A Physicochemical Consideration of Prebiotic Microenvironments for Self-Assembly and Prebiotic Chemistry
Abstract
:1. Introduction
2. Prebiotic Microenvironments and Where to Find Them
2.1. Aqueous Environments
2.1.1. Bulk Aqueous Solution
2.1.2. Sea Spray (Aqueous Aerosols)
2.1.3. Gels and Other Hygroscopic Environments
2.1.4. Ice
2.2. Alternative Liquid Environments
2.2.1. Non-Aqueous Solvents
2.2.2. Deep Eutectic Solvents
2.2.3. High Pressure Supercritical Fluids (CO2, H2O)
2.2.4. Tars
2.2.5. Inside Lipid Bilayers and Related Interfacial Assemblies
2.2.6. Condensed Droplet Microenvironments
2.3. Minerals/Rocks
2.3.1. Solid Mineral Surfaces
2.3.2. Mantle
3. Physicochemical Properties
3.1. Ionic Strength
3.2. Surface Effects
3.3. Viscosity
3.4. Specific Heat Capacity
3.5. pH
3.6. Density
3.7. Dielectric Constant
3.8. Boiling, Melting/Freezing Temperatures
3.9. Vapor Pressure
3.10. Compressibility and Stiffness
3.11. Exposure to Radiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cleaves, H.J. Prebiotic Chemistry: Geochemical Context and Reaction Screening. Life 2013, 3, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Barge, L.M. Considering Planetary Environments in Origin of Life Studies. Nat. Commun. 2018, 9, 5170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunine, J.I. Physical Conditions on the Early Earth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1721–1731. [Google Scholar] [CrossRef] [Green Version]
- Krissansen-Totton, J.; Arney, G.N.; Catling, D.C. Constraining the Climate and Ocean pH of the Early Earth with a Geological Carbon Cycle Model. Proc. Natl. Acad. Sci. USA 2018, 115, 4105–4110. [Google Scholar] [CrossRef] [Green Version]
- Van Kranendonk, M.J. Earth’s Early Atmosphere and Surface Environments: A Review. In Earth’s Early Atmosphere and Surface Environment; Geological Society of America Special Papers; Geological Society of America: Boulder, CO, USA, 2014; pp. 105–130. ISBN 9780813725048. [Google Scholar]
- Damer, B.; Deamer, D. The Hot Spring Hypothesis for an Origin of Life. Astrobiology 2020, 20, 429–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, L.-F.; Du, Z.-F.; Hao, X.-L.; Cao, L.; Luan, Z.-D.; Wang, B.; Xi, S.-C.; Lian, C.; Yan, J.; et al. Discovery of Supercritical Carbon Dioxide in a Hydrothermal System. Sci. Bull. 2020, 65, 958–964. [Google Scholar] [CrossRef]
- Balucani, N. Elementary Reactions and Their Role in Gas-Phase Prebiotic Chemistry. Int. J. Mol. Sci. 2009, 10, 2304–2335. [Google Scholar] [CrossRef]
- Rimmer, P.B.; Shorttle, O. Origin of Life’s Building Blocks in Carbon- and Nitrogen-Rich Surface Hydrothermal Vents. Life 2019, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Green, N.J.; Xu, J.; Sutherland, J.D. Illuminating Life’s Origins: UV Photochemistry in Abiotic Synthesis of Biomolecules. J. Am. Chem. Soc. 2021, 143, 7219–7236. [Google Scholar] [CrossRef]
- Cooper, G.M. Cell Membranes. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Ghosh, B.; Bose, R.; Tang, T.-Y.D. Can Coacervation Unify Disparate Hypotheses in the Origin of Cellular Life? Curr. Opin. Colloid Interface Sci. 2021, 52, 101415. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Nozawa, R.-S.; Jia, T.Z.; Saio, T.; Mori, E. Biological Phase Separation: Cell Biology Meets Biophysics. Biophys. Rev. 2020, 12, 519–539. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.G. The Lakes and Seas of Titan. Annu. Rev. Earth Planet. Sci. 2016, 44, 57–83. [Google Scholar] [CrossRef]
- Barnes, J.W.; Turtle, E.P.; Trainer, M.G.; Lorenz, R.D.; MacKenzie, S.M.; Brinckerhoff, W.B.; Cable, M.L.; Ernst, C.M.; Freissinet, C.; Hand, K.P.; et al. Science Goals and Objectives for the Dragonfly Titan Rotorcraft Relocatable Lander. Planet. Sci. J. 2021, 2, 130. [Google Scholar] [CrossRef]
- Pizzarello, S.; Shock, E. The Organic Composition of Carbonaceous Meteorites: The Evolutionary Story ahead of Biochemistry. Cold Spring Harb. Perspect. Biol. 2010, 2, a002105. [Google Scholar] [CrossRef]
- Arumainayagam, C.R.; Garrod, R.T.; Boyer, M.C.; Hay, A.K.; Bao, S.T.; Campbell, J.S.; Wang, J.; Nowak, C.M.; Arumainayagam, M.R.; Hodge, P.J. Extraterrestrial Prebiotic Molecules: Photochemistry vs. Radiation Chemistry of Interstellar Ices. Chem. Soc. Rev. 2019, 48, 2293–2314. [Google Scholar] [CrossRef] [Green Version]
- Van Dishoeck, E.F.; Herbst, E.; Neufeld, D.A. Interstellar Water Chemistry: From Laboratory to Observations. Chem. Rev. 2013, 113, 9043–9085. [Google Scholar] [CrossRef] [Green Version]
- Lasne, J.; Noblet, A.; Szopa, C.; Navarro-González, R.; Cabane, M.; Poch, O.; Stalport, F.; François, P.; Atreya, S.K.; Coll, P. Oxidants at the Surface of Mars: A Review in Light of Recent Exploration Results. Astrobiology 2016, 16, 977–996. [Google Scholar] [CrossRef] [Green Version]
- Deamer, D.; Damer, B. Can Life Begin on Enceladus? A Perspective from Hydrothermal Chemistry. Astrobiology 2017, 17, 834–839. [Google Scholar] [CrossRef]
- Cable, M.L.; Hörst, S.M.; Hodyss, R.; Beauchamp, P.M.; Smith, M.A.; Willis, P.A. Titan Tholins: Simulating Titan Organic Chemistry in the Cassini-Huygens Era. Chem. Rev. 2012, 112, 1882–1909. [Google Scholar] [CrossRef]
- Kitadai, N.; Maruyama, S. Origins of Building Blocks of Life: A Review. Geosci. Front. 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Cleaves, H.J. Prebiotic Chemistry: What We Know, What We Don’t. Evolution 2012, 5, 342–360. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic Systems Chemistry: New Perspectives for the Origins of Life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef]
- Deamer, D.; Singaram, S.; Rajamani, S.; Kompanichenko, V.; Guggenheim, S. Self-Assembly Processes in the Prebiotic Environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1809–1818. [Google Scholar] [CrossRef] [Green Version]
- Tanford, C. The Hydrophobic Effect and the Organization of Living Matter. Science 1978, 200, 1012–1018. [Google Scholar] [CrossRef]
- Das, S.; Lin, Y.-H.; Vernon, R.M.; Forman-Kay, J.D.; Chan, H.S. Comparative Roles of Charge, and Hydrophobic Interactions in Sequence-Dependent Phase Separation of Intrinsically Disordered Proteins. Proc. Natl. Acad. Sci. USA 2020, 117, 28795–28805. [Google Scholar] [CrossRef]
- Maibaum, L.; Dinner, A.R.; Chandler, D. Micelle Formation and the Hydrophobic Effect. J. Phys. Chem. B 2004, 108, 6778–6781. [Google Scholar] [CrossRef] [Green Version]
- Farmer, J.R.; Sigman, D.M.; Granger, J.; Underwood, O.M.; Fripiat, F.; Cronin, T.M.; Martínez-García, A.; Haug, G.H. Arctic Ocean Stratification Set by Sea Level and Freshwater Inputs since the Last Ice Age. Nat. Geosci. 2021, 14, 684–689. [Google Scholar] [CrossRef]
- Mast, C.B.; Schink, S.; Gerland, U.; Braun, D. Escalation of Polymerization in a Thermal Gradient. Proc. Natl. Acad. Sci. USA 2013, 110, 8030–8035. [Google Scholar] [CrossRef] [Green Version]
- Herschy, B.; Whicher, A.; Camprubi, E.; Watson, C.; Dartnell, L.; Ward, J.; Evans, J.R.G.; Lane, N. An Origin-of-Life Reactor to Simulate Alkaline Hydrothermal Vents. J. Mol. Evol. 2014, 79, 213–227. [Google Scholar] [CrossRef] [Green Version]
- Kreysing, M.; Keil, L.; Lanzmich, S.; Braun, D. Heat Flux across an Open Pore Enables the Continuous Replication and Selection of Oligonucleotides towards Increasing Length. Nat. Chem. 2015, 7, 203–208. [Google Scholar] [CrossRef]
- Dobson, C.M.; Ellison, G.B.; Tuck, A.F.; Vaida, V. Atmospheric Aerosols as Prebiotic Chemical Reactors. Proc. Natl. Acad. Sci. USA 2000, 97, 11864–11868. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, D.J.; Tervahattu, H.; Tuck, A.F.; Vaida, V. Organic Aerosols and the Origin of Life: An Hypothesis. Orig. Life Evol. Biosph. 2004, 34, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Rapf, R.J.; Perkins, R.J.; Dooley, M.R.; Kroll, J.A.; Carpenter, B.K.; Vaida, V. Environmental Processing of Lipids Driven by Aqueous Photochemistry of α-Keto Acids. ACS Cent. Sci. 2018, 4, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Fournier, R.O.; Rowe, J.J. The Deposition of Silica in Hot Springs. Bull. Volcanol. 1966, 29, 585–587. [Google Scholar] [CrossRef]
- Mamajanov, I. Wet-Dry Cycling Delays the Gelation of Hyperbranched Polyesters: Implications to the Origin of Life. Life 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Dass, A.V.; Jaber, M.; Brack, A.; Foucher, F.; Kee, T.P.; Georgelin, T.; Westall, F. Potential Role of Inorganic Confined Environments in Prebiotic Phosphorylation. Life 2018, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Campbell, T.D.; Febrian, R.; McCarthy, J.T.; Kleinschmidt, H.E.; Forsythe, J.G.; Bracher, P.J. Prebiotic Condensation through Wet–dry Cycling Regulated by Deliquescence. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mamajanov, I.; Caudan, M.; Jia, T.Z. Protoenzymes: The Case of Hyperbranched Polymer-Scaffolded ZnS Nanocrystals. Life 2020, 10, 150. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Orlando, T.M.; Leszczynski, J.; Nguyen, M.T. Theoretical Study of the Decomposition of Formamide in the Presence of Water Molecules. J. Phys. Chem. A 2013, 117, 2543–2555. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Saladino, R.; Delfino, I.; García-Ruiz, J.M.; Di Mauro, E. Prebiotic Organic Chemistry of Formamide and the Origin of Life in Planetary Conditions: What We Know and What Is the Future. Int. J. Mol. Sci. 2021, 22, 917. [Google Scholar] [CrossRef] [PubMed]
- Saitta, A.M.; Saija, F. Miller Experiments in Atomistic Computer Simulations. Proc. Natl. Acad. Sci. USA 2014, 111, 13768–13773. [Google Scholar] [CrossRef] [Green Version]
- Koike, T.; Kaneko, T.; Kobayashi, K.; Miyakawa, S.; Takano, Y. Formation of Organic Compounds from Simulated Titan Atmosphere: Perspectives of the Cassini Mission. Biol. Sci. Space 2003, 17, 188–189. [Google Scholar]
- Gerakines, P.A.; Moore, M.H.; Hudson, R.L. Ultraviolet Photolysis and Proton Irradiation of Astrophysical Ice Analogs Containing Hydrogen Cyanide. Icarus 2004, 170, 202–213. [Google Scholar] [CrossRef]
- Takano, Y.; Tsuboi, T.; Kaneko, T.; Kobayashi, K.; Marumo, K. Pyrolysis of High-Molecular-Weight Complex Organics Synthesized from a Simulated Interstellar Gas Mixture Irradiated with 3 MeV Proton Beam. Bull. Chem. Soc. Jpn. 2004, 77, 779–783. [Google Scholar] [CrossRef] [Green Version]
- Saladino, R.; Crestini, C.; Pino, S.; Costanzo, G.; Di Mauro, E. Formamide and the Origin of Life. Phys. Life Rev. 2012, 9, 84–104. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Gállego, I.; Laughlin, B.; Grover, M.A.; Hud, N.V. A Viscous Solvent Enables Information Transfer from Gene-Length Nucleic Acids in a Model Prebiotic Replication Cycle. Nat. Chem. 2017, 9, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/urea Mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Gull, M.; Zhou, M.; Fernández, F.M.; Pasek, M.A. Prebiotic Phosphate Ester Syntheses in a Deep Eutectic Solvent. J. Mol. Evol. 2014, 78, 109–117. [Google Scholar] [CrossRef]
- Chien, C.-Y.; Yu, S.-S. Ester-Mediated Peptide Formation Promoted by Deep Eutectic Solvents: A Facile Pathway to Proto-Peptides. Chem. Commun. 2020, 56, 11949–11952. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Smith, P.J.; Arroyo, C.B.; Lopez Hernandez, F.; Goeltz, J.C. Ternary Deep Eutectic Solvent Behavior of Water and Urea? Choline Chloride Mixtures. J. Phys. Chem. B 2019, 123, 5302–5306. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Serra, I.; Martín-Pintado, J.; Rivilla, V.M.; Rodríguez-Almeida, L.; Alonso Alonso, E.R.; Zeng, S.; Cocinero, E.J.; Martín, S.; Requena-Torres, M.; Martín-Domenech, R.; et al. Toward the RNA-World in the Interstellar Medium-Detection of Urea and Search of 2-Amino-Oxazole and Simple Sugars. Astrobiology 2020, 20, 1048–1066. [Google Scholar] [CrossRef]
- Kaiser, R.I.; Maity, S.; Jones, B.M. Synthesis of Prebiotic Glycerol in Interstellar Ices. Angew. Chem. Int. Ed. Engl. 2015, 54, 195–200. [Google Scholar] [CrossRef]
- Yi, R.; Tran, Q.P.; Ali, S.; Yoda, I.; Adam, Z.R.; Cleaves, H.J., 2nd; Fahrenbach, A.C. A Continuous Reaction Network That Produces RNA Precursors. Proc. Natl. Acad. Sci. USA 2020, 117, 13267–13274. [Google Scholar] [CrossRef]
- Raveendran, P.; Ikushima, Y.; Wallen, S.L. Polar Attributes of Supercritical Carbon Dioxide. Acc. Chem. Res. 2005, 38, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, S.; Tsujii, K. Supercritical Water: A Fascinating Medium for Soft Matter. Soft Matter 2007, 3, 797–803. [Google Scholar] [CrossRef]
- Guttenberg, N.; Virgo, N.; Chandru, K.; Scharf, C.; Mamajanov, I. Bulk Measurements of Messy Chemistries Are Needed for a Theory of the Origins of Life. Philos. Trans. R. Soc. A 2017, 375, 20160347. [Google Scholar] [CrossRef]
- Barranco, F.T.; Dawson, H.E. Influence of Aqueous pH on the Interfacial Properties of Coal Tar. Environ. Sci. Technol. 1999, 33, 1598–1603. [Google Scholar] [CrossRef]
- Serrano-Luginbühl, S.; Ruiz-Mirazo, K.; Ostaszewski, R.; Gallou, F.; Walde, P. Soft and Dispersed Interface-Rich Aqueous Systems That Promote and Guide Chemical Reactions. Nat. Rev. Chem. 2018, 2, 306–327. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: San Diego, CA, USA, 2010; ISBN 9780123751829. [Google Scholar]
- Hanczyc, M.M.; Mansy, S.S.; Szostak, J.W. Mineral Surface Directed Membrane Assembly. Orig. Life Evol. Biosph. 2007, 37, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bomans, P.H.H.; Müller, F.A.; Will, J.; Frederik, P.M.; de With, G.; Sommerdijk, N.A.J.M. The Role of Prenucleation Clusters in Surface-Induced Calcium Phosphate Crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Köksal, E.S.; Liese, S.; Kantarci, I.; Olsson, R.; Carlson, A.; Gözen, I. Nanotube-Mediated Path to Protocell Formation. ACS Nano 2019, 13, 6867–6878. [Google Scholar] [CrossRef] [PubMed]
- Steller, L.H.; Van Kranendonk, M.J.; Wang, A. Dehydration Enhances Prebiotic Lipid Remodeling and Vesicle Formation in Acidic Environments. ACS Cent. Sci. 2022, 8, 132–139. [Google Scholar] [CrossRef]
- Rajamani, S.; Vlassov, A.; Benner, S.; Coombs, A.; Olasagasti, F.; Deamer, D. Lipid-Assisted Synthesis of RNA-like Polymers from Mononucleotides. Orig. Life Evol. Biosph. 2008, 38, 57–74. [Google Scholar] [CrossRef]
- Fraccia, T.P.; Jia, T.Z. Liquid Crystal Coacervates Composed of Short Double-Stranded DNA and Cationic Peptides. ACS Nano 2020, 14, 15071–15082. [Google Scholar] [CrossRef]
- Poudyal, R.R.; Pir Cakmak, F.; Keating, C.D.; Bevilacqua, P.C. Physical Principles and Extant Biology Reveal Roles for RNA-Containing Membraneless Compartments in Origins of Life Chemistry. Biochemistry 2018, 57, 2509–2519. [Google Scholar] [CrossRef]
- Koga, S.; Williams, D.S.; Perriman, A.W.; Mann, S. Peptide-Nucleotide Microdroplets as a Step towards a Membrane-Free Protocell Model. Nat. Chem. 2011, 3, 720–724. [Google Scholar] [CrossRef]
- Cakmak, F.P.; Keating, C.D. Combining Catalytic Microparticles with Droplets Formed by Phase Coexistence: Adsorption and Activity of Natural Clays at the Aqueous/Aqueous Interface. Sci. Rep. 2017, 7, 3215. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Kinghorn, A.B.; Zhang, Y.; Li, Q.; Poonam, A.D.; Tanner, J.A.; Shum, H.C. Non-Associative Phase Separation in an Evaporating Droplet as a Model for Prebiotic Compartmentalization. Nat. Commun. 2021, 12, 3194. [Google Scholar] [CrossRef]
- Jia, T.Z.; Hentrich, C.; Szostak, J.W. Rapid RNA Exchange in Aqueous Two-Phase System and Coacervate Droplets. Orig. Life Evol. Biosph. 2014, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.Z.; Chandru, K.; Hongo, Y.; Afrin, R.; Usui, T.; Myojo, K.; Cleaves, H.J. Membraneless Polyester Microdroplets as Primordial Compartments at the Origins of Life. Proc. Natl. Acad. Sci. USA 2019, 116, 15830–15835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, T.Z.; Bapat, N.V.; Verma, A.; Mamajanov, I.; Cleaves, H.J.; Chandru, K. Incorporation of Basic α-Hydroxy Acid Residues into Primitive Polyester Microdroplets for RNA Segregation. Biomacromolecules 2021, 22, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Afrin, R.; Chen, C.; Sarpa, D.; Sithamparam, M.; Yi, R.; Giri, C.; Mamajanov, I.; Cleaves, H.J., II; Chandru, K.; Jia, T.Z. The Effects of Dehydration Temperature and Monomer Chirality on Primitive Polyester Synthesis and Microdroplet Assembly. Macromol. Chem. Phys. 2022, 2200235. [Google Scholar] [CrossRef]
- Mountain, G.A.; Keating, C.D. Formation of Multiphase Complex Coacervates and Partitioning of Biomolecules within Them. Biomacromolecules 2020, 21, 630–640. [Google Scholar] [CrossRef]
- Frankel, E.A.; Bevilacqua, P.C.; Keating, C.D. Polyamine/Nucleotide Coacervates Provide Strong Compartmentalization of Mg2+, Nucleotides, and RNA. Langmuir 2016, 32, 2041–2049. [Google Scholar] [CrossRef]
- Zaia, D.A.M. A Review of Adsorption of Amino Acids on Minerals: Was It Important for Origin of Life? Amino Acids 2004, 27, 113–118. [Google Scholar] [CrossRef]
- Pedreira-Segade, U.; Hao, J.; Razafitianamaharavo, A.; Pelletier, M.; Marry, V.; Le Crom, S.; Michot, L.J.; Daniel, I. How Do Nucleotides Adsorb Onto Clays? Life 2018, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, H. Adsorption of Nucleic Acid Bases, Ribose, and Phosphate by Some Clay Minerals. Life 2015, 5, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.-F. Adsorption and Polymerization of Amino Acids on Mineral Surfaces: A Review. Orig. Life Evol. Biosph. 2008, 38, 211–242. [Google Scholar] [CrossRef]
- Erastova, V.; Degiacomi, M.T.; G Fraser, D.; Greenwell, H.C. Mineral Surface Chemistry Control for Origin of Prebiotic Peptides. Nat. Commun. 2017, 8, 2033. [Google Scholar] [CrossRef] [PubMed]
- Rimola, A.; Sodupe, M.; Ugliengo, P. Role of Mineral Surfaces in Prebiotic Chemical Evolution. In Silico Quantum Mechanical Studies. Life 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Aguilar, C.D.; Cuéllar-Cruz, M. The Formation of Crystalline Minerals and Their Role in the Origin of Life on Earth. Prog. Cryst. Growth Charact. Mater. 2022, 68, 100558. [Google Scholar] [CrossRef]
- Gillams, R.J.; Jia, T.Z. Mineral Surface-Templated Self-Assembling Systems: Case Studies from Nanoscience and Surface Science towards Origins of Life Research. Life 2018, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Cleaves, H.J.; Michalkova Scott, A.; Hill, F.C.; Leszczynski, J.; Sahai, N.; Hazen, R. Mineral-Organic Interfacial Processes: Potential Roles in the Origins of Life. Chem. Soc. Rev. 2012, 41, 5502–5525. [Google Scholar] [CrossRef]
- Hazen, R.M. Chance, Necessity and the Origins of Life: A Physical Sciences Perspective. Philos. Trans. A Math. Phys. Eng. Sci. 2017, 375, 20160353. [Google Scholar] [CrossRef] [Green Version]
- Li, Y. Minerals as Prebiotic Catalysts for Chemical Evolution towards the Origin of Life. In Mineralogy; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Pasek, M.; Herschy, B.; Kee, T.P. Phosphorus: A Case for Mineral-Organic Reactions in Prebiotic Chemistry. Orig. Life Evol. Biosph. 2015, 45, 207–218. [Google Scholar] [CrossRef]
- Walton, C.R.; Shorttle, O.; Jenner, F.E.; Williams, H.M.; Golden, J.; Morrison, S.M.; Downs, R.T.; Zerkle, A.; Hazen, R.M.; Pasek, M. Phosphorus Mineral Evolution and Prebiotic Chemistry: From Minerals to Microbes. Earth Sci. Rev. 2021, 221, 103806. [Google Scholar] [CrossRef]
- Gözen, İ. Did Solid Surfaces Enable the Origin of Life? Life 2021, 11, 795. [Google Scholar] [CrossRef]
- Stolar, T.; Grubešić, S.; Cindro, N.; Meštrović, E.; Užarević, K.; Hernández, J.G. Mechanochemical Prebiotic Peptide Bond Formation*. Angew. Chem. Int. Ed. Engl. 2021, 60, 12727–12731. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Chalmers, J.H.; Lazcano, A.; Miller, S.L.; Bada, J.L. A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres. Orig. Life Evol. Biosph. 2008, 38, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M. Mantle Convection: A Review. Fluid Dyn. Res. 2008, 40, 379–398. [Google Scholar] [CrossRef]
- Frost, D.J.; Liebske, C.; Langenhorst, F.; McCammon, C.A.; Trønnes, R.G.; Rubie, D.C. Experimental Evidence for the Existence of Iron-Rich Metal in the Earth’s Lower Mantle. Nature 2004, 428, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.A.; Kim, H.-J.; Biondi, E. Prebiotic Chemistry That Could Not Not Have Happened. Life 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Labrosse, S.; Hernlund, J.W.; Coltice, N. A Crystallizing Dense Magma Ocean at the Base of the Earth’s Mantle. Nature 2007, 450, 866–869. [Google Scholar] [CrossRef]
- Hu, Q.; Kim, D.Y.; Liu, J.; Meng, Y.; Yang, L.; Zhang, D.; Mao, W.L.; Mao, H.-K. Dehydrogenation of Goethite in Earth’s Deep Lower Mantle. Proc. Natl. Acad. Sci. USA 2017, 114, 1498–1501. [Google Scholar] [CrossRef] [Green Version]
- Sastre de Vicente, M.E. The Concept of Ionic Strength Eighty Years after Its Introduction in Chemistry. J. Chem. Educ. 2004, 81, 750. [Google Scholar] [CrossRef]
- Khouri, S.J. Titrimetric Study of the Solubility and Dissociation of Benzoic Acid in Water: Effect of Ionic Strength and Temperature. Am. J. Analyt. Chem. 2015, 06, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Li, K.; Li, Y.; Liu, H.; Guo, M.; Ye, X.; Wu, Z.; Lee, K. Dyes Adsorption onto Fe3O4 -bis(trimethoxysilylpropyl)amine Composite Particles: Effects of pH and Ionic Strength on Electrostatic Interactions. ChemistrySelect 2019, 4, 617–622. [Google Scholar] [CrossRef]
- Melkikh, A.V.; Sutormina, M.I. Model of Active Transport of Ions in Cardiac Cell. J. Theor. Biol. 2008, 252, 247–254. [Google Scholar] [CrossRef]
- Millero, F.J. The Physical Chemistry of Natural Waters. Pure Appl. Chem. 1985, 57, 1015–1024. [Google Scholar] [CrossRef]
- Mekic, M.; Gligorovski, S. Ionic Strength Effects on Heterogeneous and Multiphase Chemistry: Clouds versus Aerosol Particles. Atmos. Environ. 2021, 244, 117911. [Google Scholar] [CrossRef]
- Feng, Y.; Taraban, M.; Yu, Y.B. The Effect of Ionic Strength on the Mechanical, Structural and Transport Properties of Peptide Hydrogels. Soft Matter 2012, 8, 11723–11731. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Tomić, M.; Gieselmann, M.J.; Villegas, M.A. The Critical Gelling Point in Silica Gels Containing Lithium, Sodium and Potassium. J. Non-Cryst. Solids 1989, 110, 17–25. [Google Scholar] [CrossRef]
- Sanchez-Fernandez, A.; Jackson, A.J.; Prévost, S.F.; Doutch, J.J.; Edler, K.J. Long-Range Electrostatic Colloidal Interactions and Specific Ion Effects in Deep Eutectic Solvents. J. Am. Chem. Soc. 2021, 143, 14158–14168. [Google Scholar] [CrossRef]
- Abbott, A.P.; Edler, K.J.; Page, A.J. Deep Eutectic Solvents-The Vital Link between Ionic Liquids and Ionic Solutions. J. Chem. Phys. 2021, 155, 150401. [Google Scholar] [CrossRef]
- Porras, S.P.; Kenndler, E. Formamide as Solvent for Capillary Zone Electrophoresis. Electrophoresis 2004, 25, 2946–2958. [Google Scholar] [CrossRef]
- Redondo-Morata, L.; Oncins, G.; Sanz, F. Force Spectroscopy Reveals the Effect of Different Ions in the Nanomechanical Behavior of Phospholipid Model Membranes: The Case of Potassium Cation. Biophys. J. 2012, 102, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Deamer, D. The Role of Lipid Membranes in Life’s Origin. Life 2017, 7, 5. [Google Scholar] [CrossRef]
- Walton, C.R.; Shorttle, O. Scum of the Earth: A Hypothesis for Prebiotic Multi-Compartmentalised Environments. Life 2021, 11, 976. [Google Scholar] [CrossRef]
- Huang, Y.; Barraza, K.M.; Kenseth, C.M.; Zhao, R.; Wang, C.; Beauchamp, J.L.; Seinfeld, J.H. Probing the OH Oxidation of Pinonic Acid at the Air-Water Interface Using Field-Induced Droplet Ionization Mass Spectrometry (FIDI-MS). J. Phys. Chem. A 2018, 122, 6445–6456. [Google Scholar] [CrossRef] [PubMed]
- Morasch, M.; Liu, J.; Dirscherl, C.F.; Ianeselli, A.; Kühnlein, A.; Le Vay, K.; Schwintek, P.; Islam, S.; Corpinot, M.K.; Scheu, B.; et al. Heated Gas Bubbles Enrich, Crystallize, Dry, Phosphorylate and Encapsulate Prebiotic Molecules. Nat. Chem. 2019, 11, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Hazen, R.M.; Sverjensky, D.A. Mineral Surfaces, Geochemical Complexities, and the Origins of Life. Cold Spring Harb. Perspect. Biol. 2010, 2, a002162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.; Bao, X. Fundamental Insights into Interfacial Catalysis. Chem. Soc. Rev. 2017, 46, 1770–1771. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L.; White, S.P.; Otwinowski, Z.; Yuan, W.; Gelb, M.H.; Sigler, P.B. Interfacial Catalysis: The Mechanism of Phospholipase A2. Science 1990, 250, 1541–1546. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Leclercq, L.; Clacens, J.-M.; De Campo, F.; Nardello-Rataj, V. Pickering Interfacial Catalysis for Biphasic Systems: From Emulsion Design to Green Reactions. Angew. Chem. Int. Ed. Engl. 2015, 54, 2006–2021. [Google Scholar] [CrossRef]
- Carroll, K.M.; Knoll, A.W.; Wolf, H.; Duerig, U. Explaining the Transition from Diffusion Limited to Reaction Limited Surface Assembly of Molecular Species through Spatial Variations. Langmuir 2018, 34, 73–80. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Lozoya-Colinas, A.; Gállego, I.; Grover, M.A.; Hud, N.V. Solvent Viscosity Facilitates Replication and Ribozyme Catalysis from an RNA Duplex in a Model Prebiotic Process. Nucleic Acids Res. 2019, 47, 6569–6577. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Generous, M.M.; Qureshi, B.A.; Zubair, S.M. A Comprehensive Review of Saline Water Correlations and Data: Part II—Thermophysical Properties. Arab. J. Sci. Eng. 2021, 46, 1941–1979. [Google Scholar] [CrossRef]
- Nayar, K.G.; Sharqawy, M.H.; Banchik, L.D.; Lienhard, J.H., V. Thermophysical Properties of Seawater: A Review and New Correlations That Include Pressure Dependence. Desalination 2016, 390, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Sharqawy, M.H.; Lienhard, J.H., V; Zubair, S.M. Thermophysical Properties of Seawater: A Review of Existing Correlations and Data. Desalination Water Treat. 2010, 16, 354–380. [Google Scholar] [CrossRef]
- Tumminello, P.R.; James, R.C.; Kruse, S.; Kawasaki, A.; Cooper, A.; Guadalupe-Diaz, I.; Zepeda, K.L.; Crocker, D.R.; Mayer, K.J.; Sauer, J.S.; et al. Evolution of Sea Spray Aerosol Particle Phase State across a Phytoplankton Bloom. ACS Earth Space Chem. 2021, 5, 2995–3007. [Google Scholar] [CrossRef]
- Ghaffari, Z.; Rezvani, H.; Khalilnezhad, A.; Cortes, F.B.; Riazi, M. Experimental Characterization of Colloidal Silica Gel for Water Conformance Control in Oil Reservoirs. Sci. Rep. 2022, 12, 9628. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.C. Glaciers and Ice Sheets. In The Mathematics of Models for Climatology and Environment; Springer: Berlin/Heidelberg, Germany, 1997; pp. 301–336. ISBN 9783642644726. [Google Scholar]
- Wang, Y.; Ma, C.; Liu, C.; Lu, X.; Feng, X.; Ji, X. Thermodynamic Study of Choline Chloride-Based Deep Eutectic Solvents with Water and Methanol. J. Chem. Eng. Data 2020, 65, 2446–2457. [Google Scholar] [CrossRef]
- Lemaoui, T.; Darwish, A.S.; Attoui, A.; Abu Hatab, F.; Hammoudi, N.E.H.; Benguerba, Y.; Vega, L.F.; Alnashef, I.M. Predicting the Density and Viscosity of Hydrophobic Eutectic Solvents: Towards the Development of Sustainable Solvents. Green Chem. 2020, 22, 8511–8530. [Google Scholar] [CrossRef]
- Cases, A.M.; Gómez Marigliano, A.C.; Bonatti, C.M.; Sólimo, H.N. Density, Viscosity, and Refractive Index of Formamide, Three Carboxylic Acids, and Formamide + Carboxylic Acid Binary Mixtures. J. Chem. Eng. Data 2001, 46, 712–715. [Google Scholar] [CrossRef]
- Heidaryan, E.; Hatami, T.; Rahimi, M.; Moghadasi, J. Viscosity of Pure Carbon Dioxide at Supercritical Region: Measurement and Correlation Approach. J. Supercrit. Fluids 2011, 56, 144–151. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, G.; Zhang, L.; Ma, H.; Song, F.; Cao, Q.; Zhang, X. Simple and Accurate Calculation Model of Viscosity for Supercritical CO2. J. Phys. Conf. Ser. 2021, 2076, 012030. [Google Scholar] [CrossRef]
- Zheng, H.; Yu, T.; Qu, C.; Li, W.; Wang, Y. Basic Characteristics and Application Progress of Supercritical Water. IOP Conf. Ser. Earth Environ. Sci. 2020, 555, 012036. [Google Scholar] [CrossRef]
- Wood, L.J.; Downer, M. Viscosity/temperature Equations for Coal Tar Pitches and Refined Tars. J. Appl. Chem. 2007, 15, 431–438. [Google Scholar] [CrossRef]
- Nojima, Y.; Iwata, K. Viscosity Heterogeneity inside Lipid Bilayers of Single-Component Phosphatidylcholine Liposomes Observed with Picosecond Time-Resolved Fluorescence Spectroscopy. J. Phys. Chem. B 2014, 118, 8631–8641. [Google Scholar] [CrossRef]
- Lu, T.; Spruijt, E. Multiphase Complex Coacervate Droplets. J. Am. Chem. Soc. 2020, 142, 2905–2914. [Google Scholar] [CrossRef] [Green Version]
- She, Y.; Fu, G. Viscosities of the Crust and Upper Mantle Constrained by Three-Dimensional GPS Rates in the Sichuan–Yunnan Fragment of China. Earth Planets Space 2019, 71, 33. [Google Scholar] [CrossRef]
- Yousefi, Y.; Tariku, F. Thermal Conductivity and Specific Heat Capacity of Insulation Materials at Different Mean Temperatures. J. Phys. Conf. Ser. 2021, 2069, 012090. [Google Scholar] [CrossRef]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Fazio, E.; Chen, S.-H.; Cupane, A. Specific Heat and Transport Functions of Water. Int. J. Mol. Sci. 2020, 21, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waples, D.W.; Waples, J.S. A Review and Evaluation of Specific Heat Capacities of Rocks, Minerals, and Subsurface Fluids. Part 2: Fluids and Porous Rocks. Nat. Resour. Res. 2004, 13, 123–130. [Google Scholar] [CrossRef]
- Pirajno, F. Subaerial Hot Springs and near-Surface Hydrothermal Mineral Systems Past and Present, and Possible Extraterrestrial Analogues. Geosci. Front. 2020, 11, 1549–1569. [Google Scholar] [CrossRef]
- Kitadai, N.; Nakamura, R.; Yamamoto, M.; Okada, S.; Takahagi, W.; Nakano, Y.; Takahashi, Y.; Takai, K.; Oono, Y. Thioester Synthesis through Geoelectrochemical CO2 Fixation on Ni Sulfides. Commun. Chem. 2021, 4, 37. [Google Scholar] [CrossRef]
- Yang, F.; Wang, G.; Hu, D.; Zhou, H.; Tan, X. Influence of Water-Rock Interaction on Permeability and Heat Conductivity of Granite under High Temperature and Pressure Conditions. Geothermics 2022, 100, 102347. [Google Scholar] [CrossRef]
- Feistel, R. A New Extended Gibbs Thermodynamic Potential of Seawater. Prog. Oceanogr. 2003, 58, 43–114. [Google Scholar] [CrossRef]
- Millero, F.J.; Perron, G.; Desnoyers, J.E. Heat Capacity of Seawater Solutions from 5° to 35 °C and 0.5 to 22‰ Chlorinity. J. Geophys. Res. 1973, 78, 4499–4507. [Google Scholar] [CrossRef]
- Islam, M.A.; Pal, A.; Saha, B.B. Experimental Study on Thermophysical and Porous Properties of Silica Gels. Int. J. Refrig. 2020, 110, 277–285. [Google Scholar] [CrossRef]
- Di Maggio, R.; Dirè, S.; Callone, E.; Bergamonti, L.; Lottici, P.P.; Albatici, R.; Rigon, R.; Ataollahi, N. Super-Adsorbent Polyacrylate under Swelling in Water for Passive Solar Control of Building Envelope. SN Appl. Sci. 2020, 2, 45. [Google Scholar] [CrossRef] [Green Version]
- Barnes, W.H.; Maass, O. Specific Heats and Latent Heat of Fusion of Ice. Can. J. Res. 1930, 3, 205–213. [Google Scholar] [CrossRef]
- Seo, J.; Shin, D. Enhancement of Specific Heat of Ternary Nitrate (LiNO3-NaNO3-KNO3) Salt by Doping with SiO2 Nanoparticles for Solar Thermal Energy Storage. Micro Nano Lett. 2014, 9, 817–820. [Google Scholar] [CrossRef]
- De Wit, H.G.M.; De Kruif, C.G.; Van Miltenburg, J.C. Thermodynamic Properties of Molecular Organic Crystals Containing Nitrogen, Oxygen, and Sulfur II. Molar Heat Capacities of Eight Compounds by Adiabatic Calorimetry. J. Chem. Thermodyn. 1983, 15, 891–902. [Google Scholar] [CrossRef]
- Li, W.; Yu, Z. Heat Exchangers for Cooling Supercritical Carbon Dioxide and Heat Transfer Enhancement: A Review and Assessment. Energy Rep. 2021, 7, 4085–4105. [Google Scholar] [CrossRef]
- Pioro, I.; Mokry, S. Thermophysical Properties at Critical and Supercritical Pressures. In Heat Transfer-Theoretical Analysis, Experimental Investigations and Industrial Systems; InTechOpen: Rijeka, Croatia, 2011; ISBN 9789533072265. [Google Scholar]
- Hyman, D.; Kay, W.B. Heat Capacity and Content of Tars and Pitches. Ind. Eng. Chem. 1949, 41, 1764–1768. [Google Scholar] [CrossRef]
- Marsh, D. Thermodynamics of Phospholipid Self-Assembly. Biophys. J. 2012, 102, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Heimburg, T. Mechanical Aspects of Membrane Thermodynamics. Estimation of the Mechanical Properties of Lipid Membranes close to the Chain Melting Transition from Calorimetry. Biochim. Biophys. Acta Biomembr. 1998, 1415, 147–162. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.H.; Rupley, J.A. Protein–Water Interactions. Heat Capacity of the Lysozyme–Water System. Biochemistry 1979, 18, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Waples, D.W.; Waples, J.S. A Review and Evaluation of Specific Heat Capacities of Rocks, Minerals, and Subsurface Fluids. Part 1: Minerals and Nonporous Rocks. Nat. Resour. Res. 2004, 13, 97–122. [Google Scholar] [CrossRef]
- Jaupart, C.; Labrosse, S.; Lucazeau, F.; Mareschal, J.-C. Temperatures, Heat, and Energy in the Mantle of the Earth. In Treatise on Geophysics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 223–270. ISBN 9780444538031. [Google Scholar]
- Kahlert, H.; Leito, I. Generalization of Acid-Base Diagrams Based on the Unified pH-Scale. Chemphyschem 2019, 20, 1779–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, C.; Steinkühler, J.; Gonzales, D.T.; Yandrapalli, N.; Robinson, T.; Dimova, R.; Tang, T.-Y.D. Reversible pH-Responsive Coacervate Formation in Lipid Vesicles Activates Dormant Enzymatic Reactions. Angew. Chem. Int. Ed. Engl. 2020, 59, 5950–5957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, A.; Bonfio, C.; Johnson, C.M.; Sutherland, J.D. pH-Driven RNA Strand Separation under Prebiotically Plausible Conditions. Biochemistry 2018, 57, 6382–6386. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Sánchez, R.; O’Flaherty, D.K.; Wang, A.; Coscia, F.; Petris, G.; Di Michele, L.; Cicuta, P.; Bonfio, C. Thermally Driven Membrane Phase Transitions Enable Content Reshuffling in Primitive Cells. J. Am. Chem. Soc. 2021, 143, 16589–16598. [Google Scholar] [CrossRef]
- Komatsu, G.; Senthil Kumar, P.; Goto, K.; Sekine, Y.; Giri, C.; Matsui, T. Drainage Systems of Lonar Crater, India: Contributions to Lonar Lake Hydrology and Crater Degradation. Planet. Space Sci. 2014, 95, 45–55. [Google Scholar] [CrossRef]
- Lee, J.; Kim, G. Dependence of pH in Coastal Waters on the Adsorption of Protons onto Sediment Minerals. Limnol. Oceanogr. 2015, 60, 831–839. [Google Scholar] [CrossRef]
- Dutta, S.; Sarma, D.; Nath, P. Ground and River Water Quality Monitoring Using a Smartphone-Based pH Sensor. AIP Adv. 2015, 5, 057151. [Google Scholar] [CrossRef]
- Sasaki, M. Classification of Water Types of Acid Hot-Spring Waters in Japan. J. Geotherm. Res. Soc. Jpn. 2018, 40, 235–243. [Google Scholar]
- Poddar, A.; Das, S.K. Microbiological Studies of Hot Springs in India: A Review. Arch. Microbiol. 2018, 200, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Angle, K.J.; Crocker, D.R.; Simpson, R.M.C.; Mayer, K.J.; Garofalo, L.A.; Moore, A.N.; Mora Garcia, S.L.; Or, V.W.; Srinivasan, S.; Farhan, M.; et al. Acidity across the Interface from the Ocean Surface to Sea Spray Aerosol. Proc. Natl. Acad. Sci. USA 2021, 118, e2018397118. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W.; Madeley, J.D. The Surface Properties of Silica Gels. I. Importance of pH in the Preparation from Sodium Silicate and Sulphuric Acid. J. Appl. Chem. 2007, 3, 549–556. [Google Scholar] [CrossRef]
- Balköse, D. Effect of Preparation pH on Properties of Silica Gel. J. Chem. Technol. Biotechnol. 2007, 49, 165–171. [Google Scholar] [CrossRef]
- Ülkü, S.; Balköse, D.; Baltacboğlu, H. Effect of Preparation pH on Pore Structure of Silica Gels. Colloid Polym. Sci. 1993, 271, 709–713. [Google Scholar] [CrossRef]
- Toyama, Y.; Sahara, R.; Iino, Y.; Kubota, K. PH Dependence of Rheological Properties of Gelatin Gel Mixed with Agar or Agarose. Trans. Mater. Res. Soc. Jpn. 2011, 36, 383–386. [Google Scholar] [CrossRef] [Green Version]
- Naser, J.; Mjalli, F.; Jibril, B.; Al-Hatmi, S.; Gano, Z. Potassium Carbonate as a Salt for Deep Eutectic Solvents. Int. J. Chem. Eng. Appl. 2013, 4, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Skulcova, A.; Russ, A.; Jablonsky, M.; Sima, J. The pH Behavior of Seventeen Deep Eutectic Solvents. BioResources 2018, 13, 5042–5051. [Google Scholar] [CrossRef]
- Toews, K.L.; Shroll, R.M.; Wai, C.M.; Smart, N.G. PH-Defining Equilibrium between Water and Supercritical CO2. Influence on SFE of Organics and Metal Chelates. Anal. Chem. 1995, 67, 4040–4043. [Google Scholar] [CrossRef]
- Goertz, M.P.; Goyal, N.; Montano, G.A.; Bunker, B.C. Lipid Bilayer Reorganization under Extreme pH Conditions. Langmuir 2011, 27, 5481–5491. [Google Scholar] [CrossRef]
- Petelska, A.D.; Figaszewski, Z.A. Effect of pH on the Interfacial Tension of Lipid Bilayer Membrane. Biophys. J. 2000, 78, 812–817. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.F.; Kahler, D.M. Geochemical and Mineralogical Indications of pH in Lakes and Soils in Central New Hampshire in the Early Holocene1. Limnol. Oceanogr. 1987, 32, 751–757. [Google Scholar] [CrossRef]
- Hutchison, W.; Finch, A.A.; Borst, A.M.; Marks, M.A.W.; Upton, B.G.J.; Zerkle, A.L.; Stüeken, E.E.; Boyce, A.J. Mantle Sources and Magma Evolution in Europe’s Largest Rare Earth Element Belt (Gardar Province, SW Greenland): New Insights from Sulfur Isotopes. Earth Planet. Sci. Lett. 2021, 568, 117034. [Google Scholar] [CrossRef]
- Sleep, N.H.; Bird, D.K.; Pope, E.C. Serpentinite and the Dawn of Life. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2857–2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrback, G.H.; Cady, G.H. The Liquid—Vapor Equilibrium of the System Tungsten Hexafluoride-Perfluorocycyclopentane1. J. Am. Chem. Soc. 1951, 73, 4250–4251. [Google Scholar] [CrossRef]
- Gorgolis, G.; Galiotis, C. Graphene Aerogels: A Review. 2D Mater. 2017, 4, 032001. [Google Scholar] [CrossRef]
- Mallamace, F.; Branca, C.; Broccio, M.; Corsaro, C.; Mou, C.-Y.; Chen, S.-H. The Anomalous Behavior of the Density of Water in the Range 30 K < T < 373K. Proc. Natl. Acad. Sci. USA 2007, 104, 18387–18391. [Google Scholar] [CrossRef] [Green Version]
- Schön, J.H. Density. In Developments in Petroleum Science; Developments in Petroleum Science; Elsevier: Amsterdam, The Netherlands, 2015; pp. 109–118. ISBN 9780081004043. [Google Scholar]
- Minic, Z.; Thongbam, P.D. The Biological Deep Sea Hydrothermal Vent as a Model to Study Carbon Dioxide Capturing Enzymes. Mar. Drugs 2011, 9, 719–738. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Girard, G.; Davis, R.; Peuto, A.; Bignell, N. Recommended Table for the Density of Water between 0 C and 40 C Based on Recent Experimental Reports. Metrologia 2001, 38, 301–309. [Google Scholar] [CrossRef]
- Sarangi, B.; Aggarwal, S.G.; Sinha, D.; Gupta, P.K. Aerosol Effective Density Measurement Using Scanning Mobility Particle Sizer and Quartz Crystal Microbalance with the Estimation of Involved Uncertainty. Atmos. Meas. Tech. 2016, 9, 859–875. [Google Scholar] [CrossRef] [Green Version]
- Sofieva, S.; Asmi, E.; Atanasova, N.S.; Heikkinen, A.E.; Vidal, E.; Duplissy, J.; Romantschuk, M.; Kouznetsov, R.; Kukkonen, J.; Bamford, D.H.; et al. Effects of Temperature and Salinity on Sea-Spray-Aerosol Formation Simulated with a Bubble-Generating Chamber. Atmos. Meas. Tech. Discuss. 2022, 1–40. [Google Scholar] [CrossRef]
- Timco, G.W.; Frederking, R.M.W. A Review of Sea Ice Density. Cold Reg. Sci. Technol. 1996, 24, 1–6. [Google Scholar]
- Hou, X.-J.; Yu, L.-Y.; Wang, Y.-X.; Wu, K.-J.; He, C.-H. Comprehensive Prediction of Densities for Deep Eutectic Solvents: A New Bonding-Group Interaction Contribution Scheme. Ind. Eng. Chem. Res. 2021, 60, 13127–13139. [Google Scholar] [CrossRef]
- Yam, H.; Schmitt, D.R. CO Rock Physics: A Laboratory Study. In Proceedings of the Recovery–Joint CSPG CSEG CWLS Annual Convention, Pittsburgh, PA, USA, 19–22 May 2011; pp. 1–7. [Google Scholar]
- Guo, Z.; Rüpke, L.; Tao, C. HydrothermalFoam v1.0: A 3-D Hydrothermal Transport Model for Natural Submarine Hydrothermal Systems. Geosci. Model Dev. 2020, 13, 6547–6565. [Google Scholar]
- Lewis, R.A. Hawley’s Condensed Chemical Dictionary; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 9781118135150. [Google Scholar]
- Iravani, M.A.; Deparis, J.; Davarzani, H.; Colombano, S.; Guérin, R.; Maineult, A. The Influence of Temperature on the Dielectric Permittivity and Complex Electrical Resistivity of Porous Media Saturated with DNAPLs: A Laboratory Study. J. Appl. Geophys. 2020, 172, 103921. [Google Scholar] [CrossRef]
- Rajput, M.K.; Konwar, M.; Sarma, D. Hydrophobic Natural Deep Eutectic Solvent THY-DA as Sole Extracting Agent for Arsenic (III) Removal from Aqueous Solutions. Environ. Technol. Innov. 2021, 24, 102017. [Google Scholar] [CrossRef]
- Kim, S.; Huang, J.; Lee, Y.; Dutta, S.; Yoo, H.Y.; Jung, Y.M.; Jho, Y.; Zeng, H.; Hwang, D.S. Complexation and Coacervation of like-Charged Polyelectrolytes Inspired by Mussels. Proc. Natl. Acad. Sci. USA 2016, 113, E847–E853. [Google Scholar]
- Earle, S. Physical Geology; BCcampus: Victoria, BC, Canada, 2019; ISBN 9781774200285. [Google Scholar]
- Zhuang, B.; Ramanauskaite, G.; Koa, Z.Y.; Wang, Z.-G. Like Dissolves like: A First-Principles Theory for Predicting Liquid Miscibility and Mixture Dielectric Constant. Sci. Adv. 2021, 7, eabe7275. [Google Scholar]
- Griffiths, T.R.; Pugh, D.C. Correlations among Solvent Polarity Scales, Dielectric Constant and Dipole Moment, and a Means to Reliable Predictions of Polarity Scale Values from Cu. Coord. Chem. Rev. 1979, 29, 129–211. [Google Scholar]
- Ahmad, Z. Polymer Dielectric Materials. In Dielectric Material; InTech: Vienna, Austria, 2012; ISBN 9789535107644. [Google Scholar]
- Kato, C.; Nishihara, S.; Tsunashima, R.; Tatewaki, Y.; Okada, S.; Ren, X.-M.; Inoue, K.; Long, D.-L.; Cronin, L. Quick and Selective Synthesis of Li6[α-P2W18O62]·28H2O Soluble in Various Organic Solvents. Dalton Trans. 2013, 42, 11363–11366. [Google Scholar]
- Mayer, C.; Schreiber, U.; Dávila, M.J. Selection of Prebiotic Molecules in Amphiphilic Environments. Life 2017, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Klein, L.; Swift, C. An Improved Model for the Dielectric Constant of Sea Water at Microwave Frequencies. IEEE J. Ocean. Eng. 1977, 2, 104–111. [Google Scholar] [CrossRef]
- Malmberg, C.G.; Maryott, A.A. Dielectric Constant of Water from 0 C to 100 C. J. Res. Natl. Bur. Stand. 1956, 56, 2641. [Google Scholar] [CrossRef]
- Davison, S.W.; Gentry, J.W. Differences in Diffusion Charging of Dielectric and Conducting Ultrafine Aerosols. Aerosol Sci. Technol. 1985, 4, 157–163. [Google Scholar] [CrossRef]
- Hrubesh, L.W.; Pekala, R.W. Dielectric Properties and Electronic Applications of Aerogels. In Sol-Gel Processing and Applications; Springer US: Boston, MA, USA, 1994; pp. 363–367. ISBN 9781461360988. [Google Scholar]
- Aragones, J.L.; MacDowell, L.G.; Vega, C. Dielectric Constant of Ices and Water: A Lesson about Water Interactions. J. Phys. Chem. A 2011, 115, 5745–5758. [Google Scholar] [CrossRef]
- Essex, J.W.; Jorgensen, W.L. Dielectric Constants of Formamide and Dimethylformamide via Computer Simulation. J. Phys. Chem. 1995, 99, 17956–17962. [Google Scholar] [CrossRef]
- Bass, S.J.; Nathan, W.I.; Meighan, R.M.; Cole, R.H. Dielectric Properties of Alkyl Amides. II. Liquid Dielectric Constant and Loss. J. Phys. Chem. 1964, 68, 509–515. [Google Scholar] [CrossRef]
- Leeke, G.; Santos, R.; Al-Duri, B.; Seville, J.; Smith, C.; Holmes, A.B. Solubilities of 4-Phenyltoluene, Phenylboric Acid, Biphenyl, and Iodobenzene in Carbon Dioxide from Measurements of the Relative Permittivity. J. Chem. Eng. Data 2005, 50, 1370–1374. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhou, J.; Guo, X.S.; Ji, B.B.; Zhou, W.; Li, D.H. Terahertz Spectral Properties of Coal Tar. J. Appl. Spectrosc. 2018, 85, 840–844. [Google Scholar] [CrossRef]
- Gramse, G.; Dols-Perez, A.; Edwards, M.A.; Fumagalli, L.; Gomila, G. Nanoscale Measurement of the Dielectric Constant of Supported Lipid Bilayers in Aqueous Solutions with Electrostatic Force Microscopy. Biophys. J. 2013, 104, 1257–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilger, J.P.; McLaughlin, S.G.; McIntosh, T.J.; Simon, S.A. The Dielectric Constant of Phospholipid Bilayers and the Permeability of Membranes to Ions. Science 1979, 206, 1196–1198. [Google Scholar] [CrossRef]
- Yewdall, N.A.; André, A.A.M.; Lu, T.; Spruijt, E. Coacervates as Models of Membraneless Organelles. Curr. Opin. Colloid Interface Sci. 2021, 52, 101416. [Google Scholar] [CrossRef]
- Takubo, J.; Ukai, Y.; Kuo, C.C. On the dielectric constants of rocks. Mineral. J. 1953, 1, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Spanu, L.; Harrison, B.; Sverjensky, D.A.; Galli, G. Dielectric Properties of Water under Extreme Conditions and Transport of Carbonates in the Deep Earth. Proc. Natl. Acad. Sci. USA 2013, 110, 6646–6650. [Google Scholar] [CrossRef] [Green Version]
- Attinger, D.; Frankiewicz, C.; Betz, A.R.; Schutzius, T.M.; Ganguly, R.; Das, A.; Kim, C.-J.; Megaridis, C.M. Surface Engineering for Phase Change Heat Transfer: A Review. MRS Energy Sustain. 2014, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Petrov, O.; Furó, I. A Study of Freezing–melting Hysteresis of Water in Different Porous Materials. Part I: Porous Silica Glasses. Microporous Mesoporous Mater. 2011, 138, 221–227. [Google Scholar] [CrossRef]
- Budisa, N.; Schulze-Makuch, D. Supercritical Carbon Dioxide and Its Potential as a Life-Sustaining Solvent in a Planetary Environment. Life 2014, 4, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, U.; Locker-Grütjen, O.; Mayer, C. Hypothesis: Origin of Life in Deep-Reaching Tectonic Faults. Orig. Life Evol. Biosph. 2012, 42, 47–54. [Google Scholar] [CrossRef]
- Zhang, S.J.; Duzdevich, D.; Ding, D.; Szostak, J.W. Freeze-Thaw Cycles Enable a Prebiotically Plausible and Continuous Pathway from Nucleotide Activation to Nonenzymatic RNA Copying. Proc. Natl. Acad. Sci. USA 2022, 119, e2116429119. [Google Scholar] [CrossRef]
- Mutschler, H.; Wochner, A.; Holliger, P. Freeze-Thaw Cycles as Drivers of Complex Ribozyme Assembly. Nat. Chem. 2015, 7, 502–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, T.Z.; Fraccia, T.P. Liquid Crystal Peptide/DNA Coacervates in the Context of Prebiotic Molecular Evolution. Crystals 2020, 10, 964. [Google Scholar] [CrossRef]
- Lu, T.; Nakashima, K.K.; Spruijt, E. Temperature-Responsive Peptide-Nucleotide Coacervates. J. Phys. Chem. B 2021, 125, 3080–3091. [Google Scholar] [CrossRef] [PubMed]
- Chang, H. The Myth of the Boiling Point. Sci. Prog. 2008, 91, 219–240. [Google Scholar] [CrossRef]
- Ming, F.; Chen, L.; Li, D.; Du, C. Investigation into Freezing Point Depression in Soil Caused by NaCl Solution. Water 2020, 12, 2232. [Google Scholar] [CrossRef]
- Miyake, Y. Chemical Studies of the Western Pacific Ocean. III. Freezing Point, Osmotic Pressure, Boiling Point, and Vapour Pressure of Sea Water. Bull. Chem. Soc. Jpn. 1939, 14, 58–62. [Google Scholar] [CrossRef]
- Rosen, J.M. The Boiling Point of Stratospheric Aerosols. J. Appl. Meteorol. 1971, 10, 1044–1046. [Google Scholar]
- DeMott, P.J.; Hill, T.C.J.; McCluskey, C.S.; Prather, K.A.; Collins, D.B.; Sullivan, R.C.; Ruppel, M.J.; Mason, R.H.; Irish, V.E.; Lee, T.; et al. Sea Spray Aerosol as a Unique Source of Ice Nucleating Particles. Proc. Natl. Acad. Sci. USA 2016, 113, 5797–5803. [Google Scholar] [CrossRef] [Green Version]
- Wypych, G. Handbook of Fillers; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9781927885109. [Google Scholar]
- Saladino, R.; Crestini, C.; Ciciriello, F.; Pino, S.; Costanzo, G.; Di Mauro, E. From Formamide to RNA: The Roles of Formamide and Water in the Evolution of Chemical Information. Res. Microbiol. 2009, 160, 441–448. [Google Scholar] [PubMed]
- Michael, T. Formamide [MAK Value Documentation, 2013]. In The MAK-Collection for Occupational Health and Safety; J. Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 1–26. [Google Scholar]
- Clayton, G.D. Patt’s Industrial Hygiene and Toxicology: Toxicology; J. Wiley & Sons: Hoboken, NJ, USA, 1981; Volume 2, ISBN 9780471160427. [Google Scholar]
- Chapman, J.B.; Runyon, S.E.; Shields, J.E.; Lawler, B.L.; Pridmore, C.J.; Scoggin, S.H.; Swaim, N.T.; Trzinski, A.E.; Wiley, H.N.; Barth, A.P.; et al. The North American Cordilleran Anatectic Belt. Earth Sci. Rev. 2021, 215, 103576. [Google Scholar] [CrossRef]
- Kennedy, G.C.; Higgins, G.H. Melting Temperatures in the Earth’s Mantle. In Developments in Geotectonics; Developments in geotectonics; Elsevier: Amsterdam, The Netherlands, 1972; pp. 221–232. ISBN 9780444410153. [Google Scholar]
- Canıaz, R.O.; Erkey, C. Process Intensification for Heavy Oil Upgrading Using Supercritical Water. Chem. Eng. Res. Des. 2014, 92, 1845–1863. [Google Scholar] [CrossRef]
- Marinos-Kouris, D.; Krokida, M.; Oreopoulou, V. Frying of Foods. In Handbook of Industrial Drying, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9781574446685. [Google Scholar]
- Martinotti, C.; Ruiz-Perez, L.; Deplazes, E.; Mancera, R.L. Molecular Dynamics Simulation of Small Molecules Interacting with Biological Membranes. Chemphyschem 2020, 21, 1486–1514. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Sunilkumar, K.T.; Betha, S.; Mohanvarma, M. Liposomal Drug Delivery System—A Comprehensive Review. Int. J. Drug Dev. Res. 2013, 5, 62–75. [Google Scholar]
- Lowry, C.A.; Kay, L.M. Chemical Factors Determine Olfactory System Beta Oscillations in Waking Rats. J. Neurophysiol. 2007, 98, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Chamberlin, J.C. Heavy Mineral Oil as a Permanent Non-Volatile Preservative for Valuable Biological Material. Science 1925, 61, 634–635. [Google Scholar] [CrossRef]
- Gutmann, F.; Simmons, L.M. A Theoretical Basis for the Antoine Vapor Pressure Equation. J. Chem. Phys. 1950, 18, 696–697. [Google Scholar] [CrossRef]
- Meyers, C.H. The Vapor Pressure of Liquid and Solid Carbon Dioxide (Classic Reprint); Forgotten Books: London, UK, 2018; ISBN 9781390303124. [Google Scholar]
- Orthoefer, F.T.; List, G.R. Dynamics of Frying. In Deep Frying; Elsevier: Amsterdam, The Netherlands, 2007; pp. 253–275. ISBN 9781893997929. [Google Scholar]
- Mishra, V.K.; Temelli, F.; Ooraikul, B. Vapor Pressure of Fatty Acid Esters: Correlation and Estimation. J. Food Eng. 1994, 23, 467–480. [Google Scholar] [CrossRef]
- Bonnell, D.G.R. Studies in Gels III. Vapour Pressure of Silica Gels. Trans. Faraday Soc. 1932, 28, 463. [Google Scholar] [CrossRef]
- Wexler, A. Vapor Pressure Formulation for Ice. J. Res. Natl. Bur. Stand. A Phys. Chem. 1977, 81, 5. [Google Scholar] [CrossRef]
- Girnik, I.; Aristov, Y. An Aqueous CaCl2 Solution in the Condenser/evaporator instead of Pure Water: Application for the New Adsorptive Cycle “heat from Cold”. Energies 2020, 13, 2904. [Google Scholar] [CrossRef]
- Xin, K.; Roghair, I.; Gallucci, F.; van Sint Annaland, M. Total Vapor Pressure of Hydrophobic Deep Eutectic Solvents: Experiments and Modelling. J. Mol. Liq. 2021, 325, 115227. [Google Scholar] [CrossRef]
- Bockish, M. Composition, Structure, Physical Data, and Chemical Reactions of Fats and Oils, Their Derivatives, and Their Associates. In Fats and Oils Handbook; Elsevier: Amsterdam, The Netherlands, 1998; pp. 53–120. ISBN 9780981893600. [Google Scholar]
- Matricarde Falleiro, R.M.; Akisawa Silva, L.Y.; Meirelles, A.J.A.; Krähenbühl, M.A. Vapor Pressure Data for Fatty Acids Obtained Using an Adaptation of the DSC Technique. Thermochim. Acta 2012, 547, 6–12. [Google Scholar] [CrossRef]
- Van Lente, J.; Pazos Urrea, M.; Brouwer, T.; Schuur, B.; Lindhoud, S. Complex Coacervates as Extraction Media. Green Chem. 2021, 23, 5812–5824. [Google Scholar] [CrossRef] [PubMed]
- Fegley, B., Jr.; Schaefer, L.; Kargel, J.S. Vapor Pressure, Vapor Composition and Fractional Vaporization of High Temperature Lavas on Io. LPI Contrib. 2003, 1686. [Google Scholar]
- Guo, Z.; Qin, X.; Zhang, Y.; Niu, C.; Wang, D.; Ling, Y. Numerical Investigation of the Effect of Heterogeneous Pore Structures on Elastic Properties of Tight Gas Sandstones. Front. Earth Sci. 2021, 9, 641637. [Google Scholar] [CrossRef]
- Molina, O.; Vilarrasa, V.; Zeidouni, M. Geologic Carbon Storage for Shale Gas Recovery. Energy Procedia 2017, 114, 5748–5760. [Google Scholar] [CrossRef] [Green Version]
- Lowe, D.R.; Byerly, G.R. The Terrestrial Record of Late Heavy Bombardment. New Astron. Rev. 2018, 81, 39–61. [Google Scholar] [CrossRef]
- Lamour, S.; Pallmann, S.; Haas, M.; Trapp, O. Prebiotic Sugar Formation Under Nonaqueous Conditions and Mechanochemical Acceleration. Life 2019, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Hansma, H.G. Potassium at the Origins of Life: Did Biology Emerge from Biotite in Micaceous Clay? Life 2022, 12, 301. [Google Scholar] [CrossRef]
- Shashi Menon, E. Pipeline Planning and Construction Field Manual; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Lei, J.; Liu, Z.; Yeo, J.; Ng, T.Y. Determination of the Young’s Modulus of Silica Aerogels—An Analytical–numerical Approach. Soft Matter 2013, 9, 11367. [Google Scholar] [CrossRef]
- Phair, J.W.; Tkachev, S.N.; Manghnani, M.H.; Livingston, R.A. Elastic and Structural Properties of Alkaline-Calcium Silica Hydrogels. J. Mater. Res. 2005, 20, 344–349. [Google Scholar] [CrossRef]
- Neumeier, J.J. Elastic Constants, Bulk Modulus, and Compressibility of H2O Ice Ihfor the Temperature Range 50 K–273 K. J. Phys. Chem. Ref. Data 2018, 47, 033101. [Google Scholar] [CrossRef]
- Schulson, E.M. The Structure and Mechanical Behavior of Ice. JOM 1999, 51, 21–27. [Google Scholar] [CrossRef]
- Arriaga, M.-C.S. Supercritical Thermodynamics of the Rock/Fluid Geothermal System. In Proceedings of the World Geothermal Congress 2020+1, Reykjavik, Iceland, 24–27 October 2021; pp. 1–15. [Google Scholar]
- Lumley, D.; Sherlock, D.; Daley, T.; Huang, L.; Lawton, D.; Masters, R.; Verliac, M.; White, D. Highlights of the 2009 SEG Summer Research Workshop on CO2 Sequestration. Lead. Edge 2010, 29, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Terzi, M.M.; Deserno, M.; Nagle, J.F. Mechanical Properties of Lipid Bilayers: A Note on the Poisson Ratio. Soft Matter 2019, 15, 9085–9092. [Google Scholar] [CrossRef] [PubMed]
- Picas, L.; Rico, F.; Scheuring, S. Direct Measurement of the Mechanical Properties of Lipid Phases in Supported Bilayers. Biophys. J. 2012, 102, L01–L03. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momeni Bashusqeh, S.; Rastgoo, A. Elastic Modulus of Free-Standing Lipid Bilayer. Soft Mater. 2016, 14, 210–216. [Google Scholar] [CrossRef]
- Marsden, L.H.; Neuberg, J.W.; Thomas, M.E.; Mothes, P.A.; Ruiz, M.C. Combining Magma Flow and Deformation Modeling to Explain Observed Changes in Tilt. Front. Earth Sci. 2019, 7, 219. [Google Scholar] [CrossRef] [Green Version]
- Heap, M.J.; Faulkner, D.R.; Meredith, P.G.; Vinciguerra, S. Elastic Moduli Evolution and Accompanying Stress Changes with Increasing Crack Damage: Implications for Stress Changes around Fault Zones and Volcanoes during Deformation. Geophys. J. Int. 2010, 183, 225–236. [Google Scholar] [CrossRef]
- Gutenberg, B. Elastic Constants, and Elastic Processes in the Earth. In International Geophysics; International geophysics series; Elsevier: Amsterdam, The Netherlands, 1959; pp. 165–184. ISBN 9780123106506. [Google Scholar]
- Burtch, N.C.; Heinen, J.; Bennett, T.D.; Dubbeldam, D.; Allendorf, M.D. Mechanical Properties in Metal–Organic Frameworks: Emerging Opportunities and Challenges for Device Functionality and Technological Applications. Adv. Mater. 2018, 30, 1704124. [Google Scholar] [CrossRef]
- Gough, D.O. Solar Interior Structure and Luminosity Variations. In Physics of Solar Variations; Springer: Dordrecht, The Netherlands, 1981; pp. 21–34. ISBN 9789401096355. [Google Scholar]
- Ranjan, S.; Sasselov, D.D. Influence of the UV Environment on the Synthesis of Prebiotic Molecules. Astrobiology 2016, 16, 68–88. [Google Scholar] [CrossRef] [Green Version]
- Ritson, D.; Sutherland, J.D. Prebiotic Synthesis of Simple Sugars by Photoredox Systems Chemistry. Nat. Chem. 2012, 4, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Ueno, Y.; Kitadai, N. Photochemical Synthesis of Ammonia and Amino Acids from Nitrous Oxide. Astrobiology 2022, 22, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.L.; Moreirinha, C.; Lopes, D.; Esteves, A.C.; Henriques, I.; Almeida, A.; Domingues, M.R.M.; Delgadillo, I.; Correia, A.; Cunha, A. Effects of UV Radiation on the Lipids and Proteins of Bacteria Studied by Mid-Infrared Spectroscopy. Environ. Sci. Technol. 2013, 47, 6306–6315. [Google Scholar] [CrossRef]
- Indriolo, N.; McCall, B.J. Cosmic-Ray Astrochemistry. Chem. Soc. Rev. 2013, 42, 7763–7773. [Google Scholar] [CrossRef]
- Bertaina, M.; Apel, W.D.; Arteaga-Velázquez, J.C.; Bekk, K.; Blümer, J.; Bozdog, H.; Brancus, I.M.; Buchholz, P.; Cantoni, E.; Chiavassa, A.; et al. The Cosmic Ray Energy Spectrum in the Range 1016–1018 eV Measured by KASCADE-Grande. Astrophys. Space Sci. Trans. 2011, 7, 229–234. [Google Scholar] [CrossRef]
- Airapetian, V.S.; Glocer, A.; Gronoff, G.; Hébrard, E.; Danchi, W. Prebiotic Chemistry and Atmospheric Warming of Early Earth by an Active Young Sun. Nat. Geosci. 2016, 9, 452–455. [Google Scholar] [CrossRef]
- Ebisuzaki, T.; Maruyama, S. Nuclear Geyser Model of the Origin of Life: Driving Force to Promote the Synthesis of Building Blocks of Life. Geosci. Front. 2017, 8, 275–298. [Google Scholar] [CrossRef] [Green Version]
- Adam, Z.R.; Hongo, Y.; Cleaves, H.J.; Yi, R.; Fahrenbach, A.C.; Yoda, I.; Aono, M. Estimating the Capacity for Production of Formamide by Radioactive Minerals on the Prebiotic Earth. Sci. Rep. 2018, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- Coogan, L. Did Natural Reactors Form as a Consequence of the Emergence of Oxygenic Photosynthesis during the Archean? GSA Today 2009, 19, 4–10. [Google Scholar] [CrossRef]


| Environment | Ionic Strength Range (M) | References |
|---|---|---|
| Aqueous solution | 0.1–0.8 (oceans) 0.002–6 (lakes) 0.1–17 (lagoons) 0.1–7 (seas) 0.7–6 (hydrothermal brines) | [105] |
| Sea spray | Up to 6 (marine aerosol) | [106] |
| Gels | Variable, depending on components. Salt can dramatically alter gel properties. | [107,108] |
| Deep eutectic solvents | Ranges from 0 to >1, but ionic strength may not be the relevant principle. | [109,110] |
| Pure formamide | 0.024 (commercially available pure formamide contains a significant amount of ionic impurities) | [111] |
| Lipid bilayer vesicle lumens (interior) | 0–0.6, depends on the solution in which the amphiphilic molecules self-assemble. | [112,113] |
| Condensed droplet microenvironments | Up to 15 (within coacervate droplets) | [79] |
| Solid mineral surfaces | No ionic strength for solid mineral surfaces, surface charge density may be the more relevant parameter. | |
| Environment | Typical Viscosity (mPas) | References |
|---|---|---|
| Aqueous solution | 0.89–1.00 (freshwater at room temperature) Up to 1.3 (seawater at room temperature, depending on salinity) | [123,124,125] |
| Sea spray | Ranges from 1 (sea water) to 10–10,000 during evaporation or in presence of organics | [126] |
| Gels | Ranges from 1 up to 2 × 106 (colloidal silica gel) | [127] |
| Ice | 1015 | [128] |
| Deep eutectic solvents | Variable; >100 and as high as 1700 possible | [129,130] |
| Formamide | 3.23 | [131] |
| High pressure supercritical fluids | 0.02–0.16 (CO2, depending on pressure) 2.98 (water) | [132,133,134] |
| Tars | 10–over 1010 | [135] |
| Inside lipid bilayers | 2D diffusion ~100–1000 1–1500 (heterogeneous) | [136] |
| Condensed droplet microenvironments | 100 (coacervate) | [137] |
| Solid mineral surfaces | <1.0 × 1028 (crust) | [138] |
| Mantle | 2.8 × 1025 | [138] |
| Environment | Specific Heat (kJ/Kg K) | References |
|---|---|---|
| Aqueous solution | 4.18 (freshwater) 3.6–4.18 (saltwater), at room temperature. | [124,125,145,146] |
| Sea spray | Aerosols readily evaporate; specific heat is not very relevant. | |
| Gels | 0.8–1.10 (silica gel) Specific heat for hydrogels depends on water level and temperature, for example, up to 30. | [147,148] |
| Ice | 0.4873–0.3496 (from 0 to −80 °C, respectively) | [149] |
| Deep eutectic solvent | 1.5–1.8 (example of salt eutectic) | [150] |
| Formamide | 2.39 | [151] |
| High pressure supercritical fluids | 3–30 (CO2, depending on pressure) 27–690 (water, depending on pressure) | [152,153] |
| Tars | 1.25–2 | [154] |
| Inside bilayers | 0.3–0.9; higher near melting temperature | [155,156] |
| Condensed droplet microenvironments | 1.483 | [157] |
| Solid mineral surfaces | 0.180 (bromyrite) to 1.510 (epsomite); however, most are between 0.3 and 0.9 | [158] |
| Mantle | 1.250 | [159] |
| Environment | pH Range | References |
|---|---|---|
| Aqueous solution | 6.3–7.2 (4.0 Ga ocean) | [4,166,167,168] |
| 6.5–7.7 (2.5 Ga ocean) | ||
| 8.2 (modern ocean) | ||
| 6–8 (freshwater) | ||
| Pure water is 7.0 | ||
| Hot spring environments have more variability, and can range from very acidic (less than pH 3) to somewhat alkaline (as high as pH 10). | ||
| Sea spray | Around 8.0 | [169] |
| Gels | Variable, depending on components. | [75,170,171,172,173] |
| Deep eutectic solvents | 1.2–13.5 (eutectic at room temperature; pH varies greatly between eutectics, and also changes with temperature, down to pH 0) | [174,175] |
| High pressure supercritical fluids | 2.80–2.95 (of water around scCO2) | [176] |
| Inside lipid bilayers | pH can be of a variety of ranges such as low as pH 2 or lower [177] or as high as pH 12 [178]. | |
| Condensed droplet microenvironments | Highly dependent on the components, and especially their charge states at different pH (i.e., pKa). | |
| Solid mineral surfaces | Aqueous solutions containing solid mineral surfaces are mostly acidic. However, some have been found that were alkaline (pH 8.7–9.6). | [179] |
| Mantle | Mantle-derived igneous rocks can be alkaline, while mantle-derived minerals on the seafloor (around hydrothermal systems) can be around pH 9–11 | [180,181] |
| Environment | Density (g/mL) | References |
|---|---|---|
| Aqueous solution | 0.9999749 (freshwater at 4 °C); 0.9970470 (freshwater at 25 °C) 1.025 (seawater, average; can be up to 1.09 depending on salinity) | [124,125,187] |
| Sea spray | 1.12–2.16 (at room temperature) | [188,189] |
| Gels | Lower bound is that of the solvent for dilute gels. | |
| Ice | 0.84–0.91 (sea ice) | [190] |
| Deep eutectic solvent | 0.8–1.8 (example of a eutectic between 5 and 100 °C) | [174,191] |
| Formamide | 1.129 (at 25 °C) | [131] |
| High pressure supercritical fluids | 0.1–1 (CO2, depending on temperature and pressure) ~0.1–0.326 (water, depending on temperature and pressure) | [134,192,193] |
| Tars | 1.1–1.23 | [194,195] |
| Lipid bilayers | ~0.9 for the lipid bilayer itself (e.g., decanoic acid density is 0.893 g/cm3) In the aqueous lumen, values as per ‘aqueous solution’. | [196] |
| Condensed droplet microenvironments | 1.18–1.92 | [197] |
| Solid mineral surfaces | 1.2 (kerogen) to 10.969 (uraninite); however, most are typically between 2 and 7 | [158,198] |
| Mantle | 3.4 (mantle surface, and gets larger deeper) | [198] |
| Environment | ε (unitless) | References |
|---|---|---|
| Aqueous solution | ~70–80 (decreases with increasing temperature and salinity; seawater may be slightly lower than freshwater) | [204,205] |
| Sea spray | 2.5–50 | [206] |
| Gels | 1.008–1.9 (silica gel, depending on density) | [207] |
| Ice | 30–130 (ice) | [208] |
| Deep eutectic solvent | 22.8 (one example) | [109] |
| Formamide | 105–113 (room temperature) | [209,210] |
| High pressure supercritical fluids | 1.07–1.46 (CO2, depending on temperature and pressure) | [211] |
| Tars | Up to 8 (coal tar) | [195,212] |
| Inside bilayers | 2–3, can be higher for membranes that are more permeable than phospholipids | [213,214] |
| Condensed droplet microenvironments | 40–50 | [215] |
| Solid mineral surfaces | 4.9–7.5 | [216] |
| Mantle | ~38 (water in the upper mantle at 300 km and 1000 K) | [217] |
| Environment | Boiling Temperature | Melting/Freezing Temperature | References |
|---|---|---|---|
| Aqueous solution | Freshwater (100 °C); As high as 102 °C (seawater, depending on salinity) | Freshwater (0 °C); As low as –2 °C (seawater, depending on salinity) | [124,125,226,227,228] |
| Sea spray | 70–100 °C | Close to 0 °C | [229,230] |
| Gels | 2230 °C (silica gel) | 1710 °C (silica gel) | [231] |
| Ice (eutectic) | In solid form, same as water (depending on salinity). | ||
| Formamide | 210 °C | 2–3 °C | [232,233] |
| High pressure supercritical fluids | See footnote * | ||
| Tars | 190–400 °C | [234] | |
| Inside lipid bilayers | See footnote ^ | ||
| Solid mineral surfaces | N/A | 700–900 °C | [235] |
| Mantle | N/A | ~3600 °C near the core–mantle boundary | [236] |
| Environment | Vapor Pressure (kPa) | References |
|---|---|---|
| Aqueous solution | 2.3–4.2 (freshwater, room temperature) 2.1–3.9 (seawater, room temperature, depending on salinity) | [124,125] |
| Gels | ~0.13–2.3, depending on the gel formulation and conditions. | [247] |
| Ice | 6.1 (ice at 0 °C), but decreases with decreasing temperature (for example, 0.1 at −20 °C and 0.0014 at −100 °C). | [248] |
| Deep eutectic solvent | 1.48 (CaCl2 eutectic in water at 20 °C). However, vapor pressure of other eutectics may vary depending on composition and temperature. | [249,250] |
| Formamide | 0.008 | [233] |
| Inside bilayers | Vapor pressure will be related to the vapor pressure of the bilayer components; vapor pressure typically decreases with increasing chain length (at constant temperature). | [251,252] |
| Condensed droplet microenvironments | Very low to negligible vapor pressure (ionic liquids) | [253] |
| Solid mineral surfaces | Around 0.05–0.25 (melted minerals >1900 K) Vapor pressure of solid mineral surfaces is negligible | [254] |
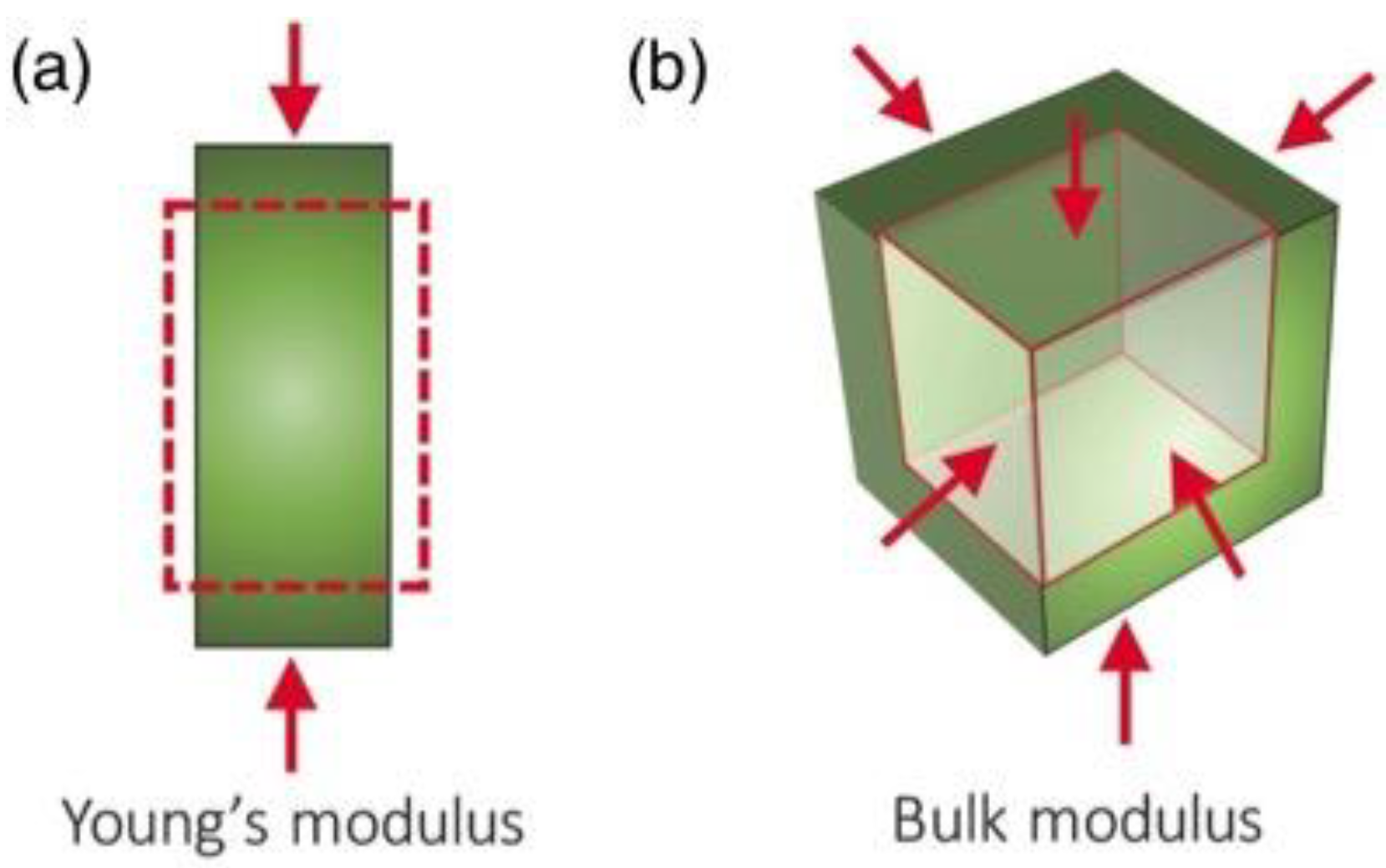
| Environment | E (GPa) * | K (GPa) | References |
|---|---|---|---|
| Aqueous solution | - | 2.1 | [260] |
| Gels | 0.05–10 (of a silica aerogel, depending on gel density) | 4–20 (of an alkaline-calcium silica hydrogel, depending on pressure) | [261,262] |
| Ice | 8.6–12 (depends on the plane) | 8.5–11.5 (depends on temperature) | [263,264] |
| High pressure supercritical fluids | - | 1 (water at room temperature and pressure) 0.1–0.7 (CO2, depending on temperature and pressure) | [192,265,266] |
| Inside bilayers | 0.02–0.03 | 0.6–0.9 (depending on temperature and location) | [267,268,269] |
| Condensed droplet microenvironments | These values will all depend on the droplet composition; “aging” is also an issue in these droplets. | ||
| Solid mineral surfaces | 6.38–288 (depending on the mineral and pressure) | 40–120 (depending on mineral and pressure) | [270,271,272] |
| Mantle | 150–720 (depending on depth) | 100–600 (depending on depth) | [272] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, A.; Yi, R.; Fahrenbach, A.C.; Wang, A.; Jia, T.Z. A Physicochemical Consideration of Prebiotic Microenvironments for Self-Assembly and Prebiotic Chemistry. Life 2022, 12, 1595. https://doi.org/10.3390/life12101595
Saha A, Yi R, Fahrenbach AC, Wang A, Jia TZ. A Physicochemical Consideration of Prebiotic Microenvironments for Self-Assembly and Prebiotic Chemistry. Life. 2022; 12(10):1595. https://doi.org/10.3390/life12101595
Chicago/Turabian StyleSaha, Arpita, Ruiqin Yi, Albert C. Fahrenbach, Anna Wang, and Tony Z. Jia. 2022. "A Physicochemical Consideration of Prebiotic Microenvironments for Self-Assembly and Prebiotic Chemistry" Life 12, no. 10: 1595. https://doi.org/10.3390/life12101595
APA StyleSaha, A., Yi, R., Fahrenbach, A. C., Wang, A., & Jia, T. Z. (2022). A Physicochemical Consideration of Prebiotic Microenvironments for Self-Assembly and Prebiotic Chemistry. Life, 12(10), 1595. https://doi.org/10.3390/life12101595








