Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Wound Healing
Abstract
:1. Introduction
- (a)
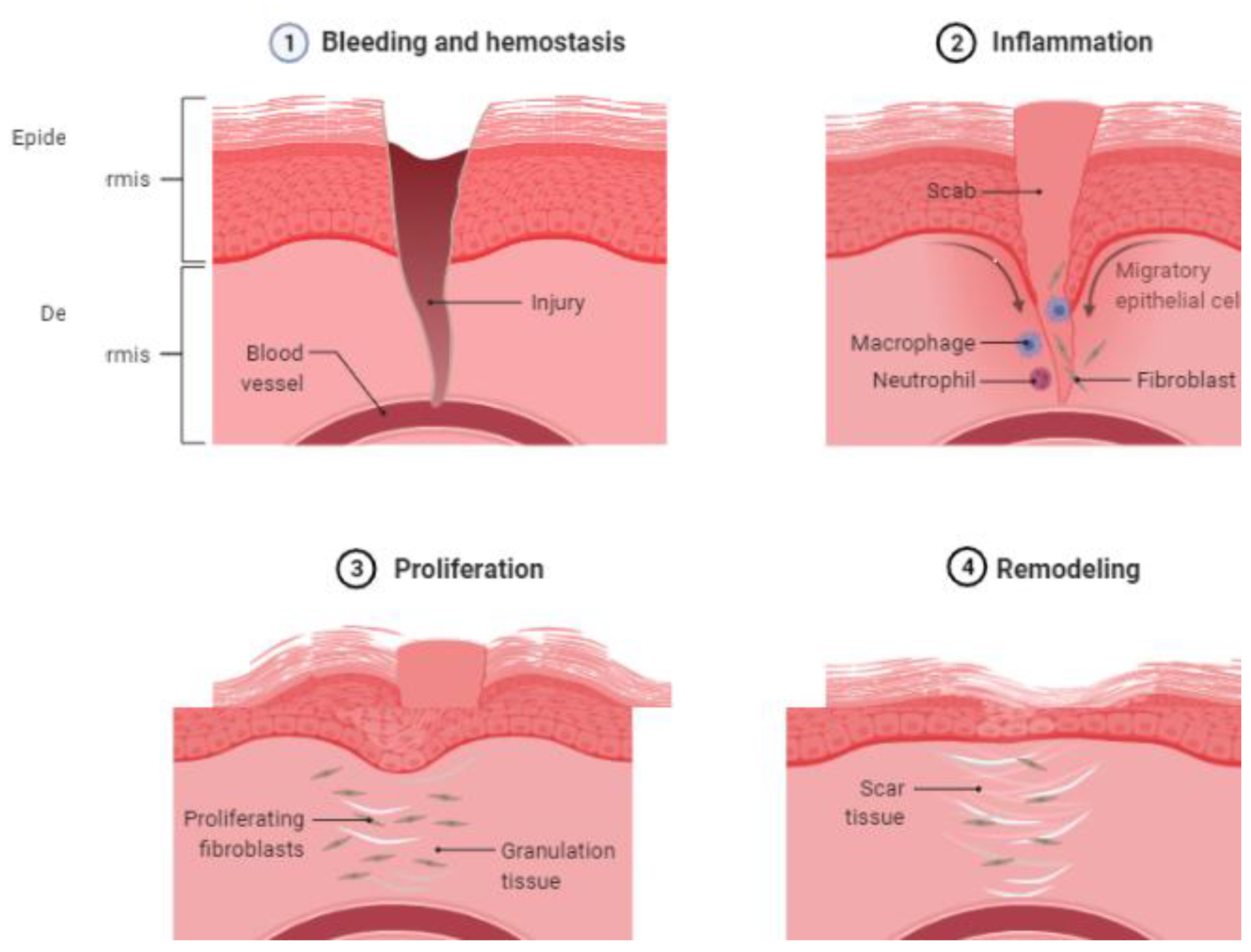
- Hemostasis: The earliest phase in wound healing starts with the formation of a platelet plug and the activation of the coagulation cascade to reduce bleeding. Platelets are activated when they come into contact with extracellular collagen, releasing growth factors that cause platelet aggregation and clumping. This is followed by the activation of the coagulation cascade.
- (b)
- Inflammation: This phase begins within 24 h of the injury and lasts up to 2 weeks, first with the recruitment of neutrophils and then macrophages. These cells release various cytokines (IL-1, -6, -8, and TNF-alpha) and growth factors (PDGF, TGF-beta, TGF-alpha, and fibroblast growth factors) to activate fibroblasts and epithelial cells. Neutrophils serve as the first line of defense. Macrophages are activated later. The classical proinflammatory pathway of macrophages is activated first, followed by the alternate macrophage pathway (M2).
- (c)
- Proliferative phase: This phase is characterized by fibroblast migration, collagen and extracellular matrix production, angiogenesis, the laying of granulation tissue, and epithelialization. Fibroblasts begin moving by first binding to the matrix components (such as fibronectin) via the integrity receptors. The direction of the fibroblast movement is determined by the concentration gradient of cytokines and growth factors. Fibroblasts secrete matrix metalloproteinase, collagenase, and gelatinase to degrade the extracellular matrix, facilitating cell migration and movement. After fibroblast migration, there is an increased production of the extracellular matrix through stimulation by TGF-β and PDGF. Damaged vasculature must be replaced by new blood vessels through angiogenesis, stimulated by VEGF, HIF, and PEGF. In epithelialization, epithelial cells around the margin of the wound lose contact inhibition and epiboly migrate into the wound area [16].
- (d)
- Remodeling: This is the final phase of the healing process, with the formation of granulation tissue. Type 3 collagen is gradually replaced by type 1 collagen.
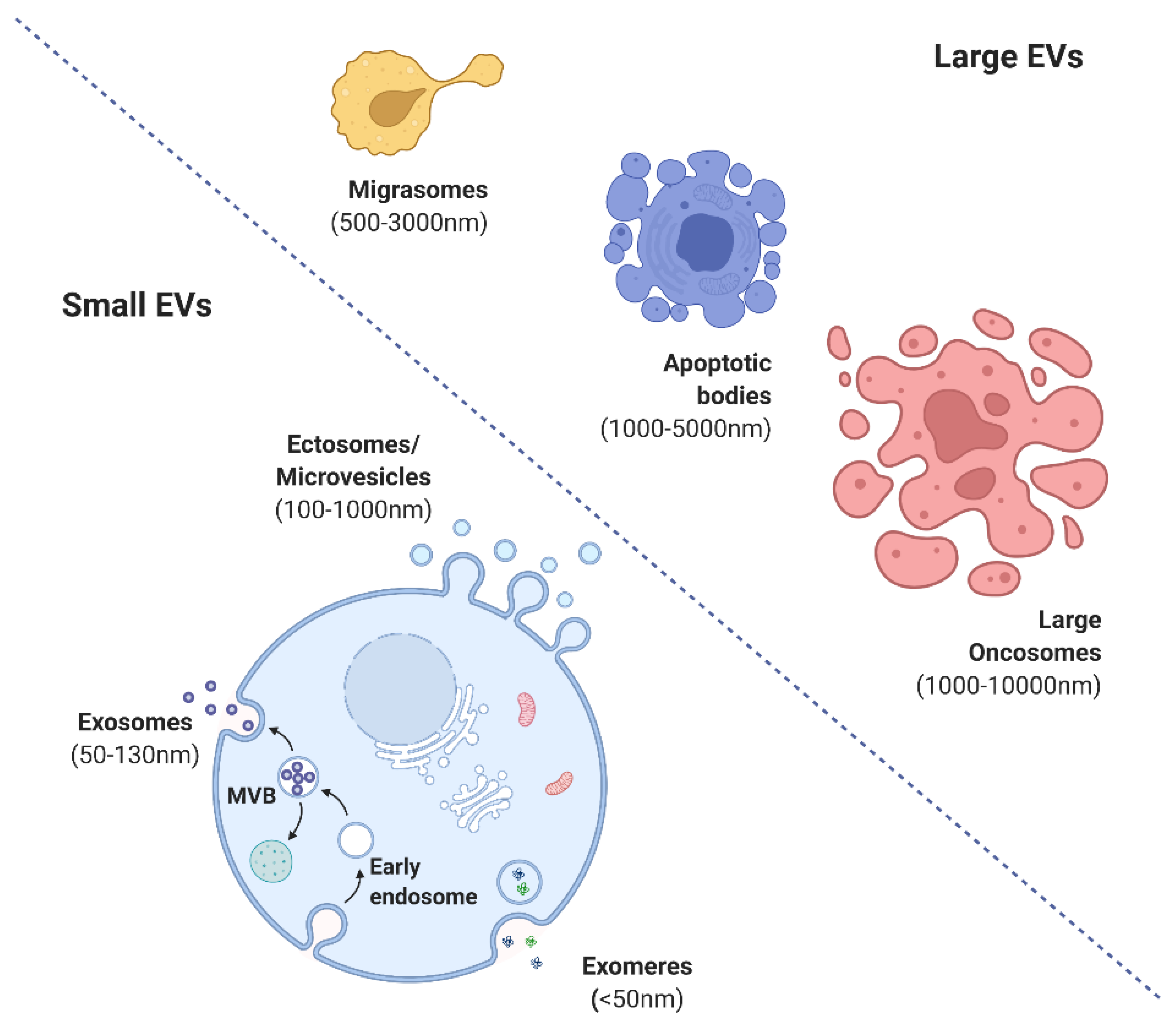
2. Forms and Functions of Extracellular Vesicles
3. Biogenesis of MSC-Derived EVs
4. Molecular Signaling Targets of EVs in Wound Healing
4.1. EVs in Hemostasis through Glycoproteins and Oxidases
4.2. EVs in Inflammation through Adhesion Molecules and ROS Products
4.3. EVs in Proliferation and the Mechanism in Wound Healing
4.4. EVs in the Remodeling of Wound Healing
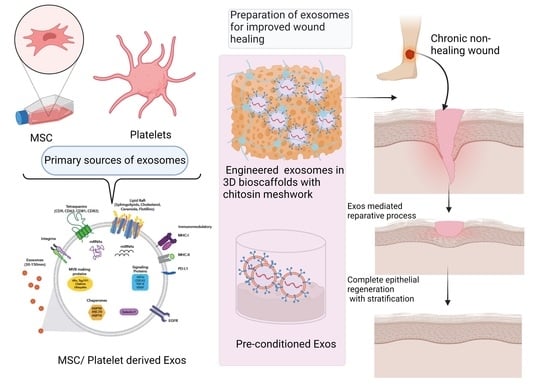
5. New Perspectives of EV-based Therapy in Wound Healing
5.1. Engineered EV Therapy
5.2. EV-Induced Immunomodulation
5.3. PRP-Derived EV Therapy
5.4. Bioscaffolds with Functionalized EV Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ping, J.Y.X.; Neupane, Y.R.; Pastorin, G. Extracellular Vesicles and Their Interplay with Biological Membranes; IntechOpen: London, UK, 2021; ISBN 978-1-80355-055-8. [Google Scholar]
- Jaiswal, R.; Sedger, L.M. Intercellular Vesicular Transfer by Exosomes, Microparticles and Oncosomes—Implications for Cancer Biology and Treatments. Front. Oncol. 2019, 9, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Khan, K.; Kim, J.-H. Biogenesis, Membrane Trafficking, Functions, and Next Generation Nanotherapeutics Medicine of Extracellular Vesicles. Int. J. Nanomed. 2021, 16, 3357–3383. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mentkowski, K.I.; Snitzer, J.D.; Rusnak, S.; Lang, J.K. Therapeutic Potential of Engineered Extracellular Vesicles. AAPS J. 2018, 20, 50. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281. [Google Scholar] [CrossRef]
- Muthu, S.; Bapat, A.; Jain, R.; Jeyaraman, N.; Jeyaraman, M. Exosomal Therapy—A New Frontier in Regenerative Medicine. Stem Cell Investig. 2021, 8, 7. [Google Scholar] [CrossRef]
- Shetgaonkar, G.G.; Marques, S.M.; DCruz, C.E.M.; Vibhavari, R.J.A.; Kumar, L.; Shirodkar, R.K. Exosomes as Cell-Derivative Carriers in the Diagnosis and Treatment of Central Nervous System Diseases. Drug Deliv. Transl. Res. 2022, 12, 1047–1079. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Enoch, S.; Leaper, D.J. Basic Science of Wound Healing. Surg.-Oxf. Int. Ed. 2005, 23, 37–42. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Derm. 2007, 127, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Jin, Y.; Hu, W.; Lian, W.; Cao, C.; Han, S.; Zhao, S.; Yuan, H.; Yang, X.; Shi, J.; et al. Exosomes Derived from Mmu_circ_0000250-Modified Adipose-Derived Mesenchymal Stem Cells Promote Wound Healing in Diabetic Mice by Inducing MiR-128-3p/SIRT1-Mediated Autophagy. Am. J. Physiol. Cell Physiol. 2020, 318, C848–C856. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Yufit, T.; Carson, P.; Fiore, D.; Falanga, J.; Lin, X.; Mamakos, L.; Falanga, V. Differential Keratin Expression during Epiboly in a Wound Model of Bioengineered Skin and in Human Chronic Wounds. Int. J. Low Extrem. Wounds 2011, 10, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular Vesicles in Physiological and Pathological Conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Hurley, J.H. ESCRTs Are Everywhere. EMBO J. 2015, 34, 2398–2407. [Google Scholar] [CrossRef] [Green Version]
- Boura, E.; Ivanov, V.; Carlson, L.-A.; Mizuuchi, K.; Hurley, J.H. Endosomal Sorting Complex Required for Transport (ESCRT) Complexes Induce Phase-Separated Microdomains in Supported Lipid Bilayers. J. Biol. Chem. 2012, 287, 28144–28151. [Google Scholar] [CrossRef]
- Shi, J.; Wei, L. Rho Kinase in the Regulation of Cell Death and Survival. Arch. Immunol. Exp. 2007, 55, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.-T.; Bhaidani, S.; Sutherland, C.; MacDonald, J.A.; Walsh, M.P. Rho-Associated Kinase and Zipper-Interacting Protein Kinase, but Not Myosin Light Chain Kinase, Are Involved in the Regulation of Myosin Phosphorylation in Serum-Stimulated Human Arterial Smooth Muscle Cells. PLoS ONE 2019, 14, e0226406. [Google Scholar] [CrossRef] [Green Version]
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.S.; Kim, J. Mesenchymal Stem Cell-Derived Exosomes: Applications in Cell-Free Therapy. Korean J. Clin. Lab. Sci. 2018, 50, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Toward Cell-Free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [Green Version]
- Zhao, A.G.; Shah, K.; Cromer, B.; Sumer, H. Mesenchymal Stem Cell-Derived Extracellular Vesicles and Their Therapeutic Potential. Stem Cells Int. 2020, 2020, 8825771. [Google Scholar] [CrossRef]
- Kauanova, S.; Urazbayev, A.; Vorobjev, I. The Frequent Sampling of Wound Scratch Assay Reveals the “Opportunity” Window for Quantitative Evaluation of Cell Motility-Impeding Drugs. Front. Cell Dev. Biol. 2021, 9, 640972. [Google Scholar] [CrossRef]
- Comfort, N.; Cai, K.; Bloomquist, T.R.; Strait, M.D.; Ferrante, A.W.; Baccarelli, A.A. Nanoparticle Tracking Analysis for the Quantification and Size Determination of Extracellular Vesicles. J. Vis. Exp. 2021, 169, e62447. [Google Scholar] [CrossRef]
- Palmieri, V.; Lucchetti, D.; Gatto, I.; Maiorana, A.; Marcantoni, M.; Maulucci, G.; Papi, M.; Pola, R.; De Spirito, M.; Sgambato, A. Dynamic Light Scattering for the Characterization and Counting of Extracellular Vesicles: A Powerful Noninvasive Tool. J. Nanopart Res. 2014, 16, 2583. [Google Scholar] [CrossRef]
- Rikkert, L.G.; Nieuwland, R.; Terstappen, L.W.M.M.; Coumans, F.A.W. Quality of Extracellular Vesicle Images by Transmission Electron Microscopy Is Operator and Protocol Dependent. J. Extracell Vesicles 2019, 8, 1555419. [Google Scholar] [CrossRef]
- Vogel, R.; Coumans, F.A.W.; Maltesen, R.G.; Böing, A.N.; Bonnington, K.E.; Broekman, M.L.; Broom, M.F.; Buzás, E.I.; Christiansen, G.; Hajji, N.; et al. A Standardized Method to Determine the Concentration of Extracellular Vesicles Using Tunable Resistive Pulse Sensing. J. Extracell. Vesicles 2016, 5, 31242. [Google Scholar] [CrossRef]
- Hartjes, T.A.; Mytnyk, S.; Jenster, G.W.; van Steijn, V.; van Royen, M.E. Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering 2019, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Royo, F.; Théry, C.; Falcón-Pérez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef]
- Liang, Y.; Lehrich, B.M.; Zheng, S.; Lu, M. Emerging Methods in Biomarker Identification for Extracellular Vesicle-based Liquid Biopsy. J. Extracell Vesicles 2021, 10, e12090. [Google Scholar] [CrossRef]
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; Franquesa, M.; Beyer, K.; Borràs, F.E. Size-Exclusion Chromatography-Based Isolation Minimally Alters Extracellular Vesicles’ Characteristics Compared to Precipitating Agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef] [Green Version]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, e8545347. [Google Scholar] [CrossRef] [Green Version]
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern Isolation and Separation Techniques for Extracellular Vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef]
- Sáenz-Cuesta, M.; Arbelaiz, A.; Oregi, A.; Irizar, H.; Osorio-Querejeta, I.; Muñoz-Culla, M.; Banales, J.M.; Falcón-Pérez, J.M.; Olascoaga, J.; Otaegui, D. Methods for Extracellular Vesicles Isolation in a Hospital Setting. Front. Immunol. 2015, 6, 50. [Google Scholar] [CrossRef]
- Spakova, T.; Janockova, J.; Rosocha, J. Characterization and Therapeutic Use of Extracellular Vesicles Derived from Platelets. Int. J. Mol. Sci. 2021, 22, 9701. [Google Scholar] [CrossRef]
- Gaspar, R.S.; Ferreira, P.M.; Mitchell, J.L.; Pula, G.; Gibbins, J.M. Platelet-Derived Extracellular Vesicles Express NADPH Oxidase-1 (Nox-1), Generate Superoxide and Modulate Platelet Function. Free Radic. Biol. Med. 2021, 165, 395–400. [Google Scholar] [CrossRef]
- Taus, F.; Meneguzzi, A.; Castelli, M.; Minuz, P. Platelet-Derived Extracellular Vesicles as Target of Antiplatelet Agents. What Is the Evidence? Front. Pharmacol. 2019, 10, 1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, S.L.; Butov, K.R.; Allaeys, I.; Canas, J.; Morad, G.; Davenport, P.; Laroche, A.; Trubina, N.M.; Italiano, J.E., Jr.; Moses, M.A.; et al. Platelet-Derived Extracellular Vesicles Infiltrate and Modify the Bone Marrow during Inflammation. Blood Adv. 2020, 4, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, F.; Liu, Z.; Zuo, K.; Wang, B.; Zhang, Y.; Han, X.; Lian, A.; Wang, Y.; Liu, M.; et al. MSC-Derived Exosomes Attenuate Cell Death through Suppressing AIF Nucleus Translocation and Enhance Cutaneous Wound Healing. Stem Cell Res. Ther. 2020, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Narauskaitė, D.; Vydmantaitė, G.; Rusteikaitė, J.; Sampath, R.; Rudaitytė, A.; Stašytė, G.; Aparicio Calvente, M.I.; Jekabsone, A. Extracellular Vesicles in Skin Wound Healing. Pharmaceuticals 2021, 14, 811. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, E.; Teixeira Alves, L.G.; Felten, M.; Mitchell, T.J.; Müller-Redetzky, H.C.; Dudek, S.M.; Witzenrath, M. Neutrophil-Derived Extracellular Vesicles Activate Platelets after Pneumolysin Exposure. Cells 2021, 10, 3581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-Derived Extracellular Vesicles: Diverse Mediators of Pathology and Therapeutics in Multiple Diseases. Cell Death Dis. 2020, 11, 924. [Google Scholar] [CrossRef]
- Neupane, K.R.; McCorkle, J.R.; Kopper, T.J.; Lakes, J.E.; Aryal, S.P.; Abdullah, M.; Snell, A.A.; Gensel, J.C.; Kolesar, J.; Richards, C.I. Macrophage-Engineered Vesicles for Therapeutic Delivery and Bidirectional Reprogramming of Immune Cell Polarization. ACS Omega 2021, 6, 3847–3857. [Google Scholar] [CrossRef]
- Zhou, X.; Brown, B.A.; Siegel, A.P.; El Masry, M.S.; Zeng, X.; Song, W.; Das, A.; Khandelwal, P.; Clark, A.; Singh, K.; et al. Exosome-Mediated Crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing. ACS Nano 2020, 14, 12732–12748. [Google Scholar] [CrossRef]
- Ahuja, A.; Kim, E.; Sung, G.-H.; Cho, J.Y. STAT3 Differentially Regulates TLR4-Mediated Inflammatory Responses in Early or Late Phases. Int. J. Mol. Sci. 2020, 21, 7675. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-Preconditioned Mesenchymal Stromal Cells Modify Macrophage Polarization for Resolution of Chronic Inflammation via Exosome-Shuttled Let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-Induced Excessive Inflammation. EBioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xu, R.; Gu, H.; Zhang, E.; Qu, J.; Cao, W.; Huang, X.; Yan, H.; He, J.; Cai, Z. Metabolic Reprogramming in Macrophage Responses. Biomark. Res. 2021, 9, 1. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-Stimulated MSC-Derived Exosomes Improve Diabetic Wound Healing through Regulating Macrophage M1 and M2 Polarization by Targeting the PTEN/AKT Pathway. Stem Cell Res. 2020, 11, 259. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.; Yousefi, M. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Novel Therapeutic Paradigm. J. Cell. Physiol. 2020, 235, 706–717. [Google Scholar] [CrossRef]
- Nasiri, G.; Azarpira, N.; Alizadeh, A.; Goshtasbi, S.; Tayebi, L. Shedding Light on the Role of Keratinocyte-Derived Extracellular Vesicles on Skin-Homing Cells. Stem Cell Res. Ther. 2020, 11, 421. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Chen, W.; Huang, P.; Bi, J. Human Keratinocyte-Derived Microvesicle MiRNA-21 Promotes Skin Wound Healing in Diabetic Rats through Facilitating Fibroblast Function and Angiogenesis. Int. J. Biochem. Cell Biol. 2019, 114, 105570. [Google Scholar] [CrossRef]
- Zaiss, D.M.; Minutti, C.M.; Knipper, J.A. Immune- and Non-immune-mediated Roles of Regulatory T-cells during Wound Healing. Immunology 2019, 157, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Keyes, B.E.; Liu, S.; Asare, A.; Naik, S.; Levorse, J.; Polak, L.; Lu, C.P.; Nikolova, M.; Pasolli, H.A.; Fuchs, E. Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell 2016, 167, 1323–1338.e14. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Sanz-Ros, J.; Román-Domínguez, A.; Gimeno-Mallench, L.; Inglés, M.; Viña, J.; Borrás, C. Extracellular Vesicles from Healthy Cells Improves Cell Function and Stemness in Premature Senescent Stem Cells by MiR-302b and HIF-1α Activation. Biomolecules 2020, 10, E957. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Pintucci, G.; Moscatelli, D.; Saponara, F.; Biernacki, P.R.; Baumann, F.G.; Bizekis, C.; Galloway, A.C.; Basilico, C.; Mignatti, P. Lack of ERK Activation and Cell Migration in FGF-2-Deficient Endothelial Cells. FASEB J. 2002, 16, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.L.; Weil, B.R.; Abarbanell, A.M.; Wang, Y.; Poynter, J.A.; Manukyan, M.C.; Meldrum, D.R. IL-6 and TGF-α Costimulate Mesenchymal Stem Cell Vascular Endothelial Growth Factor Production by ERK-, JNK-, and PI3K-Mediated Mechanisms. Shock 2011, 35, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.J.; O’Neill, C.L.; O’Doherty, T.M.; Knott, H.; Guduric-Fuchs, J.; Gardiner, T.A.; Stitt, A.W. Myeloid Angiogenic Cells Act as Alternative M2 Macrophages and Modulate Angiogenesis through Interleukin-8. Mol. Med. 2011, 17, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Jordà, M.; Vinyals, A.; Marazuela, A.; Cubillo, E.; Olmeda, D.; Valero, E.; Cano, A.; Fabra, A. Id-1 Is Induced in MDCK Epithelial Cells by Activated Erk/MAPK Pathway in Response to Expression of the Snail and E47 Transcription Factors. Exp. Cell Res. 2007, 313, 2389–2403. [Google Scholar] [CrossRef]
- Li, T.; Hu, J.; Du, S.; Chen, Y.; Wang, S.; Wu, Q. ERK1/2/COX-2/PGE2 Signaling Pathway Mediates GPR91-Dependent VEGF Release in Streptozotocin-Induced Diabetes. Mol. Vis. 2014, 20, 1109–1121. [Google Scholar]
- Wu, G.; Luo, J.; Rana, J.S.; Laham, R.; Sellke, F.W.; Li, J. Involvement of COX-2 in VEGF-Induced Angiogenesis via P38 and JNK Pathways in Vascular Endothelial Cells. Cardiovasc. Res. 2006, 69, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Berra, E.; Pagès, G.; Pouysségur, J. MAP Kinases and Hypoxia in the Control of VEGF Expression. Cancer Metastasis Rev. 2000, 19, 139–145. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, J.; Zheng, C.; Su, Y.; Bao, L.; Zhu, B.; Liu, S.; Wang, L.; Wang, X.; Wang, Y.; et al. Exosomes Released from Educated Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing via Promoting Angiogenesis. Cell Prolif. 2020, 53, e12830. [Google Scholar] [CrossRef]
- Li, B.; Luan, S.; Chen, J.; Zhou, Y.; Wang, T.; Li, Z.; Fu, Y.; Zhai, A.; Bi, C. The MSC-Derived Exosomal LncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol. Ther.-Nucleic Acids 2020, 19, 814–826. [Google Scholar] [CrossRef]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef]
- Ding, J.; Wang, X.; Chen, B.; Zhang, J.; Xu, J. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Stimulated by Deferoxamine Accelerate Cutaneous Wound Healing by Promoting Angiogenesis. Biomed. Res. Int. 2019, 2019, 9742765. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Liu, W.; Li, J.; Lu, J.; Lu, H.; Jia, W.; Liu, F. Exosomes Derived from Atorvastatin-Pretreated MSC Accelerate Diabetic Wound Repair by Enhancing Angiogenesis via AKT/ENOS Pathway. Stem Cell Res. Ther. 2020, 11, 350. [Google Scholar] [CrossRef]
- Wu, D.; Kang, L.; Tian, J.; Wu, Y.; Liu, J.; Li, Z.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells with the Stimulation of Fe3O4 Nanoparticles and Static Magnetic Field Enhance Wound Healing Through Upregulated MiR-21-5p. Int. J. Nanomed. 2020, 15, 7979–7993. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from Human Adipose Stem Cells Promote Wound Healing by Optimizing Cellular Functions via AKT and ERK Signaling Pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-Free Therapy Based on Adipose Tissue Stem Cell-Derived Exosomes Promotes Wound Healing via the PI3K/Akt Signaling Pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, F.; Gu, L.; Ji, P.; Yang, X.; Liu, M.; Tao, K.; Hu, D. Adipose Mesenchymal Stem Cell Exosomes Promote Wound Healing through Accelerated Keratinocyte Migration and Proliferation by Activating the AKT/HIF-1α Axis. J. Mol. Histol. 2020, 51, 375–383. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes Derived from Human Adipose Mesenchymal Stem Cells Attenuate Hypertrophic Scar Fibrosis by MiR-192-5p/IL-17RA/Smad Axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from Adipose-Derived Stem Cells Overexpressing Nrf2 Accelerate Cutaneous Wound Healing by Promoting Vascularization in a Diabetic Foot Ulcer Rat Model. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Xi, Z.; Chen, G.; Liu, K.; Ma, R.; Zhou, C. Extracellular Vesicle-Carried MicroRNA-27b Derived from Mesenchymal Stem Cells Accelerates Cutaneous Wound Healing via E3 Ubiquitin Ligase ITCH. J. Cell. Mol. Med. 2020, 24, 11254–11271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Hu, B.; Niu, X.; Liu, X.; Zhang, G.; Zhang, C.; Li, Q.; Wang, Y. Exosomes Derived from Human Endothelial Progenitor Cells Accelerate Cutaneous Wound Healing by Promoting Angiogenesis Through Erk1/2 Signaling. Int. J. Biol. Sci. 2016, 12, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, X.; Lv, Y.; Zheng, C.; Dong, Y.; Dou, G.; Zhu, B.; Liu, A.; Wang, W.; Zhou, J.; et al. Apoptotic Bodies Derived from Mesenchymal Stem Cells Promote Cutaneous Wound Healing via Regulating the Functions of Macrophages. Stem Cell Res. Ther. 2020, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, M.; Sun, Y.; Yang, D.; Xu, W.; Qian, H. Exosomes: Emerging Cell-Free Based Therapeutics in Dermatologic Diseases. Front. Cell Dev. Biol. 2021, 9, 736022. [Google Scholar] [CrossRef]
- Geiger, A.; Walker, A.; Nissen, E. Human Fibrocyte-Derived Exosomes Accelerate Wound Healing in Genetically Diabetic Mice. Biochem. Biophys. Res. Commun. 2015, 467, 303–309. [Google Scholar] [CrossRef]
- Roy, S.; Sen, C.K. MiRNA in Wound Inflammation and Angiogenesis. Microcirculation 2012, 19, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Marofi, F.; Alexandrovna, K.I.; Margiana, R.; Bahramali, M.; Suksatan, W.; Abdelbasset, W.K.; Chupradit, S.; Nasimi, M.; Maashi, M.S. MSCs and Their Exosomes: A Rapidly Evolving Approach in the Context of Cutaneous Wounds Therapy. Stem Cell Res. Ther. 2021, 12, 597. [Google Scholar] [CrossRef]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef]
- Wang, J.; Xia, J.; Huang, R.; Hu, Y.; Fan, J.; Shu, Q.; Xu, J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Alter Disease Outcomes via Endorsement of Macrophage Polarization. Stem Cell Res. Ther. 2020, 11, 424. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Novel Frontiers in Regenerative Medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, Y.; Pan, Y.; Zhang, L.; Gong, H.; Qi, Y.; Wang, M.; Gong, H.; Shao, M.; Wang, X.; et al. Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int. 2019, 2019, 2402916. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Liu, T.; Wang, X.; Wang, H.; Song, H.; Wang, W. Exosomes Derived from TSG-6 Modified Mesenchymal Stromal Cells Attenuate Scar Formation during Wound Healing. Biochimie 2020, 177, 40–49. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Author Correction: Exosomes Derived from Human Adipose Mensenchymal Stem Cells Accelerates Cutaneous Wound Healing via Optimizing the Characteristics of Fibroblasts. Sci. Rep. 2020, 10, 6693. [Google Scholar] [CrossRef] [Green Version]
- Tutuianu, R.; Rosca, A.-M.; Iacomi, D.M.; Simionescu, M.; Titorencu, I. Human Mesenchymal Stromal Cell-Derived Exosomes Promote In Vitro Wound Healing by Modulating the Biological Properties of Skin Keratinocytes and Fibroblasts and Stimulating Angiogenesis. Int. J. Mol. Sci. 2021, 22, 6239. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes Secreted by Human Adipose Mesenchymal Stem Cells Promote Scarless Cutaneous Repair by Regulating Extracellular Matrix Remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wen, H.; Huang, J.; Liao, P.; Liao, H.; Tu, J.; Zeng, Y. Extracellular Vesicle-enclosed MiR-486-5p Mediates Wound Healing with Adipose-derived Stem Cells by Promoting Angiogenesis. J. Cell. Mol. Med. 2020, 24, 9590–9604. [Google Scholar] [CrossRef]
- Gao, S.; Chen, T.; Hao, Y.; Zhang, F.; Tang, X.; Wang, D.; Wei, Z.; Qi, J. Exosomal MiR-135a Derived from Human Amnion Mesenchymal Stem Cells Promotes Cutaneous Wound Healing in Rats and Fibroblast Migration by Directly Inhibiting LATS2 Expression. Stem Cell Res. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, Y.; Liu, Y.; Li, X.; Tang, L.; Duan, M.; Li, J.; Zhang, G. Exosomes Derived from Human Umbilical Cord Blood Mesenchymal Stem Cells Stimulate Regenerative Wound Healing via Transforming Growth Factor-β Receptor Inhibition. Stem Cell Res. 2021, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, J.; Liu, Y.; Chen, Z.; Li, X.; Tang, L.; Li, J.; Duan, M.; Zhang, G. Human Amniotic Fluid Stem Cell-Derived Exosomes as a Novel Cell-Free Therapy for Cutaneous Regeneration. Front. Cell Dev. Biol. 2021, 9, 685873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shi, Y.; Gong, A.; Pan, Z.; Shi, H.; Yang, H.; Fu, H.; Yan, Y.; Zhang, X.; Wang, M.; et al. HucMSC Exosome-Delivered 14-3-3ζ Orchestrates Self-Control of the Wnt Response via Modulation of YAP During Cutaneous Regeneration. Stem Cells 2016, 34, 2485–2500. [Google Scholar] [CrossRef]
- Ha, D.H.; Kim, H.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Guan, S.; Witte, F. The Therapeutic Potential of MSC-EVs as a Bioactive Material for Wound Healing. Eng. Regen. 2021, 2, 182–194. [Google Scholar] [CrossRef]
- Ramasubramanian, L.; Kumar, P.; Wang, A. Engineering Extracellular Vesicles as Nanotherapeutics for Regenerative Medicine. Biomolecules 2019, 10, E48. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [Green Version]
- Severus, G.B.; Florentina, I.R.; Mihai, E.R.; Codruța, D.E.; Maria, C.S.; Dragoș, C.; Cristian, V.S. Extracellular Vesicles as Intercellular Communication Vehicles in Regenerative Medicine; IntechOpen: London, UK, 2021; ISBN 978-1-80355-055-8. [Google Scholar]
- Xie, M.; Wu, D.; Li, G.; Yang, J.; Zhang, Y.S. Exosomes Targeted towards Applications in Regenerative Medicine. Nano Sel. 2021, 2, 880–908. [Google Scholar] [CrossRef]
- Kholia, S.; Ranghino, A.; Garnieri, P.; Lopatina, T.; Deregibus, M.C.; Rispoli, P.; Brizzi, M.F.; Camussi, G. Extracellular Vesicles as New Players in Angiogenesis. Vasc. Pharmacol. 2016, 86, 64–70. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic Strategies for Enhancing Angiogenesis in Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Mao, X.; Cheng, R.; Zhang, H.; Bae, J.; Cheng, L.; Zhang, L.; Deng, L.; Cui, W.; Zhang, Y.; Santos, H.A.; et al. Self-Healing and Injectable Hydrogel for Matching Skin Flap Regeneration. Adv. Sci. 2019, 6, 1801555. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.-F.; Lian, Q. Paracrine Mechanisms of Mesenchymal Stem Cell-Based Therapy: Current Status and Perspectives. Cell Transpl. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Yuan, T.; Zhang, C.-Q. Exosomes Derived from Platelet-Rich Plasma Promote the Re-Epithelization of Chronic Cutaneous Wounds via Activation of YAP in a Diabetic Rat Model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Torreggiani, E.; Perut, F.; Roncuzzi, L.; Zini, N.; Baglìo, S.R.; Baldini, N. Exosomes: Novel Effectors of Human Platelet Lysate Activity. Eur. Cells Mater. 2014, 28, 137–151; discussion 151. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Yan, C.; Zhou, W.; Endo, Y.; Liu, J.; Hu, L.; Hu, Y.; Mi, B.; Liu, G. Circulating Exosomal MiR-20b-5p Inhibition Restores Wnt9b Signaling and Reverses Diabetes-Associated Impaired Wound Healing. Small 2020, 16, e1904044. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Hong, Y.; Liang, R.; Zhang, X.; Liao, Y.; Jiang, D.; Zhang, J.; Sheng, Z.; Xie, C.; Peng, Z.; et al. Rapid Printing of Bio-Inspired 3D Tissue Constructs for Skin Regeneration. Biomaterials 2020, 258, 120287. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Ling, Y.; Deng, W.; Xue, J.; Sun, P.; Wang, D.-A. A Novel Cell Encapsulatable Cryogel (CECG) with Macro-Porous Structures and High Permeability: A Three-Dimensional Cell Culture Scaffold for Enhanced Cell Adhesion and Proliferation. Biomed. Mater. 2019, 14, 055006. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of Stem Cell-Derived Exosomes with in Situ Hydrogel Glue as a Promising Tissue Patch for Articular Cartilage Regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A.C.; et al. Exosomes Derived from Pioglitazone-Pretreated MSCs Accelerate Diabetic Wound Healing through Enhancing Angiogenesis. J. Nanobiotechnol. 2021, 19, 150. [Google Scholar] [CrossRef]
- Zhou, Y.; Nie, W.; Zhao, J.; Yuan, X. Rapidly in Situ Forming Adhesive Hydrogel Based on a PEG-Maleimide Modified Polypeptide through Michael Addition. J. Mater. Sci. Mater. Med. 2013, 24, 2277–2286. [Google Scholar] [CrossRef]
- Tan, A.; Rajadas, J.; Seifalian, A.M. Exosomes as Nano-Theranostic Delivery Platforms for Gene Therapy. Adv. Drug Deliv. Rev. 2013, 65, 357–367. [Google Scholar] [CrossRef]
- Li, Q.; Gong, S.; Yao, W.; Yang, Z.; Wang, R.; Yu, Z.; Wei, M. Exosome Loaded Genipin Crosslinked Hydrogel Facilitates Full Thickness Cutaneous Wound Healing in Rat Animal Model. Drug Deliv. 2021, 28, 884–893. [Google Scholar] [CrossRef]
- Mi, B.; Chen, L.; Xiong, Y.; Yan, C.; Xue, H.; Panayi, A.C.; Liu, J.; Hu, L.; Hu, Y.; Cao, F.; et al. Saliva Exosomes-Derived UBE2O MRNA Promotes Angiogenesis in Cutaneous Wounds by Targeting SMAD6. J. Nanobiotechnol. 2020, 18, 68. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, B.; Xiong, Y.; Tao, R.; Panayi, A.C.; Chen, L.; Tian, W.; Xue, H.; Shi, L.; Zhang, X.; et al. Cryogenic 3D Printed Hydrogel Scaffolds Loading Exosomes Accelerate Diabetic Wound Healing. Chem. Eng. J. 2021, 426, 130634. [Google Scholar] [CrossRef]
- Bai, Y.; Han, Y.; Yan, X.-L.; Ren, J.; Zeng, Q.; Li, X.-D.; Pei, X.-T.; Han, Y. Adipose Mesenchymal Stem Cell-Derived Exosomes Stimulated by Hydrogen Peroxide Enhanced Skin Flap Recovery in Ischemia-Reperfusion Injury. Biochem. Biophys. Res. Commun. 2018, 500, 310–317. [Google Scholar] [CrossRef]
- Public Safety Alert Due to Marketing of Unapproved Stem Cell and Exosome Products. Available online: https://www.fda.gov/safety/medical-product-safety-information/public-safety-alert-due-marketing-unapproved-stem-cell-and-exosome-products (accessed on 12 September 2022).
- 21st Century Cures Act. Available online: https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act (accessed on 13 September 2021).
- Kumamoto University. Effect of Plasma Derived Exosomes on Intractable Cutaneous Wound Healing: Prospective Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT02565264?cond=Plasma+Derived+Exosomes&draw=2&rank=1 (accessed on 1 September 2022).
- Brizzi, M.F. Autologous Serum-Derived Extracellular Vesicles to Treat Venous Trophic Lesions Not Responsive to Conventional Treatments. Available online: https://clinicaltrials.gov/ct2/show/NCT04652531?cond=Serum-Derived+Extracellular+Vesicles&draw=2&rank=1 (accessed on 1 September 2022).

| Form of MSC | Extracellular Vesicles | Significance |
|---|---|---|
| BM-MSC EVs | Promote M2 macrophage polarization through transfer of miR-223. Macrophage polarization by targeting the PTEN/AKT pathway. Promote proliferation, migration, and tube formation of in vitro endothelial cells and increase the p-AKT and p-eNOS signaling pathways to produce angiogenesis in the healing wound. | |
| BM-MSC Exos | Accelerate wound healing by targeting fibroblasts via the Akt, Erk1/2, and STAT3 signaling pathways. | |
| BM-MSC Exos lncRNA H19 | Promotes wound healing in diabetic foot ulcers by upregulating PTEN via miRNA-152-3p. | |
| BM-MSC Exos + deferoxamine | Activate the PI3K/AKT signaling pathway via miR-126-mediated PTEN downregulation to stimulate angiogenesis in vitro. | |
| BM-MSC Exos + atorvastatin | Enhance angiogenesis via the AKT/eNOS pathway by upregulating miR-221-3p in endothelial cells. | |
| BM-MSC Exos + static magnetic field | Promote neovasculogenesis to enhance wound healing through miR-21-5p by targeting SPRY2 to facilitate the PI3K/AKT and ERK1/2 signaling pathways. | |
| TSG-6-modified BM-MSC EVs | Suppress scar formation by suppressing SMAD2/3 signaling and inhibiting TGF-β1, COL1, COL3, and SMA-α protein synthesis and inflammation in the wound site. | |
| Adipose tissue-derived MSCs | AD-MSC EVs | Facilitate wound healing via the AKT serine/threonine kinase 1 (AKT) and mitogen-activated protein kinase 1 (ERK) signaling pathways. Promote the proliferation and migration—and stimulate the AKT and ERK signaling—of in vitro fibroblasts, keratinocytes, and endothelial cells. Facilitate wound healing by accelerating keratinocytes and fibroblasts in an AKT/HIF-1α-dependent fashion. Attenuate hypertrophic scars and fibrosis by the miR-192-5p/IL-17RA/SMAD axis. Promote the proliferation and migration of fibroblasts and keratinocytes, receive signals from COL1, COL3, MMP1, FGF-2, and TGF-β1 mRNAs, along with the increased expression of VEGF, c-myc, MMP-9, and fibronectin. |
| AD-MSC Exos | Accelerate wound healing through the PI3K/AKT signaling pathway. Facilitate extracellular matrix remodulation in wound repair by enhancing and regulating the COL3:1, TGF-β3:TGF-β1, and MMP3:TIMP1 ratios via the ERK/MAPK signaling pathways to mitigate the minimization of scar formation. Facilitate wound healing by the overexpression of miR-486-5p by targeting the Sp5 and CCND2 expression. | |
| Exos derived from mmu_circ_0000250-modified AD-MSCs | Promoted wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. | |
| AD-MSC-derived miR-192-5p | Downregulates pro-fibrosis protein and upregulates wound healing via the inhibition of the IL-17RA/SMAD expression. | |
| Umbilical cord-derived MSCs | UC-MSC EVs | Promote the proliferation and migration of fibroblasts and keratinocytes through miR-27b, which acts by suppressing E3-ubiquitin ligase ITCH, thereby activating JUNB/IRE1α. Suppressed TGF-β-induced myofibroblast formation in a mouse skin wound model through TGF-β/SMAD2 signaling in fibroblasts. |
| UC-MSC Exos | Enhance wound healing by activating the WNT/β-catenin signaling pathway. Promote the phosphorylation of YAP by transporting the 14-3-3ζ protein, which inhibited WNT/β-catenin signal transduction, enhanced collagen deposition, and inhibited excess fibroblast expansion in burn wounds. | |
| UC-MSC Exos + pluronic F127 hydrogel | Promote neovasculogenesis by increasing the expression of VEGF and TGF-β1. | |
| Amniotic fluid-derived MSCs | AF-MSC EVs | Inhibit and suppress myofibroblast aggregation and ECM synthesis via the TGF-β pathway through miRNAs such as let-7-5p, -22-3p, -27a-3p, -21-5p, and -23a-3p. |
| Human fetal dermis-derived MSCs | Human fetal dermis-MSC EVs | induce the expression of COL1, COL3, elastin, and fibronectin by activating the NOTCH pathway. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nallakumarasamy, A.; Jeyaraman, M.; Maffulli, N.; Jeyaraman, N.; Suresh, V.; Ravichandran, S.; Gupta, M.; Potty, A.G.; El-Amin, S.F., III; Khanna, M.; et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Wound Healing. Life 2022, 12, 1733. https://doi.org/10.3390/life12111733
Nallakumarasamy A, Jeyaraman M, Maffulli N, Jeyaraman N, Suresh V, Ravichandran S, Gupta M, Potty AG, El-Amin SF III, Khanna M, et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Wound Healing. Life. 2022; 12(11):1733. https://doi.org/10.3390/life12111733
Chicago/Turabian StyleNallakumarasamy, Arulkumar, Madhan Jeyaraman, Nicola Maffulli, Naveen Jeyaraman, Veerasivabalan Suresh, Srinath Ravichandran, Manu Gupta, Anish G. Potty, Saadiq F. El-Amin, III, Manish Khanna, and et al. 2022. "Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Wound Healing" Life 12, no. 11: 1733. https://doi.org/10.3390/life12111733
APA StyleNallakumarasamy, A., Jeyaraman, M., Maffulli, N., Jeyaraman, N., Suresh, V., Ravichandran, S., Gupta, M., Potty, A. G., El-Amin, S. F., III, Khanna, M., & Gupta, A. (2022). Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Wound Healing. Life, 12(11), 1733. https://doi.org/10.3390/life12111733