Current Concepts and Future Applications of Non-Invasive Functional and Anatomical Evaluation of Coronary Artery Disease
Abstract
:1. Introduction
2. Prognostic Value and Treatment of Anatomical and Functional Significant CAD Lesions
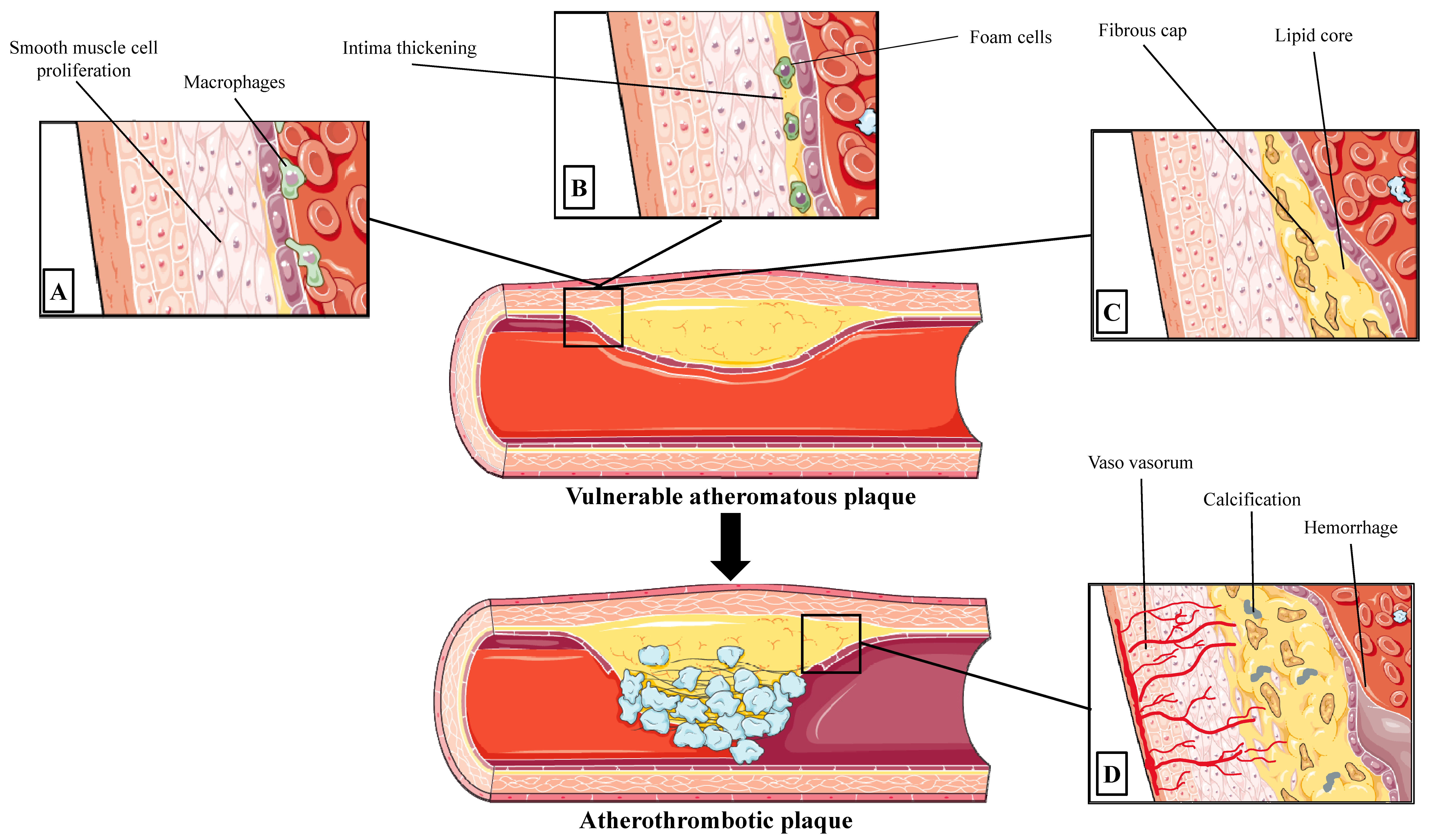
2.1. Progression to Vulnerable Coronary Atherosclerotic Lesion
2.2. Types of Atherosclerotic Lesion in Acute Coronary Syndromes
2.3. Atherosclerotic Lesions in Stable CAD
2.4. Functional Directed Treatment of CAD
3. The Role of Biomarkers in the Assessment of CAD
4. Computed Tomography
4.1. Coronary Artery Calcium
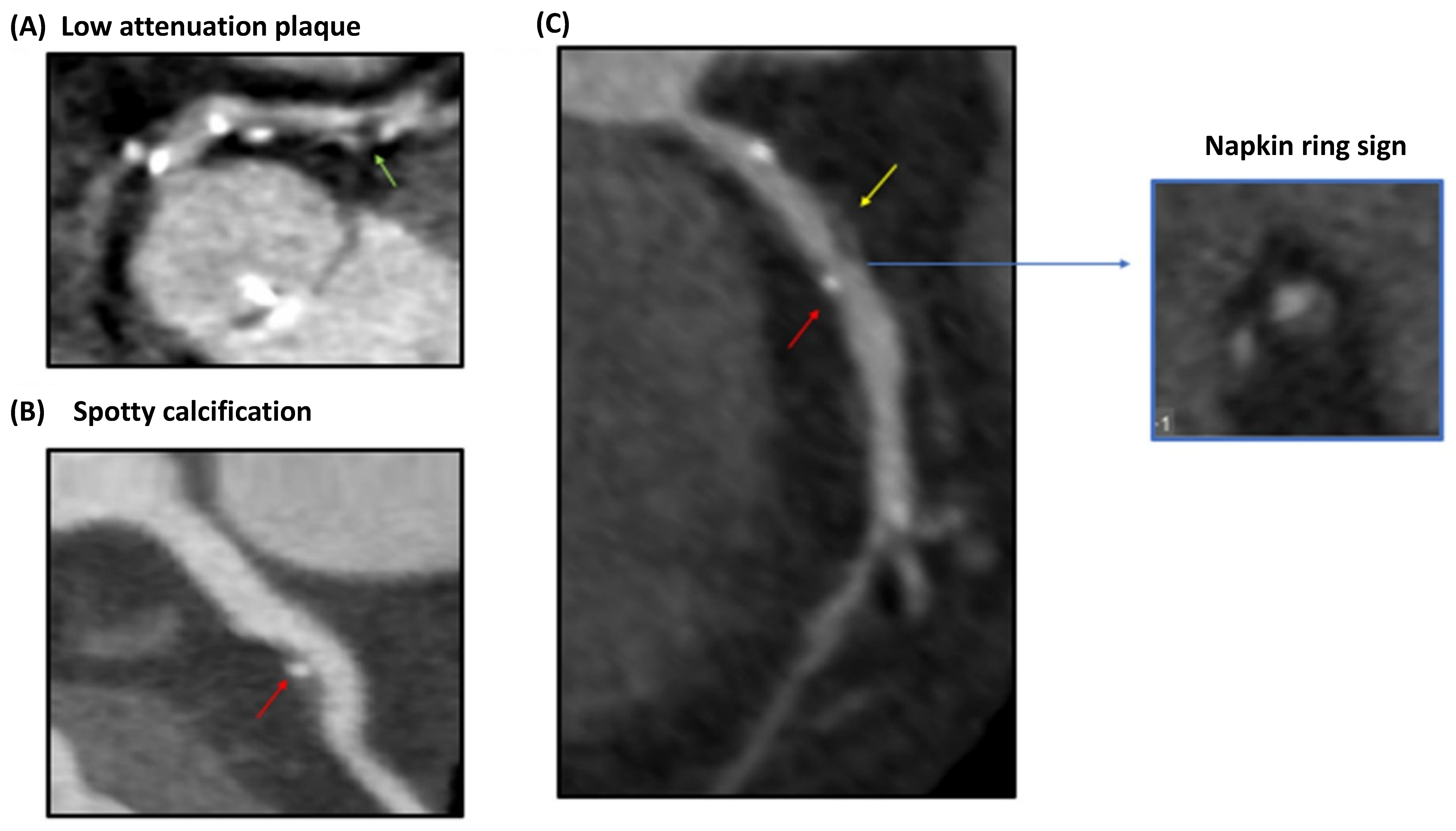
4.2. Vulnerable Plaque Assessment
4.3. Perivascular Fat Attenuation Index
4.4. CT Myocardial Perfusion
4.5. FFRCT
5. Magnetic Resonance Imaging (MRI)
5.1. General Aspects of Cardiac MRI Approach
5.2. Free-Breathing Coronary Cardiovascular Magnetic Resonance Imaging
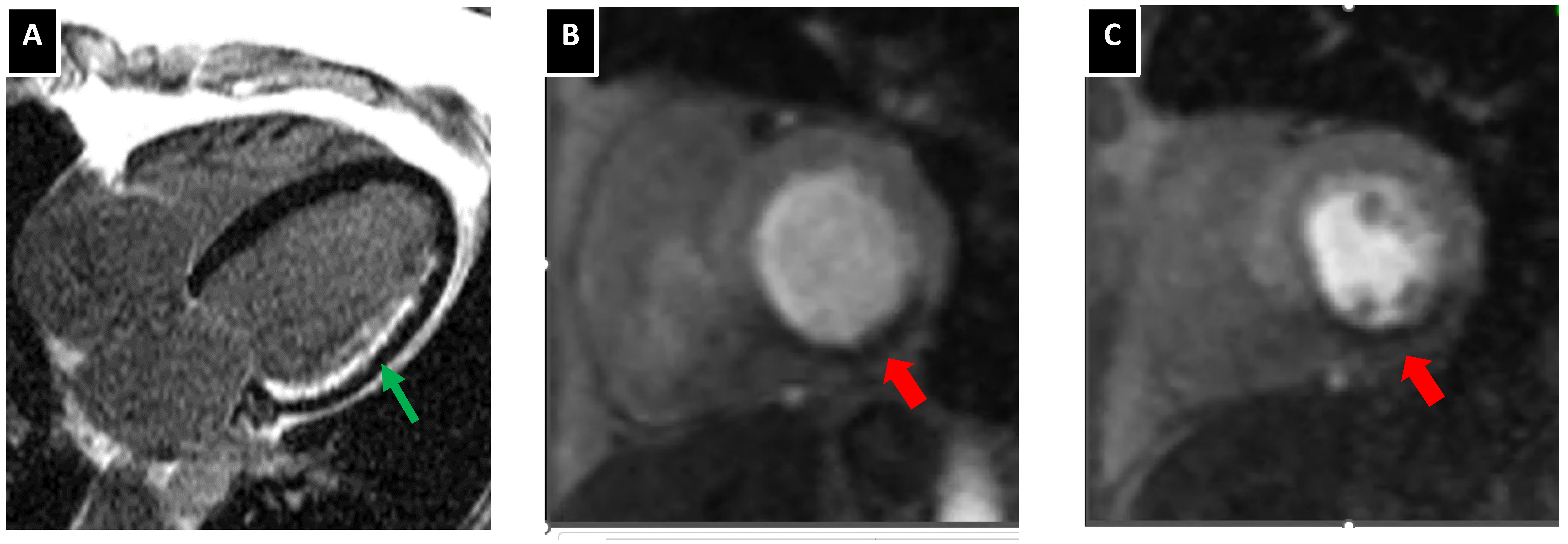
5.3. Atherosclerotic Plaque Characterization with Cardiac MRI
5.4. Myocardial Perfusion Images and Assessment of Myocardial Ischemia with Cardiac MRI
5.5. Prognostic Role of Cardiac MRI in Post-Acute Coronary Syndrome Patients
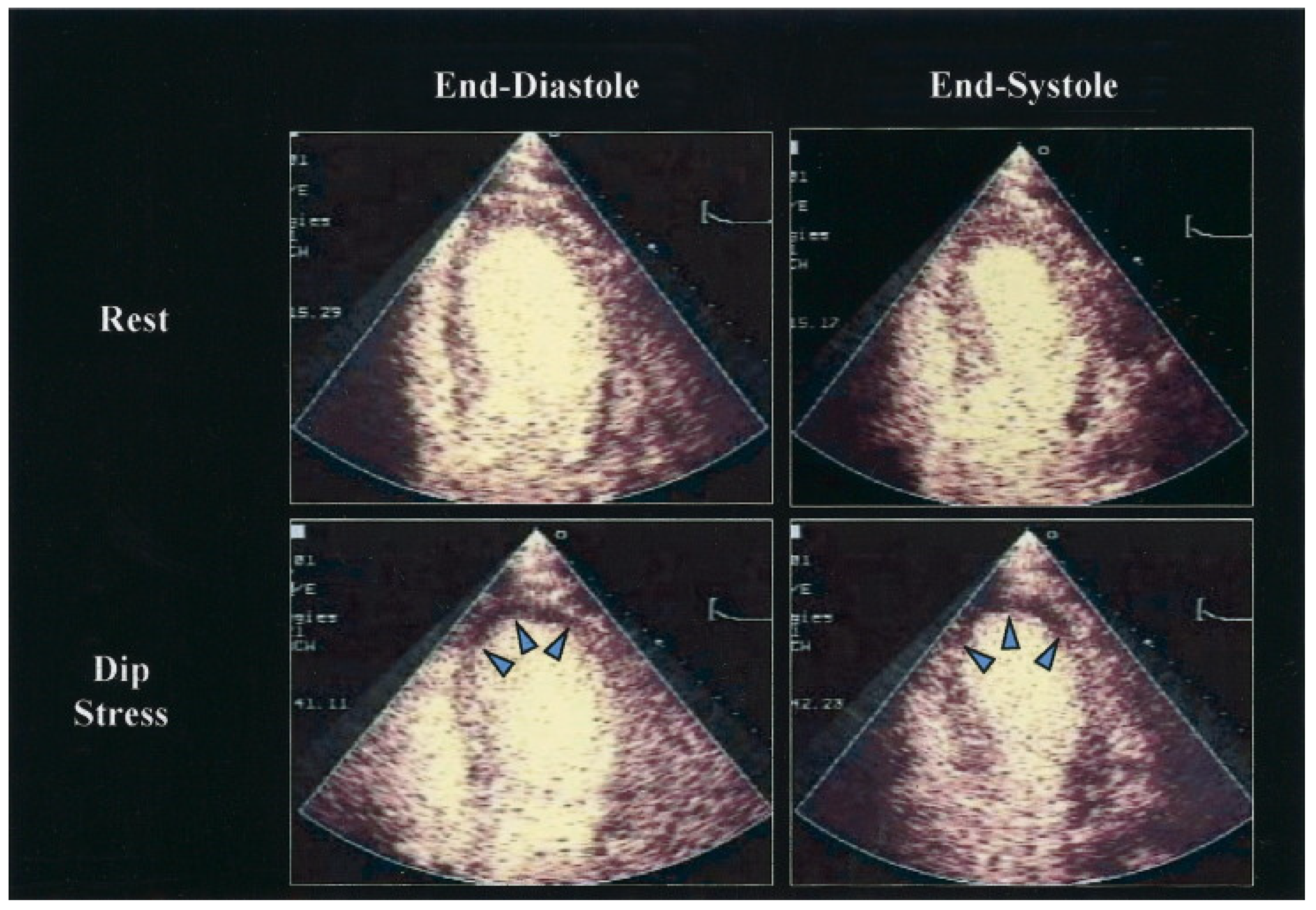
6. Stress Echocardiography
6.1. Additional Modalities to Improve Diagnostic Performance of Stress Echocardiography
6.1.1. Enhanced Stress Echocardiography with Contrast
6.1.2. Real-Time Three-Dimensional Imaging for Stress Echocardiography
6.1.3. D Strain-Speckle Tracking Imaging in Stress Echocardiography
6.2. Coronary Flow Reserve in Stress Echocardiography
6.3. Myocardial Viability Assessment
7. Nuclear Imaging
7.1. Cardiac Positron Emission Tomography
7.1.1. Vulnerable Plaque Imaging
7.1.2. Myocardial Perfusion Imaging
7.2. Cardiac Single Photon Emission Computed Tomography
8. Comprehensive Approach
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013, 22, 399–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Schatz, R.A. The History of Coronary Stenting. Interv. Cardiol. Clin. 2016, 5, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Ng, M.K. Is there a role for coronary angiography in the early detection of the vulnerable plaque? Int. J. Cardiol. 2013, 164, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [Green Version]
- Spacek, M.; Zemanek, D.; Hutyra, M.; Sluka, M.; Taborsky, M. Vulnerable atherosclerotic plaque—A review of current concepts and advanced imaging. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2018, 162, 10–17. [Google Scholar] [CrossRef]
- Stefanadis, C.; Antoniou, C.K.; Tsiachris, D.; Pietri, P. Coronary Atherosclerotic Vulnerable Plaque: Current Perspectives. J. Am. Heart Assoc. 2017, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; van Hinsbergh, V.W.M.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial permeability, LDL deposition, and cardiovascular risk factors—A review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Theofilis, P.; Sagris, M.; Antonopoulos, A.S.; Oikonomou, E.; Tsioufis, K.; Tousoulis, D. Non-Invasive Modalities in the Assessment of Vulnerable Coronary Atherosclerotic Plaques. Tomography 2022, 8, 1742–1758. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
- Michel, J.B.; Virmani, R.; Arbustini, E.; Pasterkamp, G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur. Heart J. 2011, 32, 1977–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newby, A.C. Matrix metalloproteinase inhibition therapy for vascular diseases. Vascul. Pharmacol. 2012, 56, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Babaev, V.R.; Huang, J.; Linton, E.F.; Tao, H.; Yancey, P.G. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circ. J. 2016, 80, 2259–2268. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, H.; Tang, Y.; Yao, P. Potential Mechanisms and Effects of Efferocytosis in Atherosclerosis. Front. Endocrinol. 2020, 11, 585285. [Google Scholar] [CrossRef]
- Sluimer, J.C.; Kolodgie, F.D.; Bijnens, A.P.; Maxfield, K.; Pacheco, E.; Kutys, B.; Duimel, H.; Frederik, P.M.; van Hinsbergh, V.W.; Virmani, R.; et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J. Am. Coll. Cardiol. 2009, 53, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- Falk, E.; Shah, P.K.; Fuster, V. Coronary plaque disruption. Circulation 1995, 92, 657–671. [Google Scholar] [CrossRef]
- Chan, K.H.; Chawantanpipat, C.; Gattorna, T.; Chantadansuwan, T.; Kirby, A.; Madden, A.; Keech, A.; Ng, M.K. The relationship between coronary stenosis severity and compression type coronary artery movement in acute myocardial infarction. Am. Heart J. 2010, 159, 584–592. [Google Scholar] [CrossRef]
- Libby, P.; Pasterkamp, G. Requiem for the ‘vulnerable plaque’. Eur. Heart J. 2015, 36, 2984–2987. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef]
- Iwarson, S. Do we have antibiotic drug abuse? Lakartidningen 1978, 75, 1382. [Google Scholar] [PubMed]
- Xaplanteris, P.; Fournier, S.; Pijls, N.H.J.; Fearon, W.F.; Barbato, E.; Tonino, P.A.L.; Engstrom, T.; Kaab, S.; Dambrink, J.H.; Rioufol, G.; et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N. Engl. J. Med. 2018, 379, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Mintz, G.S.; Tam, A.; McPherson, J.A.; Iniguez, A.; Fajadet, J.; Fahy, M.; Weisz, G.; De Bruyne, B.; Serruys, P.W.; et al. Prevalence, distribution, predictors, and outcomes of patients with calcified nodules in native coronary arteries: A 3-vessel intravascular ultrasound analysis from Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT). Circulation 2012, 126, 537–545. [Google Scholar] [PubMed] [Green Version]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Liu, L.; Abdu, F.A.; Yin, G.; Xu, B.; Mohammed, A.Q.; Xu, S.; Lv, X.; Luo, Y.; Zu, L.; Yang, C.; et al. Prognostic value of myocardial perfusion imaging with D-SPECT camera in patients with ischemia and no obstructive coronary artery disease (INOCA). J. Nucl. Cardiol. 2021, 28, 3025–3037. [Google Scholar] [CrossRef]
- Doukky, R.; Hayes, K.; Frogge, N.; Balakrishnan, G.; Dontaraju, V.S.; Rangel, M.O.; Golzar, Y.; Garcia-Sayan, E.; Hendel, R.C. Impact of appropriate use on the prognostic value of single-photon emission computed tomography myocardial perfusion imaging. Circulation 2013, 128, 1634–1643. [Google Scholar] [CrossRef] [Green Version]
- Senior, R.; Monaghan, M.; Becher, H.; Mayet, J.; Nihoyannopoulos, P. Stress echocardiography for the diagnosis and risk stratification of patients with suspected or known coronary artery disease: A critical appraisal. Supported by the British Society of Echocardiography. Heart 2005, 91, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Al-Lamee, R.; Thompson, D.; Dehbi, H.M.; Sen, S.; Tang, K.; Davies, J.; Keeble, T.; Mielewczik, M.; Kaprielian, R.; Malik, I.S.; et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet 2018, 391, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; Lopez-Sendon, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Bech, G.J.; De Bruyne, B.; Pijls, N.H.; de Muinck, E.D.; Hoorntje, J.C.; Escaned, J.; Stella, P.R.; Boersma, E.; Bartunek, J.; Koolen, J.J.; et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: A randomized trial. Circulation 2001, 103, 2928–2934. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, F.M.; Ferrara, A.; Johnson, N.P.; van Nunen, L.X.; Escaned, J.; Albertsson, P.; Erbel, R.; Legrand, V.; Gwon, H.C.; Remkes, W.S.; et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur. Heart J. 2015, 36, 3182–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonino, P.A.; De Bruyne, B.; Pijls, N.H.; Siebert, U.; Ikeno, F.; van’t Veer, M.; Klauss, V.; Manoharan, G.; Engstrom, T.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bruyne, B.; Pijls, N.H.; Kalesan, B.; Barbato, E.; Tonino, P.A.; Piroth, Z.; Jagic, N.; Mobius-Winkler, S.; Rioufol, G.; Witt, N.; et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avanzas, P.; Arroyo-Espliguero, R.; Garcia-Moll, X.; Kaski, J.C. Inflammatory biomarkers of coronary atheromatous plaque vulnerability. Panminerva Med. 2005, 47, 81–91. [Google Scholar] [PubMed]
- Zakynthinos, E.; Pappa, N. Inflammatory biomarkers in coronary artery disease. J. Cardiol. 2009, 53, 317–333. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Sun, K.; Zhao, R.; Hu, J.; Hao, Z.; Wang, F.; Lu, Y.; Liu, F.; Zhang, Y. Inflammatory biomarkers of coronary heart disease. Front. Biosci. 2017, 22, 504–515. [Google Scholar]
- Oikonomou, E.; Tsaplaris, P.; Anastasiou, A.; Xenou, M.; Lampsas, S.; Siasos, G.; Pantelidis, P.; Theofilis, P.; Tsatsaragkou, A.; Katsarou, O.; et al. Interleukin-1 in Coronary Artery Disease. Curr. Top. Med. Chem. 2022. [Google Scholar] [CrossRef]
- Chan, D.; Ng, L.L. Biomarkers in acute myocardial infarction. BMC Med. 2010, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Aldous, S.J. Cardiac biomarkers in acute myocardial infarction. Int. J. Cardiol. 2013, 164, 282–294. [Google Scholar] [CrossRef]
- Lubrano, V.; Balzan, S. Consolidated and emerging inflammatory markers in coronary artery disease. World J. Exp. Med. 2015, 5, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Kinlay, S.; Egido, J. Inflammatory biomarkers in stable atherosclerosis. Am. J. Cardiol. 2006, 98, 2P–8P. [Google Scholar] [CrossRef] [PubMed]
- Fichtlscherer, S.; Heeschen, C.; Zeiher, A.M. Inflammatory markers and coronary artery disease. Curr. Opin. Pharmacol. 2004, 4, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Lampsas, S.; Tsaplaris, P.; Pantelidis, P.; Oikonomou, E.; Marinos, G.; Charalambous, G.; Souvaliotis, N.; Mystakidi, V.C.; Goliopoulou, A.; Katsianos, E.; et al. The Role of Endothelial Related Circulating Biomarkers in COVID-19. A Systematic Review and Meta-analysis. Curr. Med. Chem. 2022, 29, 3790–3805. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Michalakeas, C.A.; Parissis, J.; Paraskevaidis, I.; Ntai, K.; Papadakis, I.; Anastasiou-Nana, M.; Lekakis, J. Inflammatory markers in coronary artery disease. Biofactors 2012, 38, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Oikonomou, E.; Vogiatzi, G.; Sagris, M.; Antonopoulos, A.S.; Siasos, G.; Iliopoulos, D.C.; Perrea, D.; Vavouranakis, M.; Tsioufis, K.; et al. The role of microRNA-126 in atherosclerotic cardiovascular diseases. Curr. Med. Chem. 2022. [Google Scholar] [CrossRef]
- Theofilis, P.; Vogiatzi, G.; Oikonomou, E.; Gazouli, M.; Siasos, G.; Katifelis, H.; Perrea, D.; Vavuranakis, M.; Iliopoulos, D.C.; Tsioufis, C.; et al. The Effect of MicroRNA-126 Mimic Administration on Vascular Perfusion Recovery in an Animal Model of Hind Limb Ischemia. Front. Mol. Biosci. 2021, 8, 724465. [Google Scholar] [CrossRef]
- Theofilis, P.; Oikonomou, E.; Vogiatzi, G.; Antonopoulos, A.S.; Siasos, G.; Iliopoulos, D.C.; Perrea, D.; Tsioufis, C.; Tousoulis, D. The impact of proangiogenic microRNA modulation on blood flow recovery following hind limb ischemia. A systematic review and meta-analysis of animal studies. Vascul. Pharmacol. 2021, 141, 106906. [Google Scholar] [CrossRef]
- Economou, E.K.; Oikonomou, E.; Siasos, G.; Papageorgiou, N.; Tsalamandris, S.; Mourouzis, K.; Papaioanou, S.; Tousoulis, D. The role of microRNAs in coronary artery disease: From pathophysiology to diagnosis and treatment. Atherosclerosis 2015, 241, 624–633. [Google Scholar] [CrossRef]
- Zakynthinos, G.; Siasos, G.; Oikonomou, E.; Gazouli, M.; Mourouzis, K.; Zaromitidou, M.; Tsigkou, V.; Bletsa, E.; Stampouloglou, P.; Tsouroulas, S.; et al. Exploration analysis of microRNAs -146a, -19b, and -21 in patients with acute coronary syndrome. Hellenic J. Cardiol. 2021, 62, 260–263. [Google Scholar] [CrossRef]
- Polina, I.A.; Ilatovskaya, D.V.; DeLeon-Pennell, K.Y. Cell free DNA as a diagnostic and prognostic marker for cardiovascular diseases. Clin. Chim. Acta 2020, 503, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Leta, R.; Carreras, F.; Alomar, X.; Monell, J.; Garcia-Picart, J.; Auge, J.M.; Salvador, A.; Pons-Llado, G. Non-invasive coronary angiography with 16 multidetector-row spiral computed tomography: A comparative study with invasive coronary angiography. Rev. Esp. Cardiol. 2004, 57, 217–224. [Google Scholar] [CrossRef]
- Hausleiter, J.; Meyer, T.; Hadamitzky, M.; Zankl, M.; Gerein, P.; Dorrler, K.; Kastrati, A.; Martinoff, S.; Schomig, A. Non-invasive coronary computed tomographic angiography for patients with suspected coronary artery disease: The Coronary Angiography by Computed Tomography with the Use of a Submillimeter resolution (CACTUS) trial. Eur. Heart J. 2007, 28, 3034–3041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [PubMed] [Green Version]
- Dewey, M.; Rief, M.; Martus, P.; Kendziora, B.; Feger, S.; Dreger, H.; Priem, S.; Knebel, F.; Bohm, M.; Schlattmann, P.; et al. Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: Randomised controlled trial. BMJ 2016, 355, i5441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.B.; Arbab-Zadeh, A.; Matheson, M.B.; Ostovaneh, M.R.; Vavere, A.L.; Dewey, M.; Rochitte, C.; Niinuma, H.; Laham, R.; Schuijf, J.D.; et al. Contemporary Discrepancies of Stenosis Assessment by Computed Tomography and Invasive Coronary Angiography. Circ. Cardiovasc. Imaging 2019, 12, e007720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Group, D.T.; Maurovich-Horvat, P.; Bosserdt, M.; Kofoed, K.F.; Rieckmann, N.; Benedek, T.; Donnelly, P.; Rodriguez-Palomares, J.; Erglis, A.; Stechovsky, C.; et al. CT or Invasive Coronary Angiography in Stable Chest Pain. N. Engl. J. Med. 2022, 386, 1591–1602. [Google Scholar] [CrossRef]
- Griffin, W.F.; Choi, A.D.; Riess, J.S.; Marques, H.; Chang, H.J.; Choi, J.H.; Doh, J.H.; Her, A.Y.; Koo, B.K.; Nam, C.W.; et al. AI Evaluation of Stenosis on Coronary CT Angiography, Comparison With Quantitative Coronary Angiography and Fractional Flow Reserve: A CREDENCE Trial Substudy. JACC Cardiovasc. Imaging 2022. [Google Scholar] [CrossRef]
- Daghem, M.; Newby, D.E. Detecting unstable plaques in humans using cardiac CT: Can it guide treatments? Br. J. Pharmacol. 2021, 178, 2204–2217. [Google Scholar] [CrossRef] [Green Version]
- Budoff, M.J.; Young, R.; Burke, G.; Jeffrey Carr, J.; Detrano, R.C.; Folsom, A.R.; Kronmal, R.; Lima, J.A.C.; Liu, K.J.; McClelland, R.L.; et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: The multi-ethnic study of atherosclerosis (MESA). Eur. Heart J. 2018, 39, 2401–2408. [Google Scholar] [CrossRef] [Green Version]
- Sandesara, P.B.; Mehta, A.; O’Neal, W.T.; Kelli, H.M.; Sathiyakumar, V.; Martin, S.S.; Blaha, M.J.; Blumenthal, R.S.; Sperling, L.S. Clinical significance of zero coronary artery calcium in individuals with LDL cholesterol >/=190mg/dL: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2020, 292, 224–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, N.; Erbel, R.; Mahabadi, A.A.; Rauwolf, M.; Mohlenkamp, S.; Moebus, S.; Kalsch, H.; Budde, T.; Schmermund, A.; Stang, A.; et al. Value of Progression of Coronary Artery Calcification for Risk Prediction of Coronary and Cardiovascular Events: Result of the HNR Study (Heinz Nixdorf Recall). Circulation 2018, 137, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Blaha, M.J.; Whelton, S.P.; Al Rifai, M.; Dardari, Z.; Shaw, L.J.; Al-Mallah, M.H.; Matsushita, K.; Rozanski, A.; Rumberger, J.A.; Berman, D.S.; et al. Comparing Risk Scores in the Prediction of Coronary and Cardiovascular Deaths: Coronary Artery Calcium Consortium. JACC Cardiovasc. Imaging 2021, 14, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Peri-Okonny, P.A.; Qarajeh, R.; Patel, F.S.; Sperry, B.W.; McGhie, A.I.; Thompson, R.C.; Kennedy, K.F.; Chan, P.S.; Spertus, J.A.; et al. Prognostic Relationship Between Coronary Artery Calcium Score, Perfusion Defects, and Myocardial Blood Flow Reserve in Patients With Suspected Coronary Artery Disease. Circ. Cardiovasc. Imaging 2022, 15, e012599. [Google Scholar] [CrossRef]
- Pundziute, G.; Schuijf, J.D.; Jukema, J.W.; Decramer, I.; Sarno, G.; Vanhoenacker, P.K.; Boersma, E.; Reiber, J.H.; Schalij, M.J.; Wijns, W.; et al. Evaluation of plaque characteristics in acute coronary syndromes: Non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. Eur. Heart J. 2008, 29, 2373–2381. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, M.; Tanaka, A.; Kitabata, H.; Tsujioka, H.; Kataiwa, H.; Komukai, K.; Tanimoto, T.; Takemoto, K.; Takarada, S.; Kubo, T.; et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc. Imaging 2009, 2, 1412–1419. [Google Scholar] [CrossRef]
- Ito, T.; Terashima, M.; Kaneda, H.; Nasu, K.; Matsuo, H.; Ehara, M.; Kinoshita, Y.; Kimura, M.; Tanaka, N.; Habara, M.; et al. Comparison of in vivo assessment of vulnerable plaque by 64-slice multislice computed tomography versus optical coherence tomography. Am. J. Cardiol. 2011, 107, 1270–1277. [Google Scholar] [CrossRef]
- Ito, T.; Nasu, K.; Terashima, M.; Ehara, M.; Kinoshita, Y.; Ito, T.; Kimura, M.; Tanaka, N.; Habara, M.; Tsuchikane, E.; et al. The impact of epicardial fat volume on coronary plaque vulnerability: Insight from optical coherence tomography analysis. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Wu, H.; Li, R.; Yu, L.; Zhang, J. Epicardial adipose tissue characteristics and CT high-risk plaque features: Correlation with coronary thin-cap fibroatheroma determined by intravascular ultrasound. Int. J. Cardiovasc. Imaging 2020, 36, 2281–2289. [Google Scholar] [CrossRef]
- Motoyama, S.; Kondo, T.; Sarai, M.; Sugiura, A.; Harigaya, H.; Sato, T.; Inoue, K.; Okumura, M.; Ishii, J.; Anno, H.; et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J. Am. Coll. Cardiol. 2007, 50, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Desai, M.Y.; Marwan, M.; Kotanidis, C.P.; Antonopoulos, A.S.; Schottlander, D.; Channon, K.M.; Neubauer, S.; Achenbach, S.; Antoniades, C. Perivascular Fat Attenuation Index Stratifies Cardiac Risk Associated With High-Risk Plaques in the CRISP-CT Study. J. Am. Coll. Cardiol. 2020, 76, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yu, L.; Lu, Z.; Shen, C.; Tao, X.; Zhang, J. Serial change of perivascular fat attenuation index after statin treatment: Insights from a coronary CT angiography follow-up study. Int. J. Cardiol. 2020, 319, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Nakamura, S.; Ota, H.; Ogawa, R.; Shizuka, T.; Kubo, T.; Yi, Y.; Ito, T.; Nagasawa, N.; Omori, T.; et al. Diagnostic Performance of Dynamic Myocardial Perfusion Imaging Using Dual-Source Computed Tomography. J. Am. Coll. Cardiol. 2021, 78, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- de Knegt, M.C.; Rossi, A.; Petersen, S.E.; Wragg, A.; Khurram, R.; Westwood, M.; Saberwal, B.; Mathur, A.; Nieman, K.; Bamberg, F.; et al. Stress myocardial perfusion with qualitative magnetic resonance and quantitative dynamic computed tomography: Comparison of diagnostic performance and incremental value over coronary computed tomography angiography. Eur. Heart J. Cardiovasc Imaging 2020, 22, 1452–1462. [Google Scholar] [CrossRef]
- Nous, F.M.A.; Geisler, T.; Kruk, M.B.P.; Alkadhi, H.; Kitagawa, K.; Vliegenthart, R.; Hell, M.M.; Hausleiter, J.; Nguyen, P.K.; Budde, R.P.J.; et al. Dynamic Myocardial Perfusion CT for the Detection of Hemodynamically Significant Coronary Artery Disease. JACC Cardiovasc. Imaging 2022, 15, 75–87. [Google Scholar] [CrossRef]
- Yu, L.; Lu, Z.; Dai, X.; Shen, C.; Zhang, L.; Zhang, J. Prognostic value of CT-derived myocardial blood flow, CT fractional flow reserve and high-risk plaque features for predicting major adverse cardiac events. Cardiovasc. Diagn. Ther. 2021, 11, 956–966. [Google Scholar] [CrossRef]
- Yu, M.; Shen, C.; Dai, X.; Lu, Z.; Wang, Y.; Lu, B.; Zhang, J. Clinical Outcomes of Dynamic Computed Tomography Myocardial Perfusion Imaging Combined With Coronary Computed Tomography Angiography Versus Coronary Computed Tomography Angiography-Guided Strategy. Circ. Cardiovasc. Imaging 2020, 13, e009775. [Google Scholar] [CrossRef]
- Yongguang, G.; Yibing, S.; Ping, X.; Jinyao, Z.; Yufei, F.; Yayong, H.; Yuanshun, X.; Gutao, L. Diagnostic efficacy of CCTA and CT-FFR based on risk factors for myocardial ischemia. J. Cardiothorac. Surg. 2022, 17, 39. [Google Scholar] [CrossRef]
- Chua, A.; Ihdayhid, A.R.; Linde, J.J.; Sorgaard, M.; Cameron, J.D.; Seneviratne, S.K.; Ko, B.S. Diagnostic Performance of CT-Derived Fractional Flow Reserve in Australian Patients Referred for Invasive Coronary Angiography. Heart Lung Circ. 2022, 31, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, A.T.; Meidan, T.G.; Aldrich, A.I.; Brant, N.; Squiers, J.J.; Shih, E.; Bhattal, G.; Banwait, J.K.; McCracken, J.; Kindsvater, S.; et al. Real-world validation of fractional flow reserve computed tomography in patients with stable angina: Results from the prospective AFFECTS trial. Clin. Imaging 2022, 91, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Mickley, H.; Veien, K.T.; Gerke, O.; Lambrechtsen, J.; Rohold, A.; Steffensen, F.H.; Husic, M.; Akkan, D.; Busk, M.; Jessen, L.B.; et al. Diagnostic and Clinical Value of FFRCT in Stable Chest Pain Patients With Extensive Coronary Calcification: The FACC Study. JACC Cardiovasc. Imaging 2022, 15, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Gao, Y.; Xu, B.; Yang, W.; Song, L.; Jiang, T.; Xu, L.; Hu, H.; Li, L.; Chen, W.; et al. Effect of Coronary Calcification Severity on Measurements and Diagnostic Performance of CT-FFR With Computational Fluid Dynamics: Results from CT-FFR CHINA Trial. Front. Cardiovasc. Med. 2021, 8, 810625. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, B.L.; Gaur, S.; Fairbairn, T.A.; Douglas, P.S.; Jensen, J.M.; Patel, M.R.; Ihdayhid, A.R.; Ko, B.S.H.; Sellers, S.L.; Weir-McCall, J.; et al. Prognostic value of coronary computed tomography angiographic derived fractional flow reserve: A systematic review and meta-analysis. Heart 2022, 108, 194–202. [Google Scholar] [CrossRef]
- Tang, C.X.; Qiao, H.Y.; Zhang, X.L.; Di Jiang, M.; Schoepf, U.J.; Rudzinski, P.N.; Giovagnoli, D.P.; Lu, M.J.; Li, J.H.; Wang, Y.N.; et al. Functional CAD-RADS using FFRCT on therapeutic management and prognosis in patients with coronary artery disease. Eur. Radiol. 2022, 32, 5210–5221. [Google Scholar] [CrossRef]
- Qiao, H.Y.; Tang, C.X.; Schoepf, U.J.; Bayer, R.R., 2nd; Tesche, C.; Di Jiang, M.; Yin, C.Q.; Zhou, C.S.; Zhou, F.; Lu, M.J.; et al. One-year outcomes of CCTA alone versus machine learning-based FFRCT for coronary artery disease: A single-center, prospective study. Eur. Radiol. 2022, 32, 5179–5188. [Google Scholar] [CrossRef]
- Gohmann, R.F.; Pawelka, K.; Seitz, P.; Majunke, N.; Heiser, L.; Renatus, K.; Desch, S.; Lauten, P.; Holzhey, D.; Noack, T.; et al. Combined cCTA and TAVR Planning for Ruling Out Significant CAD: Added Value of ML-Based CT-FFR. JACC Cardiovasc. Imaging 2022, 15, 476–486. [Google Scholar] [CrossRef]
- Peper, J.; Becker, L.M.; van den Berg, H.; Bor, W.L.; Brouwer, J.; Nijenhuis, V.J.; van Ginkel, D.J.; Rensing, B.; Ten Berg, J.M.; Timmers, L.; et al. Diagnostic Performance of CCTA and CT-FFR for the Detection of CAD in TAVR Work-Up. JACC Cardiovasc. Interv. 2022, 15, 1140–1149. [Google Scholar] [CrossRef]
- Brandt, V.; Schoepf, U.J.; Aquino, G.J.; Bekeredjian, R.; Varga-Szemes, A.; Emrich, T.; Bayer, R.R., 2nd; Schwarz, F.; Kroencke, T.J.; Tesche, C.; et al. Impact of machine-learning-based coronary computed tomography angiography-derived fractional flow reserve on decision-making in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Radiol. 2022, 32, 6008–6016. [Google Scholar] [CrossRef]
- Sonck, J.; Nagumo, S.; Norgaard, B.L.; Otake, H.; Ko, B.; Zhang, J.; Mizukami, T.; Maeng, M.; Andreini, D.; Takahashi, Y.; et al. Clinical Validation of a Virtual Planner for Coronary Interventions Based on Coronary CT Angiography. JACC Cardiovasc. Imaging 2022, 15, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, J.P. Cardiovascular magnetic resonance physics for clinicians: Part I. J. Cardiovasc. Magn. Reson. 2010, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Wesarg, S. Combining Short-Axis and Long-Axis Cardiac MR Images by Applying a Super-Resolution Reconstruction Algorithm; Spie Medical Imaging; SPIE: Bellingham, WA, USA, 2010; Volume 7623. [Google Scholar]
- Vijayan, S.; Barmby, D.S.; Pearson, I.R.; Davies, A.G.; Wheatcroft, S.B.; Sivananthan, M. Assessing Coronary Blood Flow Physiology in the Cardiac Catheterisation Laboratory. Curr. Cardiol. Rev. 2017, 13, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, M.S.; Bustin, A.; Hajhosseiny, R.; Yazdani, M.; Ryan, M.; Vergani, V.; Neji, R.; Kunze, K.P.; Nicol, E.; Masci, P.G.; et al. High-resolution non-contrast free-breathing coronary cardiovascular magnetic resonance angiography for detection of coronary artery disease: Validation against invasive coronary angiography. J. Cardiovasc. Magn. Reson. 2022, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Dharampal, A.; de Feyter, P.J. Coronary CT angiography for patients with suspected coronary artery disease. Heart 2014, 100, 976–984. [Google Scholar] [CrossRef]
- Dweck, M.R.; Puntman, V.; Vesey, A.T.; Fayad, Z.A.; Nagel, E. MR Imaging of Coronary Arteries and Plaques. JACC Cardiovasc. Imaging 2016, 9, 306–316. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Dzaye, O.; Steffensen, F.H.; Botker, H.E.; Jensen, J.M.; Ronnow Sand, N.P.; Kragholm, K.H.; Sorensen, H.T.; Leipsic, J.; Maeng, M.; et al. Impact of Plaque Burden Versus Stenosis on Ischemic Events in Patients With Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2020, 76, 2803–2813. [Google Scholar] [CrossRef]
- Tousoulis, D.; Siasos, G.; Maniatis, K.; Oikonomou, E.; Vlasis, K.; Papavassiliou, A.G.; Stefanadis, C. Novel biomarkers assessing the calcium deposition in coronary artery disease. Curr. Med. Chem. 2012, 19, 901–920. [Google Scholar] [CrossRef]
- Adamson, P.D.; Dweck, M.R.; Newby, D.E. The vulnerable atherosclerotic plaque: In vivo identification and potential therapeutic avenues. Heart 2015, 101, 1755–1766. [Google Scholar] [CrossRef]
- Chen, J.; Budoff, M.J.; Reilly, M.P.; Yang, W.; Rosas, S.E.; Rahman, M.; Zhang, X.; Roy, J.A.; Lustigova, E.; Nessel, L.; et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients With Chronic Kidney Disease. JAMA Cardiol. 2017, 2, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botnar, R.M.; Stuber, M.; Kissinger, K.V.; Kim, W.Y.; Spuentrup, E.; Manning, W.J. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation 2000, 102, 2582–2587. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Kawasaki, T.; Tanaka, A.; Yasuda, S.; Goto, Y.; Ishihara, M.; Nishimura, K.; Miyamoto, Y.; Node, K.; Koga, N. High-intensity signals in coronary plaques on noncontrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. J. Am. Coll. Cardiol. 2014, 63, 989–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moody, A.R.; Murphy, R.E.; Morgan, P.S.; Martel, A.L.; Delay, G.S.; Allder, S.; MacSweeney, S.T.; Tennant, W.G.; Gladman, J.; Lowe, J.; et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 2003, 107, 3047–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, T.; Koga, S.; Koga, N.; Noguchi, T.; Tanaka, H.; Koga, H.; Serikawa, T.; Orita, Y.; Ikeda, S.; Mito, T.; et al. Characterization of hyperintense plaque with noncontrast T(1)-weighted cardiac magnetic resonance coronary plaque imaging: Comparison with multislice computed tomography and intravascular ultrasound. JACC Cardiovasc. Imaging 2009, 2, 720–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, T.; Makowski, M.R.; Jankauskas, A.; Maintz, D.; Karch, M.; Schachoff, S.; Manning, W.J.; Schomig, A.; Schwaiger, M.; Botnar, R.M. Serial contrast-enhanced cardiac magnetic resonance imaging demonstrates regression of hyperenhancement within the coronary artery wall in patients after acute myocardial infarction. JACC Cardiovasc. Imaging 2009, 2, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Ginami, G.; Neji, R.; Rashid, I.; Chiribiri, A.; Ismail, T.F.; Botnar, R.M.; Prieto, C. 3D whole-heart phase sensitive inversion recovery CMR for simultaneous black-blood late gadolinium enhancement and bright-blood coronary CMR angiography. J. Cardiovasc. Magn. Reson. 2017, 19, 94. [Google Scholar] [CrossRef] [Green Version]
- Ginami, G.; Neji, R.; Phinikaridou, A.; Whitaker, J.; Botnar, R.M.; Prieto, C. Simultaneous bright- and black-blood whole-heart MRI for noncontrast enhanced coronary lumen and thrombus visualization. Magn. Reson. Med. 2018, 79, 1460–1472. [Google Scholar] [CrossRef] [Green Version]
- Demir, H.; Tan, Y.Z.; Kozdag, G.; Isgoren, S.; Anik, Y.; Ural, D.; Demirci, A.; Berk, F. Comparison of gated SPECT, echocardiography and cardiac magnetic resonance imaging for the assessment of left ventricular ejection fraction and volumes. Ann. Saudi Med. 2007, 27, 415–420. [Google Scholar] [CrossRef]
- Chitiboi, T.; Axel, L. Magnetic resonance imaging of myocardial strain: A review of current approaches. J. Magn. Reson. Imaging 2017, 46, 1263–1280. [Google Scholar] [CrossRef]
- Pezel, T.; Hovasse, T.; Lefevre, T.; Sanguineti, F.; Unterseeh, T.; Champagne, S.; Benamer, H.; Neylon, A.; Toupin, S.; Garot, P.; et al. Prognostic Value of Stress CMR in Symptomatic Patients with Coronary Stenosis on CCTA. JACC Cardiovasc. Imaging 2022, 15, 1408–1422. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M. The impacts on healthcare when coronary angiography as the reference method for diagnostic accuracy of coronary artery disease is replaced by fractional flow reserve! Eur. Heart J. 2017, 38, 999–1001. [Google Scholar] [PubMed]
- Gunning, M.G.; Anagnostopoulos, C.; Knight, C.J.; Pepper, J.; Burman, E.D.; Davies, G.; Fox, K.M.; Pennell, D.J.; Ell, P.J.; Underwood, S.R. Comparison of 201Tl, 99mTc-tetrofosmin, and dobutamine magnetic resonance imaging for identifying hibernating myocardium. Circulation 1998, 98, 1869–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Pontone, G.; Schwitter, J.; Francone, M.; Iglesias, J.F.; Barison, A.; Zalewski, J.; de Luca, L.; Degrauwe, S.; Claus, P.; et al. Long-Term Incremental Prognostic Value of Cardiovascular Magnetic Resonance after ST-Segment Elevation Myocardial Infarction: A Study of the Collaborative Registry on CMR in STEMI. JACC Cardiovasc. Imaging 2018, 11, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.F.; Pellikka, P.A.; Ryan, T.; Crouse, L.; Zoghbi, W.A. Stress echocardiography: Recommendations for performance and interpretation of stress echocardiography. Stress Echocardiography Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 1998, 11, 97–104. [Google Scholar] [CrossRef]
- Fleischmann, K.E.; Hunink, M.G.; Kuntz, K.M.; Douglas, P.S. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. JAMA 1998, 280, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.; Krishnamani, R.; Senior, R. Prognostic value of normal stress echocardiogram in patients with suspected coronary artery disease—A British general hospital experience. Int. J. Cardiol. 2004, 94, 181–186. [Google Scholar] [CrossRef]
- Sicari, R.; Pasanisi, E.; Venneri, L.; Landi, P.; Cortigiani, L.; Picano, E.; Group Echo Persantine International Cooperative Study. Stress echo results predict mortality: A large-scale multicenter prospective international study. J. Am. Coll. Cardiol. 2003, 41, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Marwick, T.H.; Case, C.; Sawada, S.; Rimmerman, C.; Brenneman, P.; Kovacs, R.; Short, L.; Lauer, M. Prediction of mortality using dobutamine echocardiography. J. Am. Coll. Cardiol. 2001, 37, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, W.F.; Zoghbi, W.A. Stress echocardiography: Current methodology and clinical applications. J. Am. Coll. Cardiol. 2005, 45, 1739–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supariwala, A.; Makani, H.; Kahan, J.; Pierce, M.; Bajwa, F.; Dukkipati, S.S.; Teixeira, J.; Chaudhry, F.A. Feasibility and prognostic value of stress echocardiography in obese, morbidly obese, and super obese patients referred for bariatric surgery. Echocardiography 2014, 31, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Edgar, L.T.; Sibole, S.C.; Underwood, C.J.; Guilkey, J.E.; Weiss, J.A. A computational model of in vitro angiogenesis based on extracellular matrix fibre orientation. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 790–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelmoneim, S.S.; Bernier, M.; Scott, C.G.; Dhoble, A.; Ness, S.A.; Hagen, M.E.; Moir, S.; McCully, R.B.; Pellikka, P.A.; Mulvagh, S.L. Safety of contrast agent use during stress echocardiography in patients with elevated right ventricular systolic pressure: A cohort study. Circ. Cardiovasc. Imaging 2010, 3, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Plana, J.C.; Mikati, I.A.; Dokainish, H.; Lakkis, N.; Abukhalil, J.; Davis, R.; Hetzell, B.C.; Zoghbi, W.A. A randomized cross-over study for evaluation of the effect of image optimization with contrast on the diagnostic accuracy of dobutamine echocardiography in coronary artery disease The OPTIMIZE Trial. JACC Cardiovasc. Imaging 2008, 1, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Dolan, M.S.; Riad, K.; El-Shafei, A.; Puri, S.; Tamirisa, K.; Bierig, M.; St Vrain, J.; McKinney, L.; Havens, E.; Habermehl, K.; et al. Effect of intravenous contrast for left ventricular opacification and border definition on sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography in technically difficult patients. Am. Heart J. 2001, 142, 908–915. [Google Scholar] [CrossRef]
- Vlassak, I.; Rubin, D.N.; Odabashian, J.A.; Garcia, M.J.; King, L.M.; Lin, S.S.; Drinko, J.K.; Morehead, A.J.; Prior, D.L.; Asher, C.R.; et al. Contrast and harmonic imaging improves accuracy and efficiency of novice readers for dobutamine stress echocardiography. Echocardiography 2002, 19, 483–488. [Google Scholar] [CrossRef]
- Huang, W.C.; Chiou, K.R.; Liu, C.P.; Lin, S.L.; Lee, D.; Mar, G.Y.; Hsiao, S.H.; Kung, M.H.; Chiou, C.W.; Lin, T.W. Comparison of real-time contrast echocardiography and low-dose dobutamine stress echocardiography in predicting the left ventricular functional recovery in patients after acute myocardial infarction under different therapeutic intervention. Int. J. Cardiol. 2005, 104, 81–91. [Google Scholar] [CrossRef]
- Porter, T.R.; Smith, L.M.; Wu, J.; Thomas, D.; Haas, J.T.; Mathers, D.H.; Williams, E.; Olson, J.; Nalty, K.; Hess, R.; et al. Patient outcome following 2 different stress imaging approaches: A prospective randomized comparison. J. Am. Coll. Cardiol. 2013, 61, 2446–2455. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Xie, T.; McCulloch, M.; Abreo, G.; Runge, M. Real-time three-dimensional dobutamine stress echocardiography in assessment stress echocardiography in assessment of ischemia: Comparison with two-dimensional dobutamine stress echocardiography. J. Am. Coll. Cardiol. 2001, 37, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Tsang, W.; Adams, D.H.; Agricola, E.; Buck, T.; Faletra, F.F.; Franke, A.; Hung, J.; de Isla, L.P.; et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 3–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemes, A.; Leung, K.Y.; van Burken, G.; van Stralen, M.; Bosch, J.G.; Soliman, O.I.; Krenning, B.J.; Vletter, W.B.; ten Cate, F.J.; Geleijnse, M.L. Side-by-side viewing of anatomically aligned left ventricular segments in three-dimensional stress echocardiography. Echocardiography 2009, 26, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Yoshitani, H.; Takeuchi, M.; Mor-Avi, V.; Otsuji, Y.; Hozumi, T.; Yoshiyama, M. Comparative diagnostic accuracy of multiplane and multislice three-dimensional dobutamine stress echocardiography in the diagnosis of coronary artery disease. J. Am. Soc. Echocardiogr. 2009, 22, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, C.; Giannopoulos, G.; Misovoulos, P.; Roussakis, G.; Christoforatou, E.; Kokkinakis, C.; Brili, S.; Stefanadis, C. Real-time three-dimensional dobutamine stress echocardiography for coronary artery disease diagnosis: Validation with coronary angiography. Heart 2007, 93, 672–675. [Google Scholar] [CrossRef]
- Berbarie, R.F.; Dib, E.; Ahmad, M. Stress echocardiography using real-time three-dimensional imaging. Echocardiography 2018, 35, 1196–1203. [Google Scholar] [CrossRef]
- Yu, A.F.; Raikhelkar, J.; Zabor, E.C.; Tonorezos, E.S.; Moskowitz, C.S.; Adsuar, R.; Mara, E.; Huie, K.; Oeffinger, K.C.; Steingart, R.M.; et al. Two-Dimensional Speckle Tracking Echocardiography Detects Subclinical Left Ventricular Systolic Dysfunction among Adult Survivors of Childhood, Adolescent, and Young Adult Cancer. BioMed Res. Int. 2016, 2016, 9363951. [Google Scholar] [CrossRef] [Green Version]
- Pellikka, P.A.; Arruda-Olson, A.; Chaudhry, F.A.; Chen, M.H.; Marshall, J.E.; Porter, T.R.; Sawada, S.G. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 1–41.e8. [Google Scholar] [CrossRef] [Green Version]
- Pastore, M.C.; Mandoli, G.E.; Aboumarie, H.S.; Santoro, C.; Bandera, F.; D’Andrea, A.; Benfari, G.; Esposito, R.; Evola, V.; Sorrentino, R.; et al. Basic and advanced echocardiography in advanced heart failure: An overview. Heart Fail Rev. 2020, 25, 937–948. [Google Scholar] [CrossRef]
- Rumbinaite, E.; Zaliaduonyte-Peksiene, D.; Viezelis, M.; Ceponiene, I.; Lapinskas, T.; Zvirblyte, R.; Vencloviene, J.; Morkunaite, K.; Bielinis, A.; Slapikas, R.; et al. Dobutamine-stress echocardiography speckle-tracking imaging in the assessment of hemodynamic significance of coronary artery stenosis in patients with moderate and high probability of coronary artery disease. Medicina 2016, 52, 331–339. [Google Scholar] [CrossRef]
- Uusitalo, V.; Luotolahti, M.; Pietila, M.; Wendelin-Saarenhovi, M.; Hartiala, J.; Saraste, M.; Knuuti, J.; Saraste, A. Two-Dimensional Speckle-Tracking during Dobutamine Stress Echocardiography in the Detection of Myocardial Ischemia in Patients with Suspected Coronary Artery Disease. J. Am. Soc. Echocardiogr. 2016, 29, 470–479.e473. [Google Scholar] [CrossRef]
- Sicari, R.; Nihoyannopoulos, P.; Evangelista, A.; Kasprzak, J.; Lancellotti, P.; Poldermans, D.; Voigt, J.U.; Zamorano, J.L.; European Association of Echocardiography. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur. J. Echocardiogr. 2008, 9, 415–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Lodato, J.A.; Furlong, K.T.; Lang, R.M.; Yoshikawa, J. Feasibility of measuring coronary flow velocity and reserve in the left anterior descending coronary artery by transthoracic Doppler echocardiography in a relatively obese American population. Echocardiography 2005, 22, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.H.; Pedersen, L.R.; Snoer, M.; Christensen, T.E.; Ghotbi, A.A.; Hasbak, P.; Kjaer, A.; Haugaard, S.B.; Prescott, E. Coronary flow velocity reserve by echocardiography: Feasibility, reproducibility and agreement with PET in overweight and obese patients with stable and revascularized coronary artery disease. Cardiovasc. Ultrasound 2016, 14, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, E.H.; Rousse, M.G.; Lowenstein, J.A. Target heart rate to determine the normal value of coronary flow reserve during dobutamine stress echocardiography. Cardiovasc. Ultrasound 2011, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Haeck, J.D.E.; Van’t Veer, M.; Zimmermann, F.M.; Neumann, F.J.; Triantafyllis, A.S.; Sjauw, K.D.; Abdel-Wahab, M.; Omerovic, E.; Boxma-de Klerk, B.M.; Pijls, N.H.J.; et al. Percutaneous Coronary Intervention vs Medical Therapy for Coronary Lesions With Positive Fractional Flow Reserve (FFR) but Preserved Pressure-Bounded Coronary Flow Reserve (CFR): A Substudy of the Randomized Compare-Acute Trial. J. Invasive Cardiol. 2021, 33, E557–E564. [Google Scholar]
- Cain, P.; Khoury, V.; Short, L.; Marwick, T.H. Usefulness of quantitative echocardiographic techniques to predict recovery of regional and global left ventricular function after acute myocardial infarction. Am. J. Cardiol. 2003, 91, 391–396. [Google Scholar] [CrossRef]
- Moller, J.E.; Hillis, G.S.; Oh, J.K.; Reeder, G.S.; Gersh, B.J.; Pellikka, P.A. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am. Heart J. 2006, 151, 419–425. [Google Scholar] [CrossRef]
- Sawada, S.G.; Dasgupta, S.; Nguyen, J.; Lane, K.A.; Gradus-Pizlo, I.; Mahenthiran, J.; Feigenbaum, H. Effect of revascularization on long-term survival in patients with ischemic left ventricular dysfunction and a wide range of viability. Am. J. Cardiol. 2010, 106, 187–192. [Google Scholar] [CrossRef]
- Ma, L.; Chen, L.; Gillam, L.; Waters, D.D.; Chen, C. Nitroglycerin enhances the ability of dobutamine stress echocardiography to detect hibernating myocardium. Circulation 1997, 96, 3992–4001. [Google Scholar] [CrossRef]
- Ling, L.H.; Christian, T.F.; Mulvagh, S.L.; Klarich, K.W.; Hauser, M.F.; Nishimura, R.A.; Pellikka, P.A. Determining myocardial viability in chronic ischemic left ventricular dysfunction: A prospective comparison of rest-redistribution thallium 201 single-photon emission computed tomography, nitroglycerin-dobutamine echocardiography, and intracoronary myocardial contrast echocardiography. Am. Heart J. 2006, 151, 882–889. [Google Scholar] [PubMed]
- Kloosterman, M.; Damman, K.; Van Veldhuisen, D.J.; Rienstra, M.; Maass, A.H. The importance of myocardial contractile reserve in predicting response to cardiac resynchronization therapy. Eur. J. Heart Fail 2017, 19, 862–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saam, T.; Rominger, A.; Wolpers, S.; Nikolaou, K.; Rist, C.; Greif, M.; Cumming, P.; Becker, A.; Foerster, S.; Reiser, M.F.; et al. Association of inflammation of the left anterior descending coronary artery with cardiovascular risk factors, plaque burden and pericardial fat volume: A PET/CT study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, F.; Cornily, J.C.; Rudd, J.H.; Machac, J.; Feldman, L.J.; Fayad, Z.A. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: A comparison with 18F-FDG PET/CT and histology. J. Nucl. Med. 2009, 50, 959–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wykrzykowska, J.; Lehman, S.; Williams, G.; Parker, J.A.; Palmer, M.R.; Varkey, S.; Kolodny, G.; Laham, R. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J. Nucl. Med. 2009, 50, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Demeure, F.; Hanin, F.X.; Bol, A.; Vincent, M.F.; Pouleur, A.C.; Gerber, B.; Pasquet, A.; Jamar, F.; Vanoverschelde, J.L.; Vancraeynest, D. A randomized trial on the optimization of 18F-FDG myocardial uptake suppression: Implications for vulnerable coronary plaque imaging. J. Nucl. Med. 2014, 55, 1629–1635. [Google Scholar] [CrossRef] [Green Version]
- Dilsizian, V.; Bacharach, S.L.; Beanlands, R.S.; Bergmann, S.R.; Delbeke, D.; Dorbala, S.; Gropler, R.J.; Knuuti, J.; Schelbert, H.R.; Travin, M.I. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J. Nucl. Cardiol. 2016, 23, 1187–1226. [Google Scholar] [CrossRef] [Green Version]
- Rubeaux, M.; Joshi, N.V.; Dweck, M.R.; Fletcher, A.; Motwani, M.; Thomson, L.E.; Germano, G.; Dey, D.; Li, D.; Berman, D.S.; et al. Motion Correction of 18F-NaF PET for Imaging Coronary Atherosclerotic Plaques. J. Nucl. Med. 2016, 57, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Dweck, M.R.; Chow, M.W.; Joshi, N.V.; Williams, M.C.; Jones, C.; Fletcher, A.M.; Richardson, H.; White, A.; McKillop, G.; van Beek, E.J.; et al. Coronary arterial 18F-sodium fluoride uptake: A novel marker of plaque biology. J. Am. Coll. Cardiol. 2012, 59, 1539–1548. [Google Scholar] [CrossRef] [Green Version]
- Moss, A.J.; Doris, M.K.; Andrews, J.P.M.; Bing, R.; Daghem, M.; van Beek, E.J.R.; Forsyth, L.; Shah, A.S.V.; Williams, M.C.; Sellers, S.; et al. Molecular Coronary Plaque Imaging Using (18)F-Fluoride. Circ. Cardiovasc. Imaging 2019, 12, e008574. [Google Scholar] [CrossRef] [Green Version]
- Kwiecinski, J.; Cadet, S.; Daghem, M.; Lassen, M.L.; Dey, D.; Dweck, M.R.; Berman, D.S.; Newby, D.E.; Slomka, P.J. Whole-vessel coronary (18)F-sodium fluoride PET for assessment of the global coronary microcalcification burden. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, A.; Alavi, A.; Thamake, S.; Amerinia, R.; Ranganathan, D.; Tworowska, I.; Delpassand, E.S. Assessment of vulnerable atherosclerotic and fibrotic plaques in coronary arteries using (68)Ga-DOTATATE PET/CT. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 65–71. [Google Scholar] [PubMed]
- Weiberg, D.; Thackeray, J.T.; Daum, G.; Sohns, J.M.; Kropf, S.; Wester, H.J.; Ross, T.L.; Bengel, F.M.; Derlin, T. Clinical Molecular Imaging of Chemokine Receptor CXCR4 Expression in Atherosclerotic Plaque Using (68)Ga-Pentixafor PET: Correlation with Cardiovascular Risk Factors and Calcified Plaque Burden. J. Nucl. Med. 2018, 59, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Tzolos, E.; Bing, R.; Andrews, J.; Macaskill, M.; Tavares, A.; MacNaught, G.; Clarke, T.; Williams, M.C.; Van Beek, E.J.R.; Koglin, N.; et al. In vivo coronary artery thrombus imaging with 18F-GP1 PET-CT. Eur. Heart J. 2021, 42 (Suppl. 1), ehab724.0261. [Google Scholar] [CrossRef]
- Buchler, A.; Munch, M.; Farber, G.; Zhao, X.; Al-Haddad, R.; Farber, E.; Rotstein, B.H. Selective Imaging of Matrix Metalloproteinase-13 to Detect Extracellular Matrix Remodeling in Atherosclerotic Lesions. Mol. Imaging Biol. 2022, 24, 93–103. [Google Scholar] [CrossRef]
- Bala, G.; Blykers, A.; Xavier, C.; Descamps, B.; Broisat, A.; Ghezzi, C.; Fagret, D.; Van Camp, G.; Caveliers, V.; Vanhove, C.; et al. Targeting of vascular cell adhesion molecule-1 by 18F-labelled nanobodies for PET/CT imaging of inflamed atherosclerotic plaques. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1001–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurster, T.H.; Landmesser, U.; Abdelwahed, Y.S.; Skurk, C.; Morguet, A.; Leistner, D.M.; Frohlich, G.; Haghikia, A.; Engel, L.C.; Schuster, A.; et al. Simultaneous [18F]fluoride and gadobutrol enhanced coronary positron emission tomography/magnetic resonance imaging for in vivo plaque characterization. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1391–1398. [Google Scholar] [CrossRef]
- Thomas, M.; Sperry, B.W.; Peri-Okonny, P.; Malik, A.O.; McGhie, A.I.; Saeed, I.M.; Chan, P.S.; Spertus, J.A.; Thompson, R.C.; Bateman, T.M.; et al. Relative Prognostic Significance of Positron Emission Tomography Myocardial Perfusion Imaging Markers in Cardiomyopathy. Circ. Cardiovasc. Imaging 2021, 14, e012426. [Google Scholar] [CrossRef]
- Patel, K.K.; Shaw, L.; Spertus, J.A.; Sperry, B.; McGhie, A.I.; Kennedy, K.; Thompson, R.C.; Chan, P.S.; Bateman, T.M. Association of Sex, Reduced Myocardial Flow Reserve, and Long-Term Mortality Across Spectrum of Atherosclerotic Disease. JACC Cardiovasc. Imaging 2022, 15, 1635–1644. [Google Scholar] [CrossRef]
- Al-Mallah, M.H.; Sitek, A.; Moore, S.C.; Di Carli, M.; Dorbala, S. Assessment of myocardial perfusion and function with PET and PET/CT. J. Nucl. Cardiol. 2010, 17, 498–513. [Google Scholar] [CrossRef] [Green Version]
- Mc Ardle, B.A.; Dowsley, T.F.; deKemp, R.A.; Wells, G.A.; Beanlands, R.S. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease? A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2012, 60, 1828–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Cai, F.; Geng, C.; Wang, Z.; Tang, X. Diagnostic Performance of CMR, SPECT, and PET Imaging for the Identification of Coronary Artery Disease: A Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 621389. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Al Badarin, F.; Chan, P.S.; Spertus, J.A.; Courter, S.; Kennedy, K.F.; Case, J.A.; McGhie, A.I.; Heller, G.V.; Bateman, T.M. Randomized Comparison of Clinical Effectiveness of Pharmacologic SPECT and PET MPI in Symptomatic CAD Patients. JACC Cardiovasc. Imaging 2019, 12, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Spertus, J.A.; Chan, P.S.; Sperry, B.W.; Thompson, R.C.; Al Badarin, F.; Kennedy, K.F.; Case, J.A.; Courter, S.; Saeed, I.M.; et al. Extent of Myocardial Ischemia on Positron Emission Tomography and Survival Benefit With Early Revascularization. J. Am. Coll. Cardiol. 2019, 74, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Nudi, F.; Iskandrian, A.E.; Schillaci, O.; Peruzzi, M.; Frati, G.; Biondi-Zoccai, G. Diagnostic Accuracy of Myocardial Perfusion Imaging With CZT Technology: Systemic Review and Meta-Analysis of Comparison With Invasive Coronary Angiography. JACC Cardiovasc. Imaging 2017, 10, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Tamarappoo, B.K.; Dey, D.; Nakazato, R.; Shmilovich, H.; Smith, T.; Cheng, V.Y.; Thomson, L.E.; Hayes, S.W.; Friedman, J.D.; Germano, G.; et al. Comparison of the extent and severity of myocardial perfusion defects measured by CT coronary angiography and SPECT myocardial perfusion imaging. JACC Cardiovasc. Imaging 2010, 3, 1010–1019. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.I.; Han, Y.; Al Rifai, M.; Alnabelsi, T.; Nabi, F.; Chang, S.M.; Cocker, M.; Schwemmer, C.; Ramirez-Giraldo, J.C.; Kleiman, N.S.; et al. Prognostic Value of Computed Tomography-Derived Fractional Flow Reserve Comparison With Myocardial Perfusion Imaging. JACC Cardiovasc. Imaging 2022, 15, 284–295. [Google Scholar] [CrossRef]
- Kuronuma, K.; Miller, R.J.H.; Otaki, Y.; Van Kriekinge, S.D.; Diniz, M.A.; Sharir, T.; Hu, L.H.; Gransar, H.; Liang, J.X.; Parekh, T.; et al. Prognostic Value of Phase Analysis for Predicting Adverse Cardiac Events Beyond Conventional Single-Photon Emission Computed Tomography Variables: Results From the REFINE SPECT Registry. Circ. Cardiovasc. Imaging 2021, 14, e012386. [Google Scholar] [CrossRef]
- Gimelli, A.; Pugliese, N.R.; Buechel, R.R.; Coceani, M.; Clemente, A.; Kaufmann, P.A.; Marzullo, P. Myocardial perfusion scintigraphy for risk stratification of patients with coronary artery disease: The AMICO registry. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 372–380. [Google Scholar] [CrossRef]
- Kuronuma, K.; Han, D.; Miller, R.J.H.; Rozanski, A.; Gransar, H.; Dey, D.; Hayes, S.W.; Friedman, J.D.; Thomson, L.; Slomka, P.J.; et al. Long-term Survival Benefit From Revascularization Compared With Medical Therapy in Patients With or Without Diabetes Undergoing Myocardial Perfusion Single Photon Emission Computed Tomography. Diabetes Care 2022. [Google Scholar] [CrossRef]
- Caobelli, F.; Haaf, P.; Haenny, G.; Pfisterer, M.; Zellweger, M.J.; Investigators, B. Prognostic value of myocardial perfusion scintigraphy in asymptomatic patients with diabetes mellitus at high cardiovascular risk: 5-year follow-up of the prospective multicenter BARDOT trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3512–3521. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.A. A clinically relevant classification of chest discomfort. J. Am. Coll. Cardiol. 1983, 1, 574–575. [Google Scholar] [CrossRef]
- Genders, T.S.; Steyerberg, E.W.; Hunink, M.G.; Nieman, K.; Galema, T.W.; Mollet, N.R.; de Feyter, P.J.; Krestin, G.P.; Alkadhi, H.; Leschka, S.; et al. Prediction model to estimate presence of coronary artery disease: Retrospective pooled analysis of existing cohorts. BMJ 2012, 344, e3485. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Orozco, L.E.; Saraste, A.; Capodanno, D.; Prescott, E.; Ballo, H.; Bax, J.J.; Wijns, W.; Knuuti, J. Impact of a decreasing pre-test probability on the performance of diagnostic tests for coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Gueret, P.; Deux, J.F.; Bonello, L.; Sarran, A.; Tron, C.; Christiaens, L.; Dacher, J.N.; Bertrand, D.; Leborgne, L.; Renard, C.; et al. Diagnostic performance of computed tomography coronary angiography (from the Prospective National Multicenter Multivendor EVASCAN Study). Am. J. Cardiol. 2013, 111, 471–478. [Google Scholar] [CrossRef]
- Siontis, G.C.; Mavridis, D.; Greenwood, J.P.; Coles, B.; Nikolakopoulou, A.; Juni, P.; Salanti, G.; Windecker, S. Outcomes of non-invasive diagnostic modalities for the detection of coronary artery disease: Network meta-analysis of diagnostic randomised controlled trials. BMJ 2018, 360, k504. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, G.; Guzic Salobir, B.; Jug, B.; Devasenapathy, N.; Alexanderson, E.; Vitola, J.; Kraft, O.; Ozkan, E.; Sharma, S.; Purohit, G.; et al. Functional compared to anatomical imaging in the initial evaluation of patients with suspected coronary artery disease: An international, multi-center, randomized controlled trial (IAEA-SPECT/CTA study). J. Nucl. Cardiol. 2017, 24, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, J.P.; Ripley, D.P.; Berry, C.; McCann, G.P.; Plein, S.; Bucciarelli-Ducci, C.; Dall’Armellina, E.; Prasad, A.; Bijsterveld, P.; Foley, J.R.; et al. Effect of Care Guided by Cardiovascular Magnetic Resonance, Myocardial Perfusion Scintigraphy, or NICE Guidelines on Subsequent Unnecessary Angiography Rates: The CE-MARC 2 Randomized Clinical Trial. JAMA 2016, 316, 1051–1060. [Google Scholar] [CrossRef] [Green Version]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Juni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef]
- Oikonomou, E.; Lampsas, S.; Theofilis, P.; Souvaliotis, N.; Papamikroulis, G.A.; Katsarou, O.; Kalogeras, K.; Pantelidis, P.; Papaioannou, T.G.; Tsatsaragkou, A.; et al. Impaired left ventricular deformation and ventricular-arterial coupling in post-COVID-19: Association with autonomic dysregulation. Heart Vessel. 2022. [Google Scholar] [CrossRef]

| Imaging Modality | Anatomical Information | Functional Evaluation | Other |
|---|---|---|---|
| CT | + | + | |
| CCTA | + Lumen area stenosis Morphological information on plaque synthesis Characterization of vulnerable plaques | − | Perivascular FAI (surrogate of inflammation) Coronary artery calcium (morphological evaluation) |
| FFRCT | − | + FFRCT ≤ 0.8 is associated with plaque features and at least moderate luminal stenosis Superior to CCTA for the detection of ischemia FFRCT > 0.8 has a high negative predictive value | |
| CTP | − | + Static: qualitative evaluation of MP Dynamic: quantitative evaluation of MP Incremental hemodynamic information compared to plain CCTA, especially in multivessel disease CTP-derived MBF flow may be predictive of adverse outcomes | |
| cMRI | + Morphological information on plaque synthesis (technically demanding) | + | |
| Free-breathing coronary cMRI | + Lumen area stenosis (moderate resolution lower than CCTA) | − | |
| Stress cMRI | − | + Excellent sensitivity and specificity for the detection of ischemia | |
| LGE cMRI | − | − | Viability assessment |
| Echocardiography | − | + | |
| Stress Echocardiography | − | + Hypokinesis detection (usually with dobutamine) perfusion evaluation (with contrast agent) coronary flow reserve (usually with adenosine) | Viability assessment |
| cPET | − | - | Inflammation (with 18-Fluorodeoxyglucose) Calcifications (with NaF) Other atherosclerotic elements (according to tracer) |
| cPET-MP | − | Qualitative: visualization of perfusion defects Quantitative: calculation of myocardial flow reserve Especially important in multivessel disease and moderate stenosis of undetermined significance Superior accuracy to SPECT | |
| Cardiac SPECT | Qualitative and quantitative evaluation of MP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oikonomou, E.; Theofilis, P.; Lampsas, S.; Katsarou, O.; Kalogeras, K.; Marinos, G.; Tsatsaragkou, A.; Anastasiou, A.; Lysandrou, A.; Gounaridi, M.-I.; et al. Current Concepts and Future Applications of Non-Invasive Functional and Anatomical Evaluation of Coronary Artery Disease. Life 2022, 12, 1803. https://doi.org/10.3390/life12111803
Oikonomou E, Theofilis P, Lampsas S, Katsarou O, Kalogeras K, Marinos G, Tsatsaragkou A, Anastasiou A, Lysandrou A, Gounaridi M-I, et al. Current Concepts and Future Applications of Non-Invasive Functional and Anatomical Evaluation of Coronary Artery Disease. Life. 2022; 12(11):1803. https://doi.org/10.3390/life12111803
Chicago/Turabian StyleOikonomou, Evangelos, Panagiotis Theofilis, Stamatios Lampsas, Ourania Katsarou, Konstantinos Kalogeras, Georgios Marinos, Aikaterini Tsatsaragkou, Artemis Anastasiou, Antonios Lysandrou, Maria-Ioanna Gounaridi, and et al. 2022. "Current Concepts and Future Applications of Non-Invasive Functional and Anatomical Evaluation of Coronary Artery Disease" Life 12, no. 11: 1803. https://doi.org/10.3390/life12111803
APA StyleOikonomou, E., Theofilis, P., Lampsas, S., Katsarou, O., Kalogeras, K., Marinos, G., Tsatsaragkou, A., Anastasiou, A., Lysandrou, A., Gounaridi, M.-I., Gialamas, I., Vavuranakis, M.-A., Tousoulis, D., Vavuranakis, M., & Siasos, G. (2022). Current Concepts and Future Applications of Non-Invasive Functional and Anatomical Evaluation of Coronary Artery Disease. Life, 12(11), 1803. https://doi.org/10.3390/life12111803








