; Lan, M.; Han, X.; Wu, J.; Wang-Pruski, G. Response of ornamental pepper to high-temperature stress and role of exogenous salicylic acid in mitigating high temperature. J. Plant Growth Regul. 2020, 39, 133–146. [Google Scholar]
- Tiwari, S.; Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, K.; Joseph, D. Antioxidant potential and phenolic compounds of brown seaweeds Turbinaria conoides and Turbinaria ornata (class: Phaeophyceae). J. Aquat. Food Prod. Technol. 2016, 25, 1249–1265. [Google Scholar] [CrossRef]
- Shuping, D.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complementary Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef]
- Li, L.; Zhu, X.-M.; Zhang, Y.-R.; Cai, Y.-Y.; Wang, J.-Y.; Liu, M.-Y.; Wang, J.-Y.; Bao, J.-D.; Lin, F.-C. Research on the Molecular Interaction Mechanism between Plants and Pathogenic Fungi. Int. J. Mol. Sci. 2022, 23, 4658. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Lc-esi-qtof-ms/ms characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Nehela, Y.; Taha, N.A.; Elzaawely, A.A.; Xuan, T.D.; Amin, M.; Ahmed, M.E.; El-Nagar, A. Benzoic acid and its hydroxylated derivatives suppress early blight of tomato (Alternaria solani) via the induction of salicylic acid biosynthesis and enzymatic and nonenzymatic antioxidant defense machinery. J. Fungi 2021, 7, 663. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crops Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- An, Y.; Sun, L.; Wang, X.; Sun, R.; Cheng, Z.; Zhu, Z.; Yan, G.; Li, Y.; Bai, J. Vanillic acid mitigates dehydration stress responses in blueberry plants. Russ. J. Plant Physiol. 2019, 66, 806–817. [Google Scholar] [CrossRef]
- Saleh, A.M.; Madany, M. Coumarin pretreatment alleviates salinity stress in wheat seedlings. Plant Physiol. Biochem. 2015, 88, 27–35. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules 2019, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, G. The Effects of Gallic Acid on the Membrane Proteome and Antioxidant System of Wheat Plants under Salt Stress. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2020. [Google Scholar]
- Mekawy, A.M.M.; Abdelaziz, M.N.; Ueda, A. Apigenin pretreatment enhances growth and salinity tolerance of rice seedlings. Plant Physiol. Biochem. 2018, 130, 94–104. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, J.; Yang, L.; Li, N.; Wang, Y.; Ao, T.; Chen, W. Foliar application of flavonoids (rutin) regulates phytoremediation efficiency of Amaranthus hypochondriacus L. by altering the permeability of cell membranes and immobilizing excess Cd in the cell wall. J. Hazard. Mater. 2022, 425, 127875. [Google Scholar]
- Zhang, X.; Ran, W.; Li, X.; Zhang, J.; Ye, M.; Lin, S.; Liu, M.; Sun, X. Exogenous Application of Gallic Acid Induces the Direct Defense of Tea Plant Against Ectropis obliqua Caterpillars. Front. Plant Sci. 2022, 13, 833489. [Google Scholar] [CrossRef] [PubMed]
- Yousif, D.Y. Effects sprayed solution of salicylic acid to prevent of wilt disease caused by Fusarium oxysporium. J. Phys. Conf. Ser. 2018, 1003, 012001. [Google Scholar] [CrossRef]
- Li, S.; Pi, J.; Zhu, H.; Yang, L.; Zhang, X.; Ding, W. Caffeic Acid in Tobacco Root Exudate Defends Tobacco Plants From Infection by Ralstonia solanacearum. Front. Plant Sci. 2021, 12, 690586. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Li, X.; Gao, L.; Zhang, Z.; Zhu, Y.; Zhu, H. Application of exogenous salicylic acid reduces disease severity of Plasmodiophora brassicae in pakchoi (Brassica campestris ssp. chinensis Makino). PLoS ONE 2021, 16, e0248648. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Lee, B.-R.; Das, P.R.; Jung, H.-i.; Kim, T.-H. Characterization of p-Coumaric acid-induced soluble and cell wall-bound phenolic metabolites in relation to disease resistance to Xanthomonas campestris pv. campestris in Chinese cabbage. Plant Physiol. Biochem. 2018, 125, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Leonberger, K.; Jackson, K.; Smith, R.; Ward Gauthier, N. Plant diseases. In Kentucky Master Gardner Manual; University of Kentucky: Lexington, KY, USA, 2016. [Google Scholar]
- Mondal, K.K. Phytopathogenic Bacteria and Plant Diseases by BS Thind. Indian Phytopathol. 2020, 73, 587. [Google Scholar] [CrossRef]
- Rajauria, G. Optimization and validation of reverse phase HPLC method for qualitative and quantitative assessment of polyphenols in seaweed. J. Pharm. Biomed. Anal. 2018, 148, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Nabi, S.U.; Yadav, M.K.; Dubey, A. Mixed infection of plant viruses: Diagnostics, interactions and impact on host. J. Plant Dis. Prot. 2021, 128, 353–368. [Google Scholar] [CrossRef]
- Mishra, J.; Srivastava, R.; Trivedi, P.K.; Verma, P.C. Effect of virus infection on the secondary metabolite production and phytohormone biosynthesis in plants. 3 Biotech 2020, 10, 547. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Sun, W.; Han, K.; Yang, M.; Si, Z.; Li, G.; Qiao, Y. Effects of exogenous salicylic acid on the resistance response of wild soybean plants (Glycine soja) infected with Soybean mosaic virus. Can. J. Plant Pathol. 2020, 42, 84–93. [Google Scholar] [CrossRef]
- Gossner, M.M.; Beenken, L.; Arend, K.; Begerow, D.; Peršoh, D. Insect herbivory facilitates the establishment of an invasive plant pathogen. ISME Commun. 2021, 1, 6. [Google Scholar] [CrossRef]
- Olate-Gallegos, C.; Barriga, A.; Vergara, C.; Fredes, C.; García, P.; Giménez, B.; Robert, P. Identification of polyphenols from chilean brown seaweeds extracts by LC-DAD-ESI-MS/MS. J. Aquat. Food Prod. Technol. 2019, 28, 375–391. [Google Scholar] [CrossRef]
- Tanna, B.; Brahmbhatt, H.R.; Mishra, A. Phenolic, flavonoid, and amino acid compositions reveal that selected tropical seaweeds have the potential to be functional food ingredients. J. Food Process. Preserv. 2019, 43, e14266. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.-J.; Kim, K.-M. Overexpression of OsF3H modulates WBPH stress by alteration of phenylpropanoid pathway at a transcriptomic and metabolomic level in Oryza sativa. Sci. Rep. 2020, 10, 14685. [Google Scholar] [CrossRef] [PubMed]

| Extraction Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Maceration | Simple to operate and inexpensive | It requires the use of lots of organic solvents which makes it not eco-friendly. It is time-consuming. | [49] |
| Soxhlet extraction method | It requires the use of a smaller volume of solvent compared to other traditional extraction methods such as maceration. The solvent can be recovered and reused. | It is not eco-friendly. It causes the degradation of thermolabile compounds. Only one sample can be processed at a time. | [49] |
| Microwave-assisted extraction | It involves the use of small volumes of solvent which makes it environmentally friendly and cost-effective. It is very fast, producing a high yield of the desired phenolic compound within a short time. | It operates under high temperature and microwave power which could denature heat-sensitive compounds. It requires extra separation procedures to remove solid impurities. | [50,51] |
| Ultrasound-assisted extraction | It also involves the use of small volumes of solvent which makes it environmentally friendly and cost-effective. It is suitable for extracting thermolabile compounds because it operates at a low temperature. The equipment used is inexpensive and easily affordable compared to other nonconventional extraction techniques. It can be scaled up for industrial applications. It is very fast, producing a high yield of the desired phenolic compound within a short period. | There may be inconsistency with the distribution of sound or mechanical waves within the medium. | [24,52] |
| Supercritical CO2 extraction | It can be separated from the extract completely without leaving toxic remains. It is very fast and produces a high yield within a very short period. It is eco-friendly because no organic solvents are used. Carbon dioxide has a low critical temperature which makes it suitable for extracting thermolabile compounds. It can be used for small-scale and large-scale purposes. The resulting extract is devoid of inorganic salts and heavy metals because they cannot be extracted by carbon dioxide. | The equipment is highly sophisticated and expensive. It cannot be used to extract polar compounds due to the low polarity of carbon dioxide. However, polar solvents such as methanol are added in small quantities to supercritical CO2 to enhance their extraction. | [14,53] |
| Supercritical water extraction | It is eco-friendly because it uses water as its nontoxic solvent. It is very fast producing a high yield within a short operating time. It can be used for extracting polar compounds. | It requires the use of highly sophisticated and expensive equipment. It operates under high temperature and pressure which could denature thermolabile compounds. | [49,54] |
| Enzyme-assisted extraction method | It can be used for small-scale and large-scale production. Toxic chemicals are not utilized during the extraction process, which makes them eco-friendly. It produces a high yield of the desired phenolic compound. It can be used in conjunction with other extraction methods to obtain a higher yield. | The enzymes used could be expensive which limits their use industrially. | [55,56] |
| Phenolic Compound | Plant Species | Type of Stress | Mechanism of Action | Reference |
|---|---|---|---|---|
| Salicylic acid | Safflower (Carthamus tinctorius L.). | Abiotic stress (drought) | Stimulated the nonenzymatic defense system. Increased synthesis of osmolytes. Increased synthesis of proline. | [87] |
| Vanillic acid | Blueberry (Vaccinium corymbosum L.) | Abiotic stress (drought) | Increased the transcription of genes encoding the synthesis of antioxidant enzymes in leaves. Increased the concentration of proline and soluble sugars. Decreased the concentration of malondialdehyde, superoxide anion, and hydrogen peroxide. Improved the relative water content. | [88] |
| p-hydroxybenzoic acid and vanillic acid | Rice (Oryza sativa) | Abiotic stress (drought) | Increased the synthesis of chlorophyll “a”, “b”, carotenoids, and total phenolic compounds. Promoted plant growth rate. Enhanced the synthesis of phytoalexin momilactone (MA and MB) which increased tolerance to drought. | [70] |
| Vanillic acid | Tomato (Solanum lycopersicum L. cv. Pusa Ruby) | Abiotic stress (salinity) | Enhanced the glyoxalase system, thus preventing the accumulation of methylglyoxal. Activated the antioxidant defense mechanism thereby preventing lipid peroxidation and accumulation of reactive oxygen species. Increased rate of photosynthesis. Regulated the cellular Na+/K+ concentration. Improved the relative water content. | [75] |
| Coumarin | Wheat (Triticum aestivum) | Abiotic stress (drought) | Enhanced the activity of peroxidase, thus preventing oxidative stress. Regulated the osmotic level in the cell by regulating cellular Na+/K+ concentration. Increased synthesis of phenylalanine ammonia-lyase enzyme which increased endogenous synthesis of phenolic compound. Improved plant growth. | [89] |
| Ferulic acid | Blueberry seedlings (Vaccinium corymbosum) | Abiotic stress (Extreme temperature) | Enhanced the transcription of genes encoding for the synthesis of antioxidant enzymes (glutathione peroxidase and superoxide dismutase) which decreased lipid peroxidation and build-up of reactive oxygen species. Increased relative water content due to increased concentration of proline and soluble sugars. | [79] |
| Salicylic acid | Vigna angularis | Abiotic stress (salinity) | Increased the relative water content due to increased synthesis of glycine betaine, proline, and soluble sugar. Enhanced the enzymatic and nonenzymatic antioxidant defense mechanism. Reduction in the cellular concentration of sodium and chloride ion. | [90] |
| Gallic acid | Wheat (Triticum aestivum L.) | Abiotic stress (salinity) | Enhanced the activity of the antioxidant enzymes, thereby reducing reactive oxygen species and lipid peroxidation. Improved plant growth. Enhanced photosynthesis by increasing the chlorophyll content. Improved the relative water content. | [91] |
| Apigenin | Rice (Oryza sativa L) | Abiotic stress (salinity) | Enhanced the activity of the enzymatic (ascorbate peroxidase and catalase) and nonenzymatic defense system (endogenous flavonoids and carotenoids) thereby preventing lipid peroxidation and accumulation of reactive oxygen species. Increases the transcription of genes encoding for the synthesis of Na+ transporter protein, thus regulating the concentration of Na+/K+ in the cells. | [92] |
| Salicylic acid | Ornamental pepper (Capsicum annuum L.) | Abiotic stress (extreme temperature) | Increased chlorophyll content increased the rate of photosynthesis. Activated the enzymatic and nonenzymatic defense mechanism, thus preventing the accumulation of reactive oxygen species. Prevented degradation of cellular structures by regulating osmotic balance. | [80] |
| Salicylic acid | Mustard plant (Brassica juncea L.Czern. & Coss. cv. Type 59) | Abiotic stress (heavy metal) | Increased rate of photosynthesis, thus improving plant growth. Increased activity of antioxidant enzymes which prevented oxidative stress. Activated the glyoxylate system (glyoxalase I and glyoxalase II enzymes) which reduced the accumulation of toxic methylglyoxal. | [57] |
| Gallic acid | Sunflower (Helianthus annuus) | Abiotic stress (heavy metal) | Prevented absorption of cadmium ion by the root. Enhanced the activity of glutathione reductase, catalase, and ascorbate peroxidase which alleviated oxidative stress and increased plant growth. | [58] |
| Rutin | Amaranthus hypochondriacus | Abiotic stress (heavy metal) | Enhanced the synthesis of glutathione and promoted the conversion of glutathione to phytoalexins which chelate metal and prevent its accumulation within the cell. Prevents degradation of the cell membrane by inhibiting lipid peroxidation. | [93] |
| Gallic acid | Tea plant (Camellia sinensis cv. Longjing 43) | Biotic stress (Ectropis obliqua larvae) | Activated the phenylpropanoid and jasmonic acid pathway which stimulated the synthesis of metabolites such as epigallocatechin-3-gallate, naringenin, and astragalin that prevented the larvae from feeding on tea plants. | [94] |
| Salicylic acid | Green pepper (Capsicum annuum) | Biotic stress (antifungal) | Stimulates some immune responses in host plants such as the expression of the pathogenesis-related (PR) gene, thus inducing system resistance against the fungi. Exhibiting fungitoxic effect on the fungi and activating the synthesis of enzymes which promote the production of defense compounds. | [95] |
| Eckol | Cabbage (Brassica oleracea) | Biotic (insect repelling) | Increased the enzyme myrosinase which prevented cabbage aphid (Brevicoryne brassicae) from attacking the leaves. | [63] |
| Caffeic acid | Tobacco (Nicotiana tobaccum) | Biotic stress (antibacterial) | Increased activity of peroxidase and phenylalanine ammonia-lyase which increased the deposit of lignin in the host cell wall, thus preventing bacteria invasion. Prevented the formation of biofilm in the plant root by inhibiting the expression of epsE and lecM genes. | [96] |
| Salicylic acid | Pakchoi (Brassicaceae) | Biotic stress (antifungal) | Promoting the activity of antioxidant enzymes by increasing the expression of the respective gene. Increased concentration of proline and soluble protein which regulates the relative water content in the root and leaves cells. | [97] |
| p-coumaric acid | Chinese cabbage (Brassica rapa var. pekinensis) | Biotic stress (antibacterial) | Promotes the expression of the CHS and HCT genes, thereby increasing the synthesis of endogenous phenolic compounds such as flavonoids, sinapic acid, and ferulic acid, which protects the plant from bacterial infection and promotes plant growth. | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aina, O.; Bakare, O.O.; Daniel, A.I.; Gokul, A.; Beukes, D.R.; Fadaka, A.O.; Keyster, M.; Klein, A. Seaweed-Derived Phenolic Compounds in Growth Promotion and Stress Alleviation in Plants. Life 2022, 12, 1548. https://doi.org/10.3390/life12101548
Aina O, Bakare OO, Daniel AI, Gokul A, Beukes DR, Fadaka AO, Keyster M, Klein A. Seaweed-Derived Phenolic Compounds in Growth Promotion and Stress Alleviation in Plants. Life. 2022; 12(10):1548. https://doi.org/10.3390/life12101548
Chicago/Turabian StyleAina, Omolola, Olalekan Olanrewaju Bakare, Augustine Innalegwu Daniel, Arun Gokul, Denzil R. Beukes, Adewale Oluwaseun Fadaka, Marshall Keyster, and Ashwil Klein. 2022. "Seaweed-Derived Phenolic Compounds in Growth Promotion and Stress Alleviation in Plants" Life 12, no. 10: 1548. https://doi.org/10.3390/life12101548
APA StyleAina, O., Bakare, O. O., Daniel, A. I., Gokul, A., Beukes, D. R., Fadaka, A. O., Keyster, M., & Klein, A. (2022). Seaweed-Derived Phenolic Compounds in Growth Promotion and Stress Alleviation in Plants. Life, 12(10), 1548. https://doi.org/10.3390/life12101548












