Diagnostic Performance of On-Site Computed Tomography Derived Fractional Flow Reserve on Non-Culprit Coronary Lesions in Patients with Acute Coronary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Coronary Computed Tomography Angiography
2.3. CCTA Segmentation and CT-FFR Simulation
2.4. Invasive Fractional Flow Reserve
2.5. Dobutamine Stress Echocardiography
2.6. Statistical Analysis
3. Results
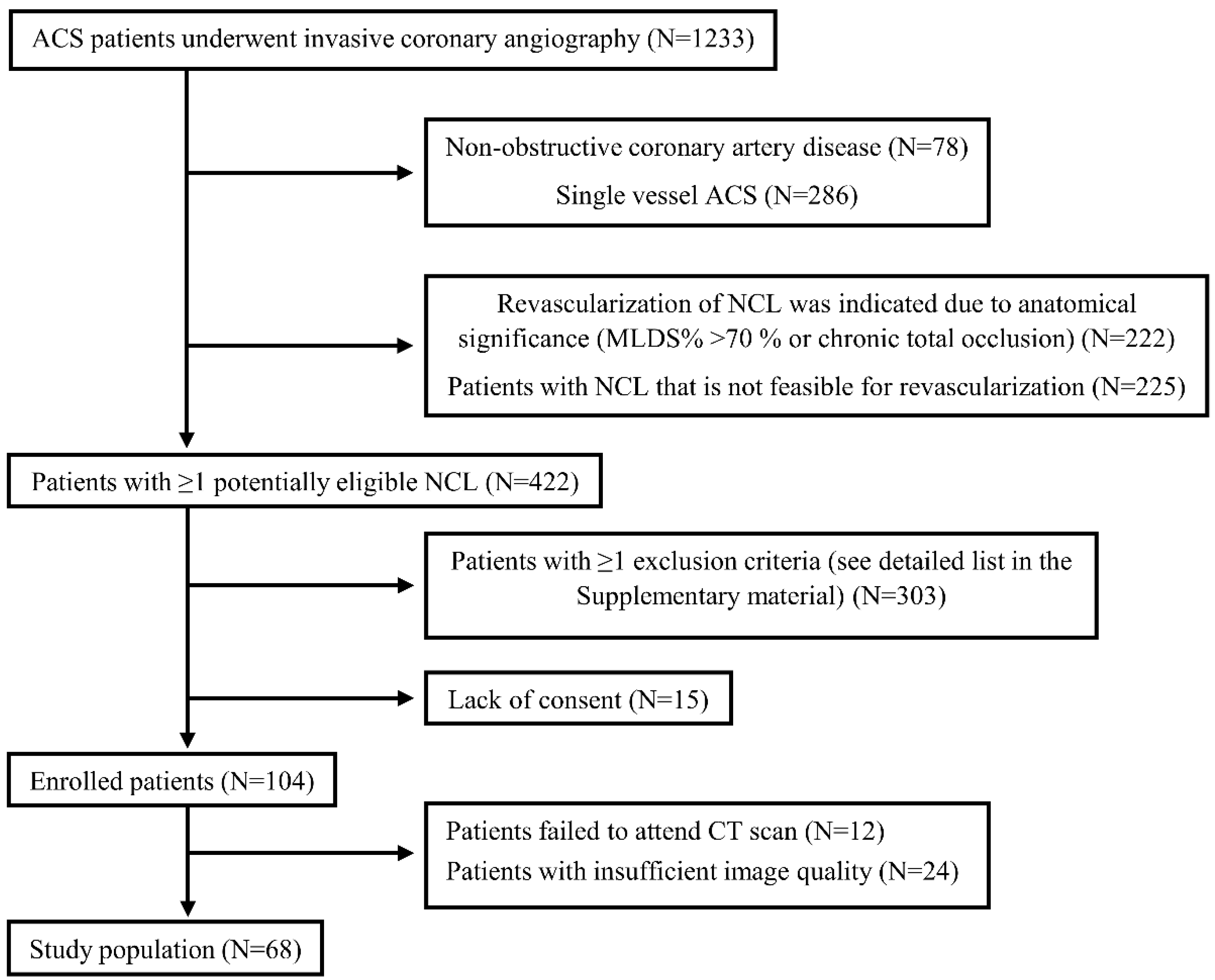
3.1. Study Population
3.2. FFRi and DSE
3.3. CT-FFR versus FFRi
3.4. CT-FFR versus DSE
4. Discussion
4.1. CT-FFR versus Invasive FFR
4.2. CT-FFR versus DSE
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, D.-W.; Clare, R.M.; Schulte, P.J.; Pieper, K.S.; Shaw, L.K.; Califf, R.M.; Ohman, E.M.; van de Werf, F.; Hirji, S.; Harrington, R.A.; et al. Extent, Location, and Clinical Significance of Non–Infarct-Related Coronary Artery Disease among Patients with ST-Elevation Myocardial Infarction. JAMA 2014, 312, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.S.; Morris, J.K.; Wald, N.J.; Chase, A.J.; Edwards, R.J.; Hughes, L.O.; Berry, C.; Oldroyd, K.G. Randomized Trial of Preventive Angioplasty in Myocardial Infarction. N. Engl. J. Med. 2013, 369, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Wood, D.A.; Storey, R.F.; Mehran, R.; Bainey, K.R.; Nguyen, H.; Meeks, B.; di Pasquale, G.; López-Sendón, J.; Faxon, D.P.; et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N. Engl. J. Med. 2019, 381, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Gershlick, A.H.; Khan, J.N.; Kelly, D.J.; Greenwood, J.P.; Sasikaran, T.; Curzen, N.; Blackman, D.J.; Dalby, M.; Fairbrother, K.L.; Banya, W.; et al. Randomized Trial of Complete versus Lesion-Only Revascularization in Patients Undergoing Primary Percutaneous Coronary Intervention for Stemi and Multivessel Disease: The CvLPRIT Trial. J. Am. Coll. Cardiol. 2015, 65, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Bauersachs, J.; Dendale, P.; Edvardsen, T.; Gale, C.P.; Jobs, A.; Lambrinou, E.; Mehilli, J.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Thim, T.; van der Hoeven, N.W.; Musto, C.; Nijveldt, R.; Götberg, M.; Engstrøm, T.; Smits, P.C.; Oldroyd, K.G.; Gershlick, A.H.; Escaned, J.; et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc. Interv. 2020, 13, 1145–1154. [Google Scholar] [CrossRef]
- Layland, J.; Oldroyd, K.G.; Curzen, N.; Sood, A.; Balachandran, K.; Das, R.; Junejo, S.; Ahmed, N.; Lee, M.M.Y.; Shaukat, A.; et al. Fractional Flow Reserve vs. Angiography in Guiding Management to Optimize Outcomes in Non-ST-Segment Elevation Myocardial Infarction: The British Heart Foundation FAMOUS-NSTEMI Randomized Trial. Eur. Heart J. 2015, 36, 100–111. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Mehta, S.; Cannon, C.P.; Fox, K.A.A.; Wallentin, L.; Boden, W.E.; Spacek, R.; Widimsky, P.; Mccullough, P.A.; Hunt, D.; Braunwald, E.; et al. Routine vs Selective Invasive Strategies. JAMA 2005, 293, 2908–2917. [Google Scholar] [CrossRef]
- Danad, I.; Raijmakers, P.G.; Driessen, R.S.; Leipsic, J.; Raju, R.; Naoum, C.; Knuuti, J.; Mäki, M.; Underwood, R.S.; Min, J.K.; et al. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol. 2017, 2, 1100–1107. [Google Scholar] [CrossRef]
- Nørgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; de Bruyne, B.; Bezerra, H.; et al. Diagnostic Performance of Noninvasive Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography in Suspected Coronary Artery Disease: The NXT Trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Erglis, A.; Doh, J.H.; Daniels, D.V.; Jegere, S.; Kim, H.S.; Dunning, A.; Defrance, T.; Lansky, A.; Leipsic, J.; et al. Diagnosis of Ischemia-Causing Coronary Stenoses by Noninvasive Fractional Flow Reserve Computed from Coronary Computed Tomographic Angiograms: Results from the Prospective Multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) Study. J. Am. Coll. Cardiol. 2011, 58, 1989–1997. [Google Scholar] [PubMed]
- Min, J.K.; Leipsic, J.; Pencina, M.J.; Berman, D.S.; Koo, B.K.; van Mieghem, C.; Erglis, A.; Lin, F.Y.; Dunning, A.M.; Apruzzese, P.; et al. Diagnostic Accuracy of Fractional Flow Reserve from Anatomic CT Angiography. JAMA 2012, 308, 1237–1245. [Google Scholar] [CrossRef]
- Donnelly, P.M.; Kolossváry, M.; Karády, J.; Ball, P.A.; Kelly, S.; Fitzsimons, D.; Spence, M.S.; Celeng, C.; Horváth, T.; Szilveszter, B.; et al. Experience with an On-Site Coronary Computed Tomography-Derived Fractional Flow Reserve Algorithm for the Assessment of Intermediate Coronary Stenoses. Am. J. Cardiol. 2018, 121, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Taylor, C.A.; Jensen, J.M.; Bøtker, H.E.; Christiansen, E.H.; Kaltoft, A.K.; Holm, N.R.; Leipsic, J.; Zarins, C.K.; Achenbach, S.; et al. FFR Derived from Coronary CT Angiography in Nonculprit Lesions of Patients with Recent STEMI. JACC Cardiovasc. Imaging 2017, 10, 424–433. [Google Scholar] [CrossRef]
- Tonino, P.A.L.; De Bruyne, B.; Pijls, N.H.J.; Siebert, U.; Ikeno, F.; van ‘t Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; Oldroyd, K.G.; et al. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef]
- Engstrøm, T.; Kelbæk, H.; Helqvist, S.; Høfsten, D.E.; Kløvgaard, L.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamäki, K.; Clemmensen, P.; et al. Complete Revascularisation versus Treatment of the Culprit Lesion Only in Patients with ST-Segment Elevation Myocardial Infarction and Multivessel Disease (DANAMI-3—PRIMULTI): An Open-Label, Randomised Controlled Trial. Lancet 2015, 386, 665–671. [Google Scholar] [CrossRef]
- Smits, P.C.; Abdel-Wahab, M.; Neumann, F.-J.; Boxma-de Klerk, B.M.; Lunde, K.; Schotborgh, C.E.; Piroth, Z.; Horak, D.; Wlodarczak, A.; Ong, P.J.; et al. Fractional Flow Reserve–Guided Multivessel Angioplasty in Myocardial Infarction. N. Engl. J. Med. 2017, 376, 1234–1244. [Google Scholar] [CrossRef]
- Carlos, M.E.; Smart, S.C.; Wynsen, J.C.; Sagar, K.B. Dobutamine Stress Echocardiography for Risk Stratification After Myocardial Infarction. Circulation 1997, 95, 1402–1410. [Google Scholar] [CrossRef]
- Greco, C.A.; Salustri, A.; Seccareccia, F.; Ciavatti, M.; Biferali, F.; Valtorta, C.; Guzzardi, G.; Falcone, M.; Palamara, A. Prognostic Value of Dobutamine Echocardiography Early After Uncomplicated Acute Myocardial Infarction: A Comparison with Exercise Electrocardiography. J. Am. Coll. Cardiol. 1997, 29, 261–267. [Google Scholar] [CrossRef]
- Neglia, D.; Rovai, D.; Caselli, C.; Pietila, M.; Teresinska, A.; Aguadé-Bruix, S.; Pizzi, M.N.; Todiere, G.; Gimelli, A.; Schroeder, S.; et al. Detection of Significant Coronary Artery Disease by Noninvasive Anatomical and Functional Imaging. Circ. Cardiovasc. Imaging 2015, 8, e002179. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Mickley, H.; Crea, F.; van de Werf, F.; et al. Fourth Universal Definition of Myocardial Infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Leipsic, J.; Abbara, S.; Achenbach, S.; Cury, R.; Earls, J.P.; Mancini, G.B.J.; Nieman, K.; Pontone, G.; Raff, G.L. SCCT Guidelines for the Interpretation and Reporting of Coronary CT Angiography: A Report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 2014, 8, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Bertella, E.; Mushtaq, S.; Loguercio, M.; Cortinovis, S.; Baggiano, A.; Conte, E.; Annoni, A.; Formenti, A.; Beltrama, V.; et al. Coronary Artery Disease: Diagnostic Accuracy of CT Coronary Angiography-a Comparison of High and Standard Spatial Resolution Scanning. Radiology 2014, 271, 688–694. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Han, H.; Tan, R.-S.; Chai, P.; Fam, J.M.; Teo, L.; Chin, C.Y.; Ong, C.C.; Low, R.; Chandola, G.; et al. Diagnostic Performance of Fractional Flow Reserve from CT Coronary Angiography with Analytical Method. Front. Cardiovasc. Med. 2021, 8, 739633. [Google Scholar] [CrossRef]
- Sousa-Uva, M.; Neumann, F.J.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. J. Cardiothorac. Surg. 2019, 55, 4–90. [Google Scholar] [CrossRef]
- Toth, G.G.; Johnson, N.P.; Jeremias, A.; Pellicano, M.; Vranckx, P.; Fearon, W.F.; Barbato, E.; Kern, M.J.; Pijls, N.H.J.; de Bruyne, B. Standardization of Fractional Flow Reserve Measurements. J. Am. Coll. Cardiol. 2016, 68, 742–753. [Google Scholar] [CrossRef]
- de Bruyne, B.; Pijls, N.H.J.; Kalesan, B.; Barbato, E.; Tonino, P.a.L.; Piroth, Z.; Jagic, N.; Möbius-Winkler, S.; Mobius-Winckler, S.; Rioufol, G.; et al. Fractional Flow Reserve-Guided PCI versus Medical Therapy in Stable Coronary Disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef]
- Sicari, R.; Nihoyannopoulos, P.; Evangelista, A.; Kasprzak, J.; Lancellotti, P.; Poldermans, D.; Voigt, J.U.; Zamorano, J.L. Stress Echocardiography Expert Consensus Statement. Eur. J. Echocardiogr. 2008, 9, 415–437. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults (2015). Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Broderick, T.M.; Bourdillon, P.D.V.; Ryan, T.; Feigenbaum, H.; Dillon, J.C.; Armstrong, W.F. Comparison of Regional and Global Left Ventricular Function by Serial Echocardiograms After Reperfusion in Acute Myocardial Infarction. J. Am. Soc. Echocardiogr. 1989, 2, 315–323. [Google Scholar] [CrossRef]
- Takeuchi, M.; Miyazaki, C.; Yoshitani, H.; Otani, S.; Sakamoto, K.; Yoshikawa, J. Assessment of Coronary Flow Velocity with Transthoracic Doppler Echocardiography during Dobutamine Stress Echocardiography. J. Am. Coll. Cardiol. 2001, 38, 117–123. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Weissler-Snir, A.; Gurevitz, C.; Assali, A.; Vaknin-Assa, H.; Bental, T.; Lador, A.; Yavin, H.; Perl, L.; Kornowski, R.; Lev, E. Prognosis of STEMI Patients with Multi-Vessel Disease Undergoing Culprit-Only PCI without Significant Residual Ischemia on Non-Invasive Stress Testing. PLoS ONE 2015, 10, e0138474. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [PubMed]
- Ahres, A.; Jablonkai, B.; Schrancz, Á.; Balogh, Z.; Kenessey, A.; Baranyai, T.; Őze, Á.; Szigeti, Z.; Rubóczky, G.; Nagybaczoni, B.; et al. Patients with Moderate Non-Culprit Coronary Lesions of Recent Acute Coronary Syndrome a Comparison of Fractional Flow Reserve and Dobutamine Stress Echocardiography. Int. Heart J. 2021, 62, 952–961. [Google Scholar] [CrossRef]
- Kwon, S.S.; Chung, E.C.; Park, J.S.; Kim, G.T.; Kim, J.W.; Kim, K.H.; Shin, E.S.; Shim, E.B. A Novel Patient-Specific Model to Compute Coronary Fractional Flow Reserve. Prog. Biophys. Mol. Biol. 2014, 116, 48–55. [Google Scholar] [CrossRef]
- Ntalianis, A.; Sels, J.W.; Davidavicius, G.; Tanaka, N.; Muller, O.; Trana, C.; Barbato, E.; Hamilos, M.; Mangiacapra, F.; Heyndrickx, G.R.; et al. Fractional Flow Reserve for the Assessment of Nonculprit Coronary Artery Stenoses in Patients with Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2010, 3, 1274–1281. [Google Scholar] [CrossRef]
- Duguay, T.M.; Tesche, C.; Vliegenthart, R.; de Cecco, C.N.; Lin, H.; Albrecht, M.H.; Varga-Szemes, A.; de Santis, D.; Ebersberger, U.; Bayer, R.R.; et al. Coronary Computed Tomographic Angiography-Derived Fractional Flow Reserve Based on Machine Learning for Risk Stratification of Non-Culprit Coronary Narrowings in Patients with Acute Coronary Syndrome. Am. J. Cardiol. 2017, 120, 1260–1266. [Google Scholar] [CrossRef]
- Padro, T.; Manfrini, O.; Bugiardini, R.; Canty, J.; Cenko, E.; de Luca, G.; Duncker, D.J.; Eringa, E.C.; Koller, A.; Tousoulis, D.; et al. ESC Working Group on Coronary Pathophysiology and Microcirculation Position Paper on “Coronary Microvascular Dysfunction in Cardiovascular Disease”. Cardiovasc. Res. 2020, 116, 741–755. [Google Scholar] [CrossRef]
- Murai, T.; Yonetsu, T.; Kanaji, Y.; Usui, E.; Hoshino, M.; Hada, M.; Hamaya, R.; Kanno, Y.; Lee, T.; Kakuta, T. Prognostic Value of the Index of Microcirculatory Resistance after Percutaneous Coronary Intervention in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. Catheter. Cardiovasc. Interv. 2018, 92, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Nijjer, S.S.; Dewaard, G.A.; Sen, S.; van de Hoef, T.P.; Petraco, R.; Echavarría-Pinto, M.; van Lavieren, M.A.; Meuwissen, M.; Danad, I.; Knaapen, P.; et al. Coronary Pressure and Flow Relationships in Humans: Phasic Analysis of Normal and Pathological Vessels and the Implications for Stenosis Assessment: A Report from the Iberian-Dutch-English (IDEAL) Collaborators. Eur. Heart J. 2016, 37, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- Brosh, D.; Higano, S.T.; Lennon, R.J.; Holmes, D.R.; Lerman, A. Effect of Lesion Length on Fractional Flow Reserve in Intermediate Coronary Lesions. Am. Heart J. 2005, 150, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, T.; Hasegawa, T.; Nishimura, S.; Nakata, S.; Kataoka, T.; Ehara, S.; Hanatani, A.; Shimada, K.; Yoshiyama, M. Impact of Lesion Length on Functional Significance in Intermediate Coronary Lesions. Clin. Cardiol. 2013, 36, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Choi, G.; Koo, B.K.; Hwang, D.; Park, J.; Zhang, J.; Kim, K.J.; Tong, Y.; Kim, H.J.; Grady, L.; et al. Identification of High-Risk Plaques Destined to Cause Acute Coronary Syndrome Using Coronary Computed Tomographic Angiography and Computational Fluid Dynamics. JACC Cardiovasc. Imaging 2019, 12, 1032–1043. [Google Scholar] [CrossRef]
- Celeng, C.; Leiner, T.; Maurovich-Horvat, P.; Merkely, B.; de Jong, P.; Dankbaar, J.W.; van Es, H.W.; Ghoshhajra, B.B.; Hoffmann, U.; Takx, R.A.P. Anatomical and Functional Computed Tomography for Diagnosing Hemodynamically Significant Coronary Artery Disease: A Meta-Analysis. JACC Cardiovasc. Imaging 2019, 12, 1316–1325. [Google Scholar] [CrossRef]
- Aziz, W.; Claridge, S.; Ntalas, I.; Gould, J.; de Vecchi, A.; Razeghi, O.; Toth, D.; Mountney, P.; Preston, R.; Rinaldi, C.A.; et al. Emerging Role of Cardiac Computed Tomography in Heart Failure. ESC Heart Fail. 2019, 6, 909–920. [Google Scholar] [CrossRef]
- Toth, G.G.; de Bruyne, B.; Rusinaru, D.; di Gioia, G.; Bartunek, J.; Pellicano, M.; Vanderheyden, M.; Adjedj, J.; Wijns, W.; Pijls, N.H.J.; et al. Impact of Right Atrial Pressure on Fractional Flow Reserve Measurements. JACC Cardiovasc. Interv. 2016, 9, 453–459. [Google Scholar] [CrossRef]
- Martin, S.S.; Mastrodicasa, D.; van Assen, M.; de Cecco, C.N.; Bayer, R.R.; Tesche, C.; Varga-Szemes, A.; Fischer, A.M.; Jacobs, B.E.; Sahbaee, P.; et al. Value of Machine Learning–Based Coronary Ct Fractional Flow Reserve Applied to Triple-Rule-out Ct Angiography in Acute Chest Pain. Radiol. Cardiothorac. Imaging 2020, 2, e190137. [Google Scholar] [CrossRef]
- Gurunathan, S.; Ahmed, A.; Vamvakidou, A.; Ramzy, I.S.; Akhtar, M.; Ali, A.; Karogiannis, N.; Zidros, S.; Balaji, G.; Young, G.; et al. Diagnostic Concordance and Clinical Outcomes in Patients Undergoing Fractional Flow Reserve and Stress Echocardiography for the Assessment of Coronary Stenosis of Intermediate Severity. J. Am. Soc. Echocardiogr. 2018, 31, 180–186. [Google Scholar] [CrossRef]
- Johnson, N.P.; Kirkeeide, R.L.; Gould, K.L. Is Discordance of Coronary Flow Reserve and Fractional Flow Reserve Due to Methodology or Clinically Relevant Coronary Pathophysiology? JACC Cardiovasc. Imaging 2012, 5, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Ryan, K.A.; Murphy, S.A.; Mesley, R.; Marble, S.J.; Giugliano, R.P.; Cannon, C.P.; Antman, E.M.; Braunwald, E. Impaired Coronary Blood Flow in Nonculprit Arteries in the Setting of Acute Myocardial Infarction. J. Am. Coll. Cardiol. 1999, 34, 974–982. [Google Scholar] [CrossRef]
- Cuculi, F.; de Maria, G.L.; Meier, P.; Dall’Armellina, E.; de Caterina, A.R.; Channon, K.M.; Prendergast, B.D.; Choudhury, R.C.; Forfar, J.C.; Kharbanda, R.K.; et al. Impact of Microvascular Obstruction on the Assessment of Coronary Flow Reserve, Index of Microcirculatory Resistance, and Fractional Flow Reserve After ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2014, 64, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Gregorini, L.; Marco, J.; Kozàkovà, M.; Palombo, C.; Anguissola, G.B.; Marco, I.; Bernies, M.; Cassagneau, B.; Distante, A.; Bossi, I.M.; et al. α-Adrenergic Blockade Improves Recovery of Myocardial Perfusion and Function After Coronary Stenting in Patients with Acute Myocardial Infarction. Circulation 1999, 99, 482–490. [Google Scholar] [CrossRef]
- Jung, P.H.; Rieber, J.; Stork, S.; Hoyer, C.; Erhardt, I.; Nowotny, A.; Voelker, W.; Weidemann, F.; Ertl, G.; Klauss, V.; et al. Effect of Contrast Application on Interpretability and Diagnostic Value of Dobutamine Stress Echocardiography in Patients with Intermediate Coronary Lesions: Comparison with Myocardial Fractional Flow Reserve. Eur. Heart J. 2008, 29, 2536–2543. [Google Scholar] [CrossRef]
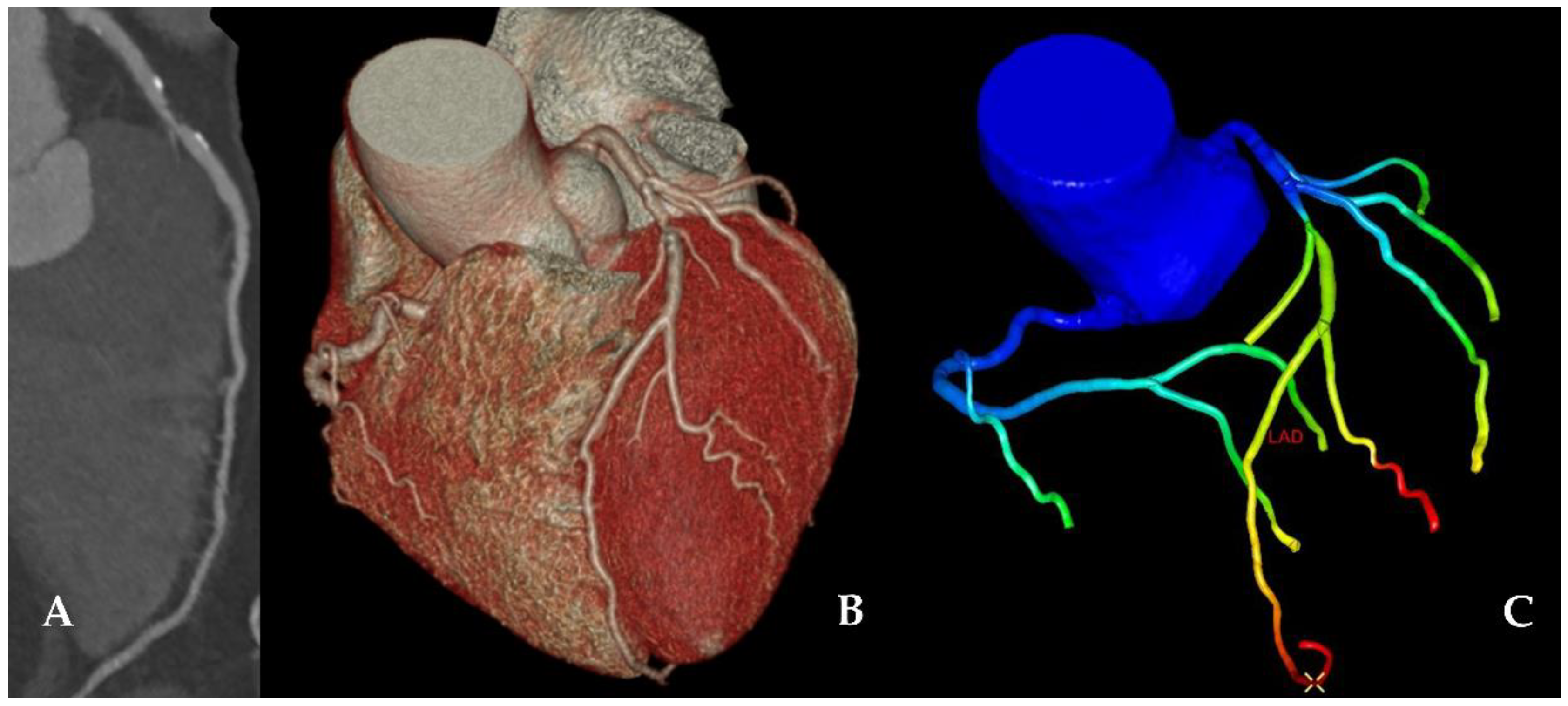


| n = 68 | |
| Demography and cardiovascular risk factors | |
| Female (n, %) | 22 (32.4) |
| Age (years) | 57.1 ± 10.1 |
| BMI (kg/m2) | 28.1 ± 4.1 |
| Hypertension (n, %) | 44 (64.7) |
| Diabetes mellitus (n, %) | 16 (23.5) |
| Hyperlipidaemia (n, %) | 36 (52.9) |
| Current smoker (n, %) | 40 (58.8) |
| Family history of cardiovascular disease (n, %) | 34 (50.0) |
| Medication | |
| ASA (n, %) | 67 (98.5) |
| P2Y12 ADP receptor blocker (n, %) | 68 (100) |
| Beta-blocker (n, %) | 67 (98.5) |
| Statin (n, %) | 67 (98.5) |
| LVEF (%) | 47.3 ± 8.4 |
| LVEF < 50% (n, %) | 32 (47.1) |
| Overall | LAD | LCx | RCA | p | |
|---|---|---|---|---|---|
| n = 89 | n = 52 | n = 15 | n = 22 | ||
| Lesion length (mm) | 22.5 ± 13.4 | 24.0 ± 14.2 | 19.1 ± 9.0 | 21.4 ± 13.9 | 0.511 |
| MLDS% | 39.4 ± 16.2 | 39.5 ± 16.0 | 42.0 ± 20.4 | 37.4 ± 14.0 | 0.707 |
| MLDS% ≥ 50% (n, %) | 20 (22.5) | 12 (23.1) | 4 (26.7) | 4 (18.2) | 0.821 |
| CT-FFR value | 0.85 ± 0.09 | 0.86 ± 0.09 | 0.87 ± 0.09 | 0.84 ± 0.10 | 0.479 |
| CT-FFR drop | 0.12 ± 0.09 | 0.12 ± 0.09 | 0.11 ± 0.09 | 0.11 ± 0.10 | 0.763 |
| CT-FFR value ≤ 0.80 (n, %) | 24 (27.0) | 14 (26.9) | 4 (26.7) | 6 (27.3) | 0.999 |
| FFRi Value | Regional WMSI at Peak Stress | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate Model 1 | Multivariate Model 2 | Univariate | |||||
| Unstandardized Beta | p | Unstandardized Beta | p | Unstandardized Beta | p | Unstandardized Beta | p | |
| Lesion length | −0.001 | 0.177 | - | - | - | - | 0.000 | 0.956 |
| MLDS% | −0.001 | 0.007 | 0.000 | 0.454 | −0.001 | 0.233 | −0.004 | 0.221 |
| CT-FFR value | 0.366 | <0.001 | 0.334 | <0.001 | −0.440 | 0.441 | ||
| CT-FFR drop | −0.337 | <0.001 | - | - | −0.289 | 0.002 | 0.403 | 0.494 |
| LAD | LCx | RCA | |
|---|---|---|---|
| n = 52 | n = 15 | n = 22 | |
| Lesion length | 0.54 (0.39–0.68) * | 0.89 (0.62–0.99) * | 0.59 (0.36–0.79) |
| MLDS% | 0.57 (0.42–0.71) # | 0.89 (0.62–0.99) # | 0.66 (0.43–0.84) |
| CT-FFR value | 0.75 (0.61–0.86) | 0.91 (0.65–1.0) | 0.79 (0.57–0.93) |
| CT-FFR drop | 0.77 (0.63–0.88) | 0.91 (0.65–1.0) | 0.70 (0.47–0.87) |
| STEMI | NSTE-ACS | p | |
|---|---|---|---|
| n = 57 | n = 32 | ||
| Lesion length | 0.58 (0.44–0.71) | 0.58 (0.40–0.76) | 0.951 |
| MLDS% | 0.64 (0.51–0.77) | 0.62 (0.43–0.78) | 0.837 |
| CT-FFR value | 0.82 (0.70–0.91) | 0.65 (0.46–0.81) | 0.141 |
| CT-FFR drop | 0.81 (0.68–0.90) | 0.70 (0.52–0.85) | 0.373 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahres, A.; Simon, J.; Jablonkai, B.; Nagybaczoni, B.; Baranyai, T.; Apor, A.; Kolossvary, M.; Merkely, B.; Maurovich-Horvat, P.; Szilveszter, B.; et al. Diagnostic Performance of On-Site Computed Tomography Derived Fractional Flow Reserve on Non-Culprit Coronary Lesions in Patients with Acute Coronary Syndrome. Life 2022, 12, 1820. https://doi.org/10.3390/life12111820
Ahres A, Simon J, Jablonkai B, Nagybaczoni B, Baranyai T, Apor A, Kolossvary M, Merkely B, Maurovich-Horvat P, Szilveszter B, et al. Diagnostic Performance of On-Site Computed Tomography Derived Fractional Flow Reserve on Non-Culprit Coronary Lesions in Patients with Acute Coronary Syndrome. Life. 2022; 12(11):1820. https://doi.org/10.3390/life12111820
Chicago/Turabian StyleAhres, Abdelkrim, Judit Simon, Balazs Jablonkai, Bela Nagybaczoni, Tamas Baranyai, Astrid Apor, Marton Kolossvary, Bela Merkely, Pal Maurovich-Horvat, Balint Szilveszter, and et al. 2022. "Diagnostic Performance of On-Site Computed Tomography Derived Fractional Flow Reserve on Non-Culprit Coronary Lesions in Patients with Acute Coronary Syndrome" Life 12, no. 11: 1820. https://doi.org/10.3390/life12111820
APA StyleAhres, A., Simon, J., Jablonkai, B., Nagybaczoni, B., Baranyai, T., Apor, A., Kolossvary, M., Merkely, B., Maurovich-Horvat, P., Szilveszter, B., & Andrassy, P. (2022). Diagnostic Performance of On-Site Computed Tomography Derived Fractional Flow Reserve on Non-Culprit Coronary Lesions in Patients with Acute Coronary Syndrome. Life, 12(11), 1820. https://doi.org/10.3390/life12111820






