Application of Potassium after Waterlogging Improves Quality and Productivity of Soybean Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Land Preparation and Layout
2.3. Experimental Treatments and Design
2.4. Seed Sowing and Crop Culture
2.5. Imposition of WL Stress
2.6. Harvesting and Sampling Crops
2.7. Quantification of Yield Data
2.8. Determination of Seed Quality Data
2.9. Determination of Seed Coat Leakage in Seeds
2.10. Determination of Nutrient Composition in Seeds
2.11. Statistical Analysis
3. Results
3.1. Plant Height and Pod Production
3.2. Production Seeds Plant−1 and 100-Seed Weight
3.3. Grain and Straw Yield of Soybean
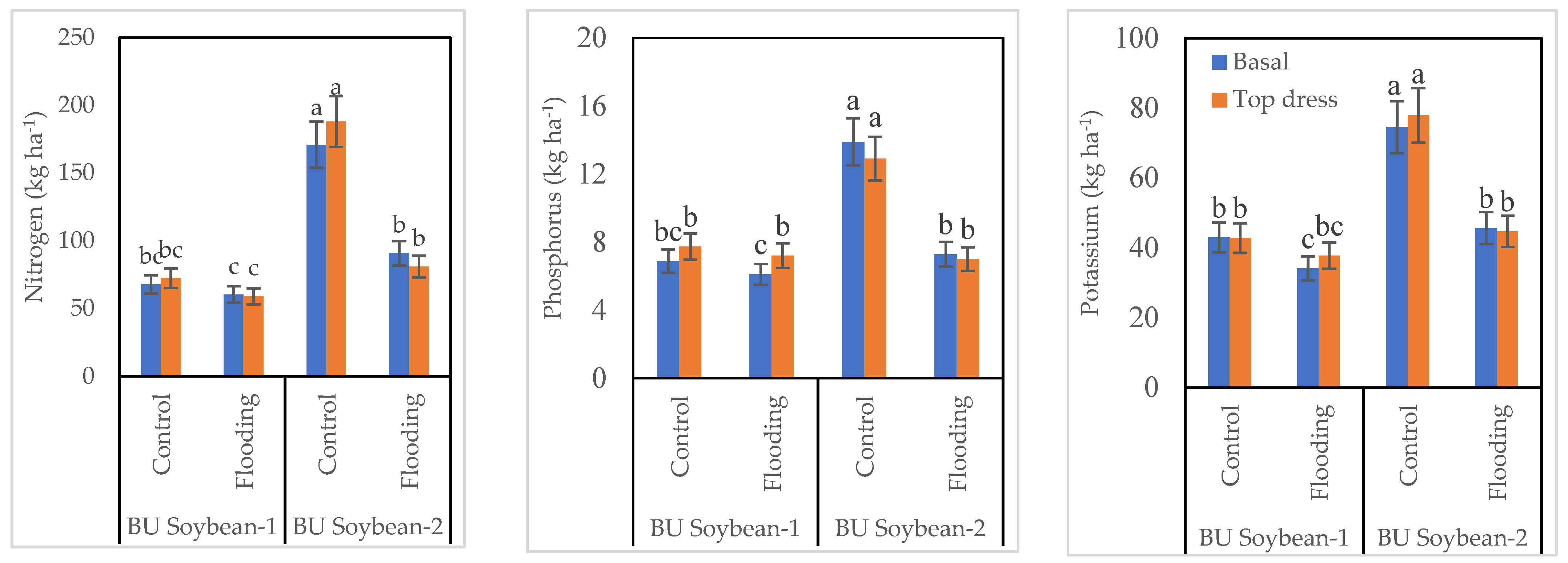
3.4. Nutrient Accumulation in Soybean Seed
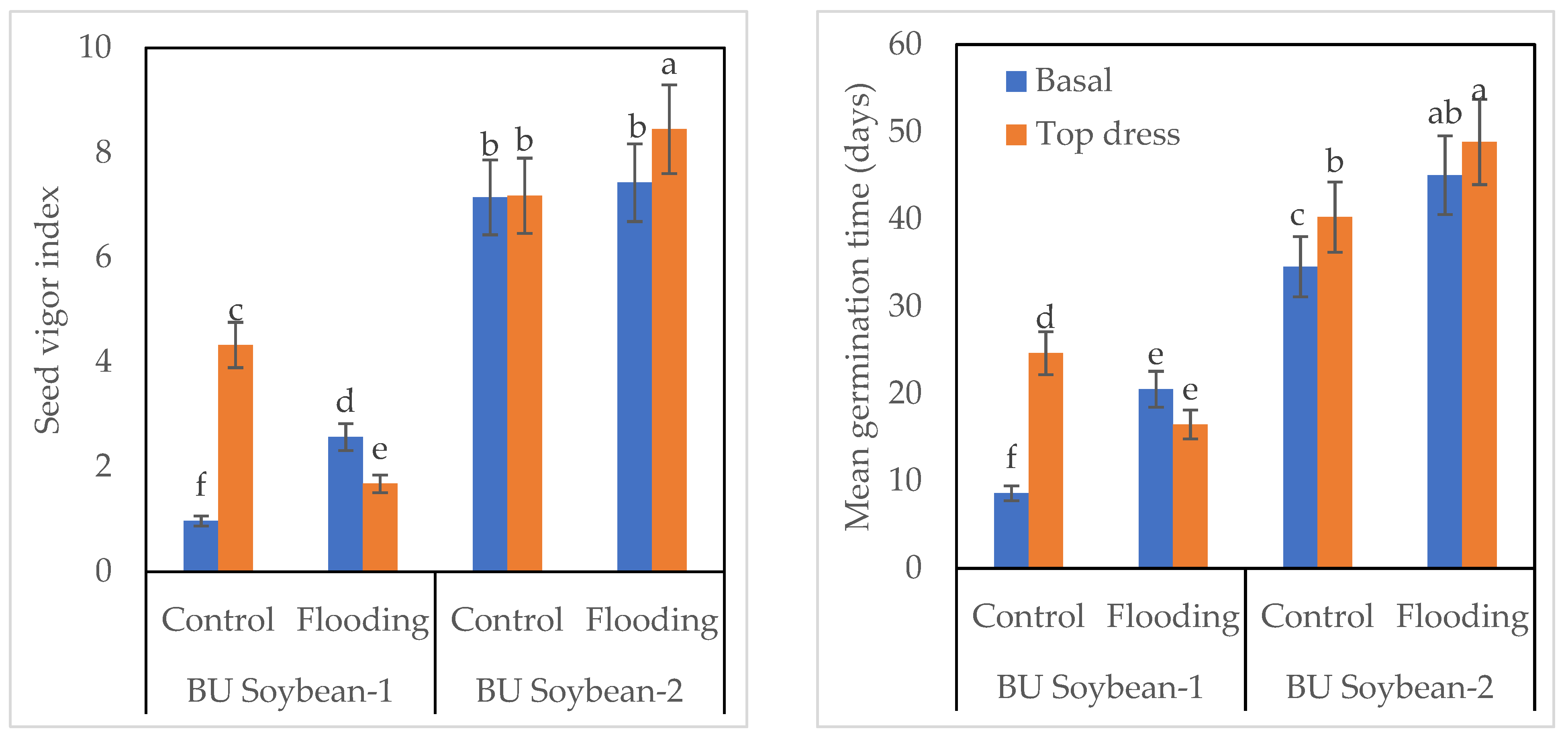
3.5. Protein Content and EC of Soybean Seed
3.6. Germination and Seed Vigor Index of Soybean
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satter, M.A.; Rahman, M.M.; Rashid, M.H.; Ali, M.S.; Alam, M.S. Krishi Projukti Hatboi (Handbook on Agro-Technology); Bangladesh Agricultural Research Institute: Gazipur, Bangladesh, 2005. [Google Scholar]
- BBS (Bangladesh Bureau of Statistics). Yearbook of Agricultural Statistics-2021; Bangladesh Bureau of Statistics: Dhaka, Bangladesh, 2021. [Google Scholar]
- Mannan, M.A.; Karim, M.A.; Haque, M.M.; Khaliq, Q.A.; Higuchi, H.; Nawata, E. Response of soybean to salinity: I. Genotypic variations in salt tolerance at the vegetative stage. Trop. Agric. Dev. 2012, 56, 117–122. [Google Scholar]
- Hossain, M.S.; Mamun, M.A.A.; Khaliq, Q.A.; Higuchi, H.; Nawata, E.; Karim, M.A. Waterlogging induced changes in morpho-physiology of soybean. Res. Trop. Agric. 2019, 1, 53–54. [Google Scholar]
- Sarker, U.; Oba, S. Polyphenol and Flavonoid Profiles and Radical Scavenging Activity in Selected Leafy Vegetable Amaranthus gangeticus. BMC Plant Biol. 2020, 20, 499. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Nutritional and Bioactive Constituents and Scavenging Capacity of Radicals in Amaranthus hypochondriacus. Sci. Rep. 2020, 10, 19962. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S.; Ercisli, S.; Assouguem, A.; Alotaibi, A.; Ullah, R. Bioactive Phytochemicals and Quenching Activity of Radicals in Selected Drought-Resistant Amaranthus tricolor Vegetable Amaranth. Antioxidants 2022, 11, 578. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Antioxidant Constituents of Three Selected Red and Green Color Amaranthus Leafy Vegetable. Sci. Rep. 2019, 9, 18233. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S.; Daramy, M.A. Nutrients, Minerals, Antioxidant Pigments and Phytochemicals, and Antioxidant Capacity of the Leaves of Stem Amaranth. Sci. Rep. 2020, 10, 3892. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Nutrients, Minerals, Pigments, Phytochemical, and Radical Scavenging Activity in Amaranthus blitum Leafy Vegetable. Sci. Rep. 2020, 10, 3868. [Google Scholar] [CrossRef]
- Siamabele, B. The significance of soybean production in the face of changing climates in Africa. Cogent Food Agric. 2021, 7, 1933745. [Google Scholar] [CrossRef]
- BBS (Bangladesh Bureau of Statistics). Statistical Pocket Book of Bangladesh; Bangladesh Bureau of Statistics, Ministry of Planning, Government of People’s Republic of Bangladesh: Dhaka, Bangladesh, 2020; p. 117. [Google Scholar]
- Terzic, D.; Popovic, V.; Tatic, M.; Vasileva, V.; Dekic, V.; Ugrenovic, V.; Popovic, S.; Avdic, P. Soybean area, yield and production in world. XXII Eco-Conference, 2018. Ecol. Mov. Novi Sad 2018, 8, 135–145. [Google Scholar]
- Miah, A.A.; Karim, M.A.; Mamun, M.A.A.; Khan, M.A.R.; Akter, N.; Haque, M.M. Planting time effect on phenology and yield of early maturing dwarf soybean genotypes. Bangladesh J. Ecol. 2020, 2, 19–24. [Google Scholar]
- Pociecha, E.; Kościelniak, J.; Filek, W. Effects of root flooding and stage of development on the growth and photosynthesis of field bean (Vicia faba L.). Acta Physiol. Plant. 2008, 30, 529–535. [Google Scholar] [CrossRef]
- Celik, G.; Turhan, E. Genotypic variation in growth and physiological responses of common bean (Phaseolus vulgaris L.) seedlings to flooding. Afr. J. Biotechnol. 2011, 10, 7372–7380. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Hossain, M.S.; Fujita, M. Soybean production and environmental stresses. In Environmental Stresses in Soybean Production; Academic Press: Cambridge, MA, USA, 2016; pp. 61–102. [Google Scholar]
- Sarker, U.; Oba, S. Salinity Stress Enhances Color Parameters, Bioactive Leaf Pigments, Vitamins, Polyphenols, Flavonoids and Antioxidant Activity in Selected Amaranthus Leafy Vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, Superoxide Dismutase and Ascorbate-Glutathione Cycle Enzymes Confer Drought Tolerance of A. tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The Response of Salinity Stress-Induced A. tricolor to Growth, Anatomy, Physiology, Non-Enzymatic and Enzymatic Antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought Stress Effects on Growth, ROS Markers, Compatible Solutes, Phenolics, Flavonoids, and Antioxidant Activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought Stress Enhances Nutritional and Bioactive Compounds, Phenolic Acids and Antioxidant Capacity of Amaranthus Leafy Vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Oba, S. Salinity Stress Accelerates Nutrients, Dietary Fiber, Minerals, Phytochemicals and Antioxidant Activity in Amaranthus tricolor Leaves. PLoS ONE 2018, 13, 0206388. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Response of Nutrients, Minerals, Antioxidant Leaf Pigments, Vitamins, Polyphenol, Flavonoid and Antioxidant Activity in Selected Vegetable Amaranth under Four Soil Water Content. Food Chem. 2018, 252, 72–83. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Augmentation of Leaf Color Parameters, Pigments, Vitamins, Phenolic Acids, Flavonoids and Antioxidant Activity in Selected Amaranthus tricolor under Salinity Stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.N.; Sarker, U.; Raihan, M.S.; Al-Huqail, A.A.; Siddiqui, M.H.; Oba, S. Influence of Salinity Stress on Color Parameters, Leaf Pigmentation, Polyphenol and Flavonoid Contents, and Antioxidant Activity of Amaranthus lividus Leafy Vegetables. Molecules 2022, 27, 1821. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Lin, Y.P.; Oba, S.; Yoshioka, Y.; Ken, H. Prospects and potentials of underutilized leafy Amaranths as vegetable use for health promotion. Plant Physiol. Biochem. 2022, 182, 104–123. [Google Scholar] [CrossRef]
- Sarker, U.; Rabbani, M.G.; Oba, S.; Eldehna, W.M.; Al-Rashood, S.T.; Mostafa, N.M.; Eldahshan, O.A. Phytonutrients, Colorant Pigments, Phytochemicals, and Antioxidant Potential of Orphan Leafy Amaranthus Species. Molecules 2022, 27, 2899. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S.; Alsanie, W.F.; Gaber, A. Characterization of Phytochemicals, Nutrients, and Antiradical Potential in Slim Amaranth. Antioxidants 2022, 11, 1089. [Google Scholar] [CrossRef]
- Sarker, U.; Iqbal, M.A.; Hossain, M.N.; Oba, S.; Ercisli, S.; Muresan, C.C.; Marc, R.A. Colorant Pigments, Nutrients, Bioactive Components, and Antiradical Potential of Danta Leaves (Amaranthus lividus). Antioxidants 2022, 11, 1206. [Google Scholar] [CrossRef]
- Hassan, J.; Rajib, M.M.R.; Sarkar, U.; Akter, M.; Khan, M.; Khandaker, S.; Khalid, F.; Rahman, G.; Ercisli, S.; Muresan, C.; et al. Optimizing textile dyeing wastewater for tomato irrigation through physiochemical, plant nutrient uses and pollution load index of irrigated soil. Sci. Rep. 2022, 12, 10088. [Google Scholar] [CrossRef]
- Sarker, U.; Azam, M.G.; Talukder, M.Z.A. Genetic Variation in Mineral Profiles, Yield Contributing Agronomic Traits, and Foliage Yield of Stem Amaranth. Genetika 2022, 54, 91–108. [Google Scholar] [CrossRef]
- Jung, G.; Matsunami, T.; Oki, Y.; Kokubun, M. Effects of waterlogging on nitrogen fixation and photosynthesis in supernodulating soybean cultivar Kanto 100. Plant Prod. Sci. 2008, 11, 291–297. [Google Scholar] [CrossRef]
- Matsunami, T.; Jung, G.H.; Oki, Y.; Kokubun, M. Effect of waterlogging during vegetative stage on growth and yield in supernodulating soybean cultivar sakukei 4. Plant Prod. Sci. 2007, 10, 112–121. [Google Scholar] [CrossRef]
- Rhine, M.D.; Stevens, G.; Shannon, G.; Wrather, A.; Sleper, D. Yield and nutritional responses to waterlogging of soybean cultivars. Irrig. Sci. 2010, 28, 135–142. [Google Scholar] [CrossRef]
- Komatsu, S.; Makino, T.; Yasue, H. Proteomic and biochemical analyses of the cotyledon and root of flooding-stressed soybean plants. PLoS ONE 2013, 8, e65301. [Google Scholar] [CrossRef] [PubMed]
- Ara, R.; Mannan, M.A.; Khaliq, Q.A.; Miah, M.U. Waterlogging tolerance of soybean. Bangladesh Agron. J. 2016, 18, 105–109. [Google Scholar] [CrossRef]
- Sullivan, M.; Van Toai, T.; Fausey, N.; Beuerlein, J.; Parkinson, R.; Soboyejo, A. Evaluating on-farm flooding impacts on soybean. Crop Sci. 2001, 41, 93–100. [Google Scholar] [CrossRef]
- Amin, M.R.; Karim, M.A.; Khaliq, Q.A.; Islam, M.R.; Aktar, S. The influence of waterlogging period on yield and yield components of mungbean (Vigna radiata L. Wilczek). Agriculturists 2017, 15, 88–100. [Google Scholar] [CrossRef]
- Divito, G.A.; Sadras, V.O. How do phosphorus, potassium and sulphur affect plant growth and biological nitrogen fixation in crop and pasture legumes? A meta-analysis. Field Crop. Res. 2014, 156, 161–171. [Google Scholar] [CrossRef]
- Amin, R. Nutrient Management and Yield Performance of Mungbean Genotypes under Soil Flooding Conditions. Ph.D. Thesis, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh, 2014. [Google Scholar]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.; Rengel, Z. Phosphorus starvation boosts carboxylate secretion in P-deficient genotypes of Lupinus angustifolius with contrasting root structure. Crop Past. Sci. 2013, 64, 588–599. [Google Scholar] [CrossRef]
- Minjian, C.; Haiqiu, Y.; Hongkui, Y.; Chunji, J. Difference in tolerance to potassium deficiency between two maize inbred lines. Plant Prod. Sci. 2007, 10, 42–46. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Wei, J.; Li, C.; Li, Y.; Jiang, G.; Cheng, G.; Zheng, Y. Effects of external potassium (K) supply on drought tolerances of two contrasting winter wheat cultivars. PLoS ONE 2013, 8, e69737. [Google Scholar] [CrossRef]
- Amanullah, A.; Iqbal, A.; Iqbal, M. Impact of potassium rates and their application time on dry matter partitioning, biomass and harvest index of maize (Zea mays) with and without cattle dung application. Emir. J. Food Agric. 2015, 27, 447–453. [Google Scholar] [CrossRef]
- ISTA (International Seed Testing Association). Available online: https://www.seedtest.org/en/home.html (accessed on 31 January 2019).
- Agrawal, R.L. Seed Technology; Oxford & IBH Publishing Co., Pvt., Ltd.: New Delhi, India, 2005; pp. 565–590. [Google Scholar]
- Marcos Filho, J.; Vieira, R.D. Seed vigor tests: Procedures-conductivity tests. In Seed Vigor Tests Handbook; Association of Official Seed Analysts: Ithaca, NY, USA, 2009; pp. 186–200. [Google Scholar]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice, 3rd ed.; International Rice Research Institute: Manila, Philippines, 1976. [Google Scholar]
- Kulsum, U.; Sarker, U.; Rasul, M.G. Genetic variability, heritability and interrelationship in salt-tolerant lines of T. Aman rice. Genetika 2022, 54, 761–776. [Google Scholar] [CrossRef]
- Hasan, M.J.; Kulsum, M.U.; Sarker, U.; Matin, M.Q.I.; Shahin, N.H.; Kabir, M.S.; Ercisli, S.; Marc, R.A. Assessment of GGE, AMMI, Regression, and Its Deviation Model to Identify Stable Rice Hybrids in Bangladesh. Plants 2022, 11, 2336. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedure for Agricultural Research, 2nd ed.; John Willey and Sons: Singapore, 1984; pp. 28–192. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Kim, Y.; Seo, C.W.; Khan, A.L.; Mun, B.G.; Shahzad, R.; Ko, J.W.; Lee, I.J. Ethylene mitigates waterlogging stress by regulating glutathione biosynthesis-related transcripts in soybeans. BioRxiv 2018, 252312. [Google Scholar] [CrossRef]
- Jin-Woong, C.H.O.; Ji, H.C.; Yamakawa, T. Comparison of photosynthetic response of two soybean cultivars to soil flooding. J. Facul. Agric. Kyushu Univ. 2006, 51, 227–232. [Google Scholar]
- Sathi, K.S.; Masud, A.A.C.; Falguni, M.R.; Ahmed, N.; Rahman, K.; Hasanuzzaman, M. Screening of Soybean Genotypes for Waterlogging Stress Tolerance and Understanding the Physiological Mechanisms. Adv. Agric. 2022, 2022, 5544665. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Ahmad, M.S.A.; Ashraf, M.; Al-Qurainy, F.; Ashraf, M.Y. Alleviation of waterlogging stress in upland cotton (Gossypium hirsutum L.) by exogenous application of potassium in soil and as a foliar spray. Crop Past. Sci. 2011, 62, 25–38. [Google Scholar] [CrossRef]
- Vyas, A.K.; Billore, S.D.; Ramesh, A.; Joshi, O.P.; Gupta, G.K.; Sharma, A.N.; Imas, P. Role of potassium in balanced fertilization of soybean-wheat cropping system. In Proceedings of the Regional Seminar on Recent Advances in Potassium Nutrition Management for Soybean Based Cropping Systems, Indore, India, 28–29 September 2007; National Research Centre for Soybean: Indore, India, 2008; pp. 28–29. [Google Scholar]
- Solaiman, Z.; Colmer, T.D.; Loss, S.P.; Thomson, B.D.; Siddique, K.H.M. Growth responses of cool-season grain legumes to transient waterlogging. Aust. J. Agric. Res. 2007, 58, 406–412. [Google Scholar] [CrossRef]
- Kuswantoro, H. Response of soybean genotypes to waterlogging. J. Agron. Indones. 2011, 39, 19–23. [Google Scholar]
- Vineela, V. Effect of Waterlogging on Growth and Yield of Green Gram (Vigna radiata L.). Ph.D. Thesis, Acharya N.G. Ranga Agricultural University, Rajendra Nagarp, Hyderabad, India, 2013. [Google Scholar]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Nitrogen. In Principles of Plant Nutrition; Springer: Dordrecht, The Netherlands, 2001; pp. 397–434. [Google Scholar]
- Alam, M.R.; Ali, M.A.; Molla, M.S.H.; Momin, M.A.; Mannan, M.A. Evaluation of different levels of potassium on the yield and protein content of wheat in the high Ganges River floodplain soil. Bangladesh J. Agric. Res. 2009, 34, 97–104. [Google Scholar] [CrossRef]
- Khan, A.A.; Inamullah; Jan, M.T. Impact of various nitrogen and potassium levels and application methods on grain yield and yield attributes of wheat. Sarhad J. Agric. 2014, 30, 35–46. [Google Scholar]
- Khan, A.; Wang, L.; Ali, S.; Tung, S.A.; Hafeez, A.; Yang, G. Optimal planting density and sowing date can improve cotton yield by maintaining reproductive organ biomass and enhancing potassium uptake. Field Crop. Res. 2017, 214, 164–174. [Google Scholar] [CrossRef]
- Ahmed, A.; Aftab, S.; Hussain, S.; Nazir Cheema, H.; Liu, W.; Yang, F.; Yang, W. Nutrient accumulation and distribution assessment in response to potassium application under maize–soybean intercropping system. Agronomy 2020, 10, 725. [Google Scholar] [CrossRef]
- Ahmad, M.; Riaz, A.; Ishaque, M.; Malik, A.U. Response of maize hybrids to varying potassium application in Pakistan. Pak. J. Agric. Sci. 2009, 46, 179–184. [Google Scholar]
- Islam, M.R.; Akter, N.; Parvej, S.S.; Haque, K.S. Growth and yield response of mungbean (Vigna radiata L. Wilczek) genotypes to wet puddling, flooding and saturated soil culture. J. Plant Sci. 2014, 2, 311–316. [Google Scholar]
- Uddin, S.; Sarkar, M.; Rahman, M. Effect of nitrogen and potassium on yield of dry direct-seeded rice cv. NERICA 1 in aus season. Int. J. Agron. Plant Prod. 2013, 4, 69–75. [Google Scholar]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture–status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, J.; Pan, Y.; Lu, P.; Li, X.; Cong, R.; Ren, T. Anatomical variation of mesophyll conductance under potassium deficiency has a vital role in determining leaf photosynthesis. Plant Cell Environ. 2016, 39, 2428–2439. [Google Scholar] [CrossRef]
- Farhad, I.S.M.; Islam, M.N.; Hoque, S.; Bhuiyan, M.S.I. Role of potassium and sulphur on the growth, yield and oil content of soybean (Glycine max L.). Acad. J. Plant Sci. 2010, 3, 99–103. [Google Scholar]
- Beutler, A.N.; Giacomeli, R.; Alberto, C.M.; Silva, V.N.; da Silva Neto, G.F.; Machado, G.A.; Santos, A.T.L. Soil hydric excess and soybean yield and development in Brazil. Aust. J. Crop Sci. 2014, 8, 1461–1466. [Google Scholar]
- Koger, C.H.; Zablotowicz, R.M.; Weaver, M.A.; Tucker-Patterson, M.R.; Krutz, J.L.; Walker, T.W.; Street, J.E. Effect of winter flooding on weeds, soybean yield, straw degradation, and soil chemical and biochemical characteristics. Am. J. Plant Sci. 2013, 4, 10–18. [Google Scholar] [CrossRef]
- Youn, J.T.; Van, K.J.; Lee, J.E.; Kim, W.H.; Yun, H.T.; Kwon, Y.U.; Lee, S.H. Waterlogging Effects on Nitrogen Accumulation and N2 Fixation of Super nodulating Soybean Mutants. J. Crop Sci. Biotechnol. 2008, 11, 111–118. [Google Scholar]
- Ullah, M.J. Effect of waterlogging on growth and yield of mungbean cv. kanti (Vigna radiata). Legume Res. Int. J. 2006, 29, 196–200. [Google Scholar]
- Nguyen, V.T.; Vuong, T.D.; VanToai, T.; Lee, J.D.; Wu, X.; Mian, M.R.; Nguyen, H.T. Mapping of quantitative trait loci associated with resistance to Phytophthora sojae and flooding tolerance in soybean. Crop Sci. 2012, 52, 2481–2493. [Google Scholar] [CrossRef]
- Dong, H.; Kong, X.; Li, W.; Tang, W.; Zhang, D. Effects of plant density and nitrogen and potassium fertilization on cotton yield and uptake of major nutrients in two fields with varying fertility. Field Crop. Res. 2010, 119, 106–113. [Google Scholar] [CrossRef]
- Ali, S.; Hafeez, A.; Ma, X.; Tung, S.A.; Chattha, M.S.; Shah, A.N.; Yang, G. Equal potassium-nitrogen ratio regulated the nitrogen metabolism and yield of high-density late-planted cotton (Gossypium hirsutum L.) in Yangtze River valley of China. Ind. Crop. Prod. 2019, 129, 231–241. [Google Scholar] [CrossRef]
- Board, J.E. Waterlogging effects on plant nutrient concentrations in soybean. J. Plant Nutr. 2008, 31, 828–838. [Google Scholar] [CrossRef]
- Mohammadi, G.R. The effect of seed priming on plant traits of late-spring seeded soybean (Glycine max L.). Am. Eurasian J. Agric. Environ. Sci. 2009, 5, 322–326. [Google Scholar]
- Taiz, L.; Zeiger, E. Responses and adaptations to abiotic stress. In Plant Physiology, 5th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2010; pp. 755–778. [Google Scholar]
- Vodnik, D.; Strajnar, P.; Jemc, S.; Maček, I. Respiratory potential of maize (Zea mays L.) roots exposed to hypoxia. Environ. Exp. Bot. 2009, 65, 107–110. [Google Scholar] [CrossRef]
- Colmer, T.D.; Greenway, H. Ion transport in seminal and adventitious roots of cereals during O2 deficiency. J. Exp. Bot. 2011, 62, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Smethurst, C.F.; Garnett, T.; Shabala, S. Nutritional and chlorophyll fluorescence responses of lucerne (Medicago sativa) to waterlogging and subsequent recovery. Plant Soil 2005, 270, 31–45. [Google Scholar] [CrossRef]
- Elzenga, J.T.M.; van Veen, H. Waterlogging and plant nutrient uptake. In Waterlogging Signaling and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 23–35. [Google Scholar]
- Wuebker, E.F.; Mullen, R.E.; Koehler, K. Flooding and temperature effects on soybean germination. Crop Sci. 2001, 41, 1857–1861. [Google Scholar] [CrossRef]
- Yaklich, R.W.; Abdul-Baki, A.A. Variability in Metabolism of Individual Axes of Soybean Seeds and Its Relationship to Vigor 1. Crop Sci. 1975, 15, 424–426. [Google Scholar] [CrossRef]
- Maryam, A.; Nasreen, S. A review: Water logging effects on morphological, anatomical, physiological and biochemical attributes of food and cash crops. Int. J. Water Resour. Environ. Sci. 2012, 1, 113–120. [Google Scholar]
- Parolin, P. Seed germination and early establishment of 12 tree species from nutrient-rich and nutrient-poor Central Amazonian floodplains. Aquat. Bot. 2001, 70, 89–103. [Google Scholar] [CrossRef]
- Jitsuyama, Y. Hypoxia-responsive root hydraulic conductivity infuences soybean cultivar-specifc waterlogging tolerance. Am. J. Plant Sci. 2017, 8, 770. [Google Scholar] [CrossRef]
- Collaku, A.; Harrison, S.A. Losses in wheat due to waterlogging. Crop Sci. 2002, 42, 444–450. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and management strategies for crop production in Waterlogged/Flooded soils: A review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef]
- Wu, C.; Mozzoni, L.A.; Moseley, D.; Hummer, W.; Ye, H.; Chen, P.; Nguyen, H. Genome-wide association mapping of fooding tolerance in soybean. Mol. Breed. 2020, 40, 4. [Google Scholar] [CrossRef]


| Months and Metrological Events | January | February | March | April | May | June |
|---|---|---|---|---|---|---|
| Average temperature (°C) | 17.8 | 20.0 | 25.2 | 28.3 | 29.0 | 30.2 |
| Maximum temperature (°C) | 28.8 | 26.5 | 32.3 | 34.7 | 33.4 | 33.4 |
| Minimum temperature (°C) | 12.8 | 13.5 | 18.5 | 21.8 | 24.8 | 26.7 |
| Relative humidity (%) | 87 | 73 | 80 | 82 | 84 | 84.5 |
| Total precipitation (mm) | 31.8 | 2.3 | 16 | 40.2 | 290.5 | 416.3 |
| Soybean Varieties | Growing Condition | Plant Height (cm) | Pods Plant−1 | Pod Length (cm) | |||
|---|---|---|---|---|---|---|---|
| Basal K | Top Dress K | Basal K | Top Dress K | Basal K | Top Dress K | ||
| BU Soybean-1 | Control | 25.20 c | 24.28 c | 18.86 | 18.53 | 3.98 ab | 3.56 c |
| WL | 24.78 c | 24.48 c | 14.40 | 14.73 | 3.76 b | 3.63 c | |
| BU Soybean-2 | Control | 42.83 b | 41.20 b | 14.46 | 15.66 | 4.31 a | 3.78 b |
| WL | 48.14 a | 48.86 a | 14.93 | 16.20 | 4.16 a | 4.18 a | |
| Soybean Varieties | WL | Seeds Pod−1 | Seeds Plant−1 | 100-Seed Weight (g) | |||
|---|---|---|---|---|---|---|---|
| Basal K | Top Dress K | Basal K | Top Dress K | Basal K | Top Dress K | ||
| BU Soybean-1 | Control | 2.66 | 2.45 | 50.54 a | 45.48 ab | 11.81 c | 11.10 c |
| WL | 2.33 | 2.00 | 32.98 c | 30.40 c | 8.88 d | 9.75 d | |
| BU Soybean-2 | Control | 2.80 | 2.13 | 42.26 ab | 39.08 b | 19.73 a | 22.04 a |
| WL | 2.60 | 2.60 | 40.50 ab | 33.09 c | 13.81 c | 16.44 b | |
| Soybean Varieties | Flooding | Protein (%) | EC (μS cm−1 g−1) | Seed Weight (mg seed−1) | |||
|---|---|---|---|---|---|---|---|
| Basal K | Top Dress K | Basal K | Top Dress K | Basal K | Top Dress K | ||
| BU Soybean-1 | Control | 30.19 b | 31.48 b | 108 a | 82 c | 118.13 b | 111.06 b |
| WL | 28.85 c | 28.48 c | 89 c | 92 b | 88.86 c | 97.50 c | |
| BU Soybean-2 | Control | 38.60 a | 39.35 a | 106 b | 92 b | 197.33 a | 220.40 a |
| WL | 32.61 b | 31.83 b | 129 a | 125 a | 138.16 ab | 164.46 ab | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamun, M.A.A.; Julekha; Sarker, U.; Mannan, M.A.; Rahman, M.M.; Karim, M.A.; Ercisli, S.; Marc, R.A.; Golokhvast, K.S. Application of Potassium after Waterlogging Improves Quality and Productivity of Soybean Seeds. Life 2022, 12, 1816. https://doi.org/10.3390/life12111816
Mamun MAA, Julekha, Sarker U, Mannan MA, Rahman MM, Karim MA, Ercisli S, Marc RA, Golokhvast KS. Application of Potassium after Waterlogging Improves Quality and Productivity of Soybean Seeds. Life. 2022; 12(11):1816. https://doi.org/10.3390/life12111816
Chicago/Turabian StyleMamun, Muhammad Abdullah Al, Julekha, Umakanta Sarker, Muhammad Abdul Mannan, Mohammad Mizanur Rahman, Md. Abdul Karim, Sezai Ercisli, Romina Alina Marc, and Kirill S. Golokhvast. 2022. "Application of Potassium after Waterlogging Improves Quality and Productivity of Soybean Seeds" Life 12, no. 11: 1816. https://doi.org/10.3390/life12111816
APA StyleMamun, M. A. A., Julekha, Sarker, U., Mannan, M. A., Rahman, M. M., Karim, M. A., Ercisli, S., Marc, R. A., & Golokhvast, K. S. (2022). Application of Potassium after Waterlogging Improves Quality and Productivity of Soybean Seeds. Life, 12(11), 1816. https://doi.org/10.3390/life12111816









.jpg)


