1. Introduction
A requisite for the emergence of life on the early Earth was the presence of water and organic compounds—primarily amino acids, nucleobases, sugars, and their respective precursors [
1]. Sugars are considered one of the most essential molecules for all living organisms as they are vital in contemporary metabolism and the synthesis of other compounds, such as amino acids and nucleotides [
2]. The previous functions of these biomolecules suggest that they were synthesized during the initial stages of the origin of life; therefore, to gain insight into the chemical processes that might have taken place on early Earth, investigating the mechanisms of abiotic sugar production is essential. The formose reaction, which involves the condensation of formaldehyde under basic conditions, is the most well-known of the reaction pathways in sugar synthesis and is dependent on the presence of inorganic catalysts, most commonly CaCO
3 and Ca(OH)
2 [
3]. Alternative proposals for sugar synthesis include (A) anaerobic formose-like reactions with low-molecular-weight aldehydes in the presence of thiols [
4] and ammonia catalysts [
2,
5], and (B) aldehyde condensation reactions catalyzed by mineral surfaces [
3,
6,
7] Nevertheless, sugars are only by-products of these reactions, with the main organic compounds involving a wide variety of straight-chain and branched sugars and aldols. Since most of these reactions occur in environments that promote the breakdown of these organic compounds, studies must be carried out to propose alternate sugar synthesis reactions under plausible prebiotic conditions [
1,
8].
Researchers have proposed glyceraldehyde (C
3H
6O
3) as an alternative to formaldehyde in the formose reaction for the synthesis of sugars, acting as a source of energy and monomers in aldehyde condensation reactions. In addition, glyceraldehyde can lead to sugar and sugar-like molecules through condensation reactions catalyzed by mineral surfaces [
6] and possible nucleobases via reactions with ammonia under anaerobic conditions [
9]. Alternatively, when glyceraldehyde is exposed to ionizing radiation, it acts as a source of molecules relevant to abiotic synthesis [
10,
11]. The decomposition products include glycolaldehyde, formaldehyde, malondialdehyde, and pyruvaldehyde. Further, glyceraldehyde can originate from both internal and external sources. Dihydroxyacetone, the ketone form of this aldehyde, may have reached early Earth through exogenous delivery from cometary and meteoritic debris [
12,
13]. Conversely, a by-product of formose-like processes, glyceraldehyde is produced internally and may have originated from endogenous sources in the Earth’s early environment [
4].
All the previously discussed organic precursors could have been synthesized on Earth when the planet became habitable, as early as 10–20 million years after the Moon-forming impact [
14]. Abiotic synthesis might have taken place in aqueous solutions in hydrothermal systems, in the atmosphere, or at the water–mineral interface of the Earth’s surface environments [
14] during the Hadean (~4.54–4.0 Ga) and early Archean (~3.9 Ga) eons, when the planet’s atmosphere may have predominantly comprised CO
2 [
15,
16]. Among these possibilities, hydrothermal systems have physicochemical traits that are believed to be crucial for the development of life [
1]. Hydrothermal fluids are rich in ions, which can act as catalysts for chemical reactions and form metal complexes with organic molecules in solution [
17]. Additionally, studies have revealed that hydrothermal systems provide concentration, redox, and temperature gradients that can be utilized for the abiotic synthetic processes of biomolecule precursors [
1]. The thermal energy of these systems could also be used to start chemical reactions. Therefore, these geological environments are considered prime candidates for simulation experiments of abiotic synthesis under plausible early Earth geochemical conditions.
The Kverkfjöll Volcano and the Hveradalur Geothermal Area, Central Iceland: Analog of an Ancient Hydrothermal System
The Kverkfjöll volcano, located in Central Iceland, consists of a mountain massif of rocks of evolved basaltic composition, with an area of surface geothermal activity covering approximately 25 km
2. This area displays a wide range of temperatures, pH values, and chemical compositions and has been extensively investigated [
18]. Its western part, the Hveradalur geothermal area [
19], is home to hydrothermal fluids of low pH (1–5) and low temperatures (<50 °C). This zone is of particular interest to the present work, acting as an analog of a low-temperature primitive early Archean (~3.9 Ga) hydrothermal area (
Figure 1). Although representing a novel concept, using analog environments in prebiotic chemistry experiments shows great potential [
13], as it allows researchers to reproduce the physicochemical conditions of geological environments that may have existed on ancient Earth. Analog environments also provide insight into the possible mineralogy of early Earth environments by allowing investigations into the distribution of mineral phases under the physicochemical conditions of interest.
Goethite (FeOOH), an iron(III) oxyhydroxide, is one of the mineral phases that potentially existed on primitive Earth and is of special relevance in studies involving the abiotic synthesis of organic molecules. It can act as a catalyst for aldehyde condensation reactions [
6] and amino acid surface polymerization [
20] and, therefore, possibly plays an important role in the abiotic synthesis of biomolecule precursors. Research has indicated that the sedimentary deposits of the early hydrothermal systems on Earth may have contained this mineral [
21,
22] Further, reference [
19] reported the formation of goethite by hydrothermal modification of an iron meteorite under simulated hydrothermal conditions, thus demonstrating the mineral’s stability under conditions of low pH and high temperatures.
In the present work, we took quantitative measurements of remnant DL-glyceraldehyde in sample solutions after exposure to the simulated physicochemical conditions of an analog of a primitive Earth hydrothermal area, the Kverkfjöll geothermal field, using goethite as a catalyst for the mineral–water interface reactions. Through these measurements, we aimed to investigate the stability of glyceraldehyde and identify its decomposition products.
2. Materials and Methods
2.1. Reagents
All the reagents were of the highest commercial purity available, obtained from Sigma-Aldrich
®, St. Louis, MO, USA, and included the following: DL-glyceraldehyde (>90%), acetaldehyde (>99.5%), pyruvaldehyde (40 wt. % in H
2O), glyoxal (40 wt. % in H
2O), glycolaldehyde (>90%), orcinol (>99%), D-glucose (99.9%), D-ribose (99.9%), and FeCl
3 (95%). Methanol-free formaldehyde was prepared from paraformaldehyde according to the method described by [
23]. Acetonitrile (HPLC), ethanol (HPLC), H
2SO
4 (95–97%), HCl (37 wt. % in H
2O), HNO
3 (70%), KOH (>85%), and 2,4-dinitrophenylhydrazine (DNPH) (97%) were obtained from Merck Co.
®, Kenilworth, NJ, USA. Natural iron(III) hydroxide oxide samples were collected in Durango, Mexico, and were characterized by X-ray diffraction, Raman, and X-ray Photoemission spectroscopy (XPS). To eliminate organic contamination, the goethite samples were treated with a 3% HNO
3 solution (acid wash), followed by two additional washes with Milli-Q
® (Merck Millipore, Burlington, MA, USA) water (deionized) and a 3% KOH solution. After the acidic and basic washes, the mineral samples were rinsed with Milli-Q
® water and dried in a desiccator at room temperature for 24 h.
2.2. Preparation of Samples
To simulate the Hveradalur geothermal site’s physicochemical conditions, a 1 × 10−2 mol/L, aqueous DL-glyceraldehyde solution was prepared using deionized water; the solution was acidified with 36 mM H2SO4 (pH 2). The samples were degasified with Ar to remove dissolved O2 in the solution. For the mineral–water interface reactions in the simulated environment, the system was mixed in 15-mL polyallomer centrifuge tubes with mineral (Goethite) powder (Beckman Coulter®, Brea, CA, USA). Goethite powder (180 mg), previously prepared from natural mineral samples by mechanical grinding in an agate mortar to an approximate grain size of 125 nm, was combined with 5 mL aliquots of the degasified acidic DL-glyceraldehyde standard solutions. The system was saturated with argon to create an anoxic atmosphere, and the polyallomer tubes were sealed to preserve the system’s atmospheric integrity. For the XPS measurements, small Goethite sheets (5 × 5 mm) were prepared. The sheets’ surfaces were polished with a SiC paste and cleaned with an additional acidic and basic wash.
2.3. Sorption Experiments
The DL-glyceraldehyde/goethite samples were heated to 50 °C by placing the polyallomer tubes inside a water recirculation system. The experimental system was exposed to this thermal treatment at defined time intervals (120, 240, 360, and 480 min). After each time interval had elapsed, the mineral was separated from the aqueous phase using an Allegra XL-90 centrifuge (Beckman Coulter®, Brea, CA, USA) at 25,000 rpm at room temperature for 15 min. The goethite powder was desiccated and preserved for further analysis. The collected supernatants were filtered using 12-µm Acrodisc syringe filters (Whatman, Chicago, IL, USA) and stored at 0 °C for the subsequent HPLC measurements. The formation of new organic compounds in the sample supernatants was monitored by UV–Vis spectrophotometry, HPLC–UV, and HPLC-ESIMS; this sample analysis was repeated for each time interval used in the sorption experiments. For the sorption on goethite sheets, the same experimental procedure was repeated, combining the goethite sheet with 5 mL aliquots of DL-glyceraldehyde standard solutions, modifying the time intervals for the thermal treatment (300 and 600 min).
2.4. Carbonyl Compound Measurements
Using HPLC–UV, the aldehydes, as their 2,4-dinitrophenylhydrazine (DNPH) derivatives, were identified. The derivatization of the carbonyl compounds required reacting 5 mL aliquots of the sample supernatants with 5 mL aliquots of the DNPH reagent (0.4 mg DNPH dissolved in 2 mL H2SO4, 3 mL H2O, and 25 mL of ethanol) for 12 h. The carbonyl derivatives precipitated as yellow-orange crystals, which were filtered, dried, recrystallized, and redissolved in acetonitrile for subsequent analysis. The elution times of the carbonyl DNPH derivatives of formaldehyde, acetaldehyde, glyoxal, pyruvaldehyde, and DL-glyceraldehyde (as standard compounds) were measured.
2.5. Analysis of Samples
2.5.1. HPLC–UV Analysis
For the HPLC–UV analysis of the carbonyl compounds, we used a Knauer
®, Berlin, Germany) Azura P 4.1S HPLC pump equipped with a Knauer
® Azura UVD 2.1S UV detector. The derivatives of the carbonyl compounds were monitored by measuring their absorbance at 360 nm. The derivatives were separated in a Beckman
® Ultrasphere C-18 ODS column (250 × 4.6 mm) using isocratic elution at 1.0 mL/min with a mobile phase comprising 70% acetonitrile and 30% water. The elution times of the standard carbonyl–DNPH derivatives are presented in
Table 1.
The elution times in the sample supernatants, which were unknown, were identified by comparing them with those of the standard aldehydes and carbohydrates. The quantitation of the remnant DL-glyceraldehyde in the experimental samples was determined by measuring the absorbance of DL-glyceraldehyde–DNPH at 360 nm as a function of time, employing the Beer–Lambert law to calculate the sample concentration. The calibration curve was constructed using the glyceraldehyde–DNPH standards, with a concentration interval of 1 × 10−3 to 1 × 10−5 mol/L.
2.5.2. UV–Vis Spectroscopy
For the UV–Vis spectroscopic analysis, we used a Cary 100 spectrophotometer (Varian, Palo Alto, CA, USA). The supernatants of the DL-glyceraldehyde solutions after sorption were analyzed, monitoring their absorbance at 285 nm, which corresponds to DL-glyceraldehyde in solution. A concentrated (0.2 mol/L) solution was used to increase the sensitivity of the analyte to UV detection.
2.5.3. X-ray Diffraction Analysis
The purity of the natural goethite samples was measured by X-ray diffraction (XRD) using an Empyrean diffractometer (Malvern Panalytical®, Malvern, Worcs, UK) equipped with a PIXcel3D detector, with filtered Fe radiation of 60 kV at 2θ angles of 4° to 70°. The samples were prepared by grinding the goethite with an agate mortar to a particle size of less than <75 µm. The mineral powder was then pressed onto the diffractometer’s aluminum sample holder. The software HighScore (Malvern Panalytical®, Malvern, Worcs, UK), the Inorganic Crystal Structure Database (ICSD), and the International Center for Diffraction Data (ICDD) database were used for the mineral phase identification of the measured samples.
2.5.4. Raman Spectroscopy Analysis
To study goethite and DL-glyceraldehyde, Raman spectroscopy was used to record their spectra. This analytical technique was also used to study the adsorption of organic compounds on the mineral surface. The experimental samples, in powder form, were pressed between two NaCl discs, and the Raman probe was used to collect the spectra of the samples. The Raman spectra were recorded using an Optosky ATR 3000 portable Raman spectrometer. The Raman probe containing a Class IIIB laser was positioned at an operating distance from the sample of 6 mm; the laser power was kept at a constant power value of 400 mW to avoid damage to the sample. The Raman frequencies were accurate to ±5 cm−1.
2.5.5. XPS Analysis
The XPS spectra of the goethite mineral sample were recorded before glyceraldehyde adsorption and after adsorption for 300 and 600 min from the solution. The XPS analysis of the samples was carried out in an ultra-high vacuum chamber equipped with a hemispherical electron analyzer using an Al Kα X-ray source (1486.6 eV) with an aperture of 7 mm × 20 mm. The base pressure in the chamber was 5 × 10−8 mbar, and the experiments were performed at room temperature. The peak decomposition in different components was shaped, after background subtraction, as a convolution of Lorentzian and Gaussian curves. For deconvolution, we applied the criterion of using the lowest number of components for the fit. Binding energies were calibrated against the adventitious carbon component set to 285.0 eV for the goethite samples. The following core-level peaks were recorded under the same experimental conditions: C (1s), Fe (2 p3/2), and O (1s). We did not observe any beam radiation damage to the samples’ surfaces during the data acquisition.
2.5.6. HPLC-ESIMS Analysis (Sugar-like Compound Measurements)
We define sugar-like compounds as molecules with five or six carbon atoms and multiple OH/CO substituents and isomers of carbohydrates. For the analysis of these molecules, HPLC–ESIMS was used. The analysis was carried out with a Waters® 515 HPLC pump coupled with a Waters® SQ-2 Single Quadrupole Mass Detector system, with electrospray ionization in negative (ESI-) mode. The cone voltage was 23 V, the capillary voltage was 1.46 kV, and the desolvation temperature was 350 °C. The sugar-like compounds were separated in a GL Sciences®, Torrance, CA, USA Inerstil NH2 5 μm column (4.6 × 250 mm), specifically designed for the separation of carbohydrates, using isocratic elution at 0.8 mL/min with a mobile phase comprising 80% acetonitrile and 20% water.
4. Discussion
Investigations into the stability of DL-glyceraldehyde in aqueous solutions have seldom been conducted, and those that have focused on the role of this organic compound as a source of aldehyde monomers in autocatalytic reactions in sugar synthesis [
5,
6,
9] and its stability under high-energy radiation fields in simulated prebiotic environments [
10,
11]. The novelty of the current work lies in the insight provided into the possible role of DL-glyceraldehyde as a source of aldehydes and C5/C6 compounds in simulated hydrothermal surface areas, where acidic conditions and variable temperatures are dominant. DL-glyceraldehyde in solution undergoes interconversion between different chemical species that exist in a complex equilibrium [
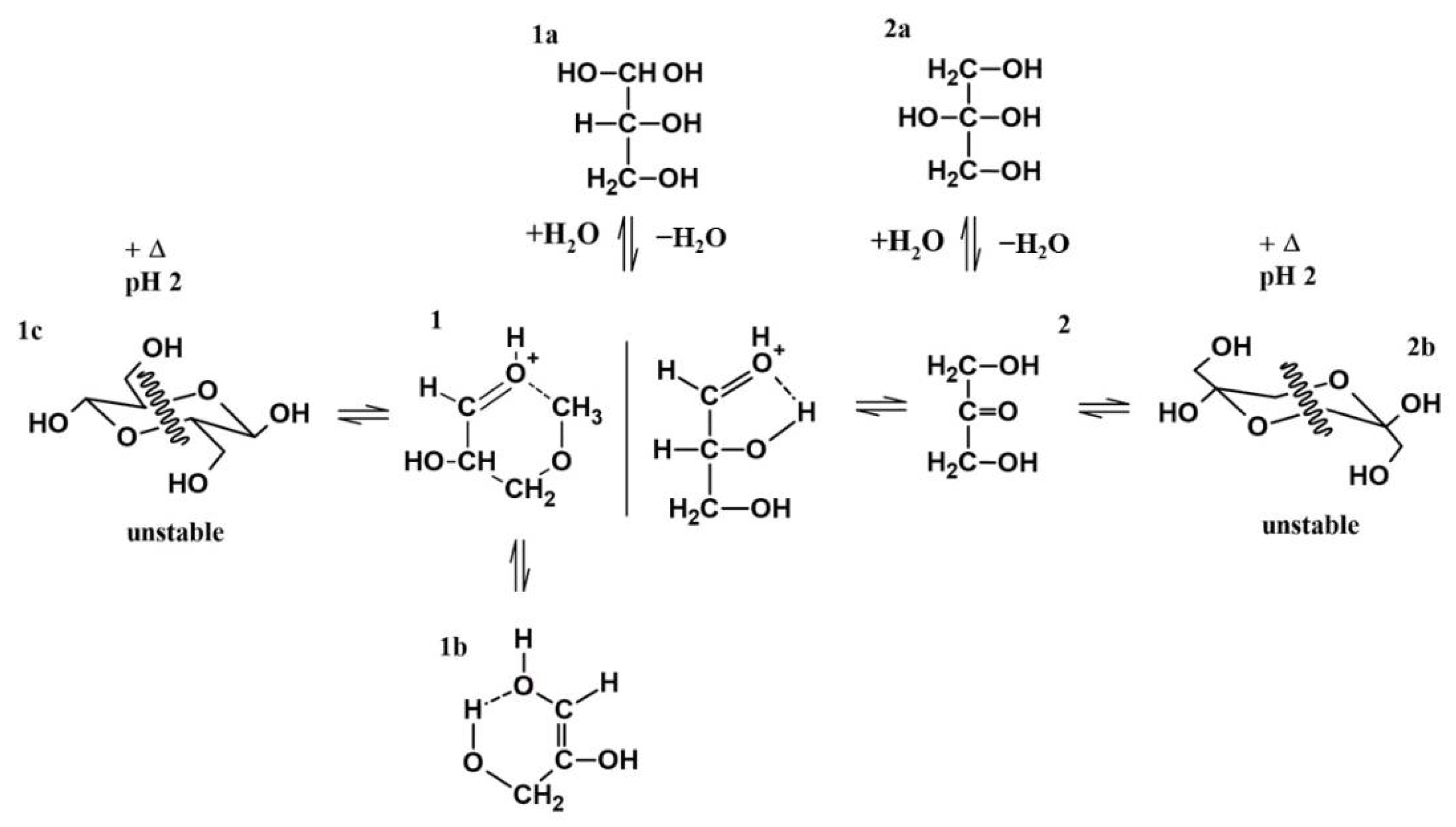
24]. Under the experimental conditions, the dimeric cyclic hemiacetal forms of DL-glyceraldehyde are unstable; consequently, the keto and enol species are dominant (
Figure 15). Intramolecular hydrogen bonds between the carbonyl carbon and the C-2 and C-3 hydroxyl hydrogens (1) stabilize the ketone form, shifting the equilibrium toward these species and ensuring that the enediol (1b) and hydrated forms (1a) are not dominant in solution. Therefore, it is highly probable that these molecules are not the dominant form of glyceraldehyde present in the solution. This chemical equilibrium is ubiquitous, regardless of whether the sample is in crystalline form or solution [
24]. The Raman spectrum shows that crystalline DL-glyceraldehyde exists as the enol form (
Figure 16, 1b), which contains the functional group C=C. The UV absorption peak of 285 nm (
Figure 9) indicates the presence of this isomer in the solution. [
29]. Derivatization with DNPH of DL-glyceraldehyde in solution shows that the carbonyl isomer is formed when the crystalline form is dissolved in water (
Figure 12). Due to the complex keto enol tautomerism of the sample in solution, additional carbonyl compounds are present. However, the concentration of the impurities is low enough when compared to DL-glyceraldehyde-DNPH.
The decomposition products of DL-glyceraldehyde, when under acidic thermal conditions, are formaldehyde (CH
2O), acetaldehyde (C
2H
4O), glyoxal (C
2H
2O
2), and pyruvaldehyde (C
3H
4O
2) (
Figure 11). Additionally, one C5 and four C6 compounds, isomers of pentose and hexose with a molecular weight of 150 and 180 g/mol, respectively, were detected in the experimental samples (
Figure 14). The formation of these isomers can be explained by the acid-catalyzed aldol condensation of the aldehydes with their respective enol forms. The protonated DL-glyceraldehyde reacts via the addition of 1,2-enediol (glyoxal isomer), leading to an aldol condensation product, a pentose isomer (
Figure 16a). The addition of the keto and aldol forms of DL-glyceraldehyde can yield hexose isomers as condensation products (
Figure 16b).
The instability of this molecule in the experimental conditions is due to the displacement of the hemiacetal equilibrium. In the presence of heat and aqueous acid, the formation of hemiacetals, which act as protective chemical groups of carbonyl compounds through the reduction of their reactivity, is hindered. This process increases the reactivity of the linear carbonyl compounds in solution, which, therefore, promotes the synthesis of different organic compounds. Nevertheless, the aldol condensation reaction is not selective to specific C5 and C6 compounds, and multiple products could form via crossed aldol reactions (i.e., condensation between two different aldehydes/ketones [
30]). This is demonstrated by the detection of the C5′ and C6
1–4′ compounds (
Figure 14) which are isomers of a pentose and a hexose. The lack of selectivity towards specific carbohydrates will yield a mixture of sugar-like compounds. The initial concentration of the stock solution hinders the formation of these compounds. C5′ and C6
1–4′ were detected only when the concentration of the stock DL-glyceraldehyde solution was increased to 0.2 mol/L, up from 0.01 mol/L. A high concentration of the initial aldehyde is required to obtain high yields of the C5′ and C6
1–4′ compounds. Therefore, the total yield of this compound must be low. In a hot, acidic environment, most of the DL-glyceraldehyde readily decomposes into low-molecular-weight aldehydes (<90 g/mol). Further analysis with higher-resolution techniques is required to reveal the structural features of the detected compounds.
Previous studies have investigated the role of goethite in carbohydrate synthesis. For example, reference [
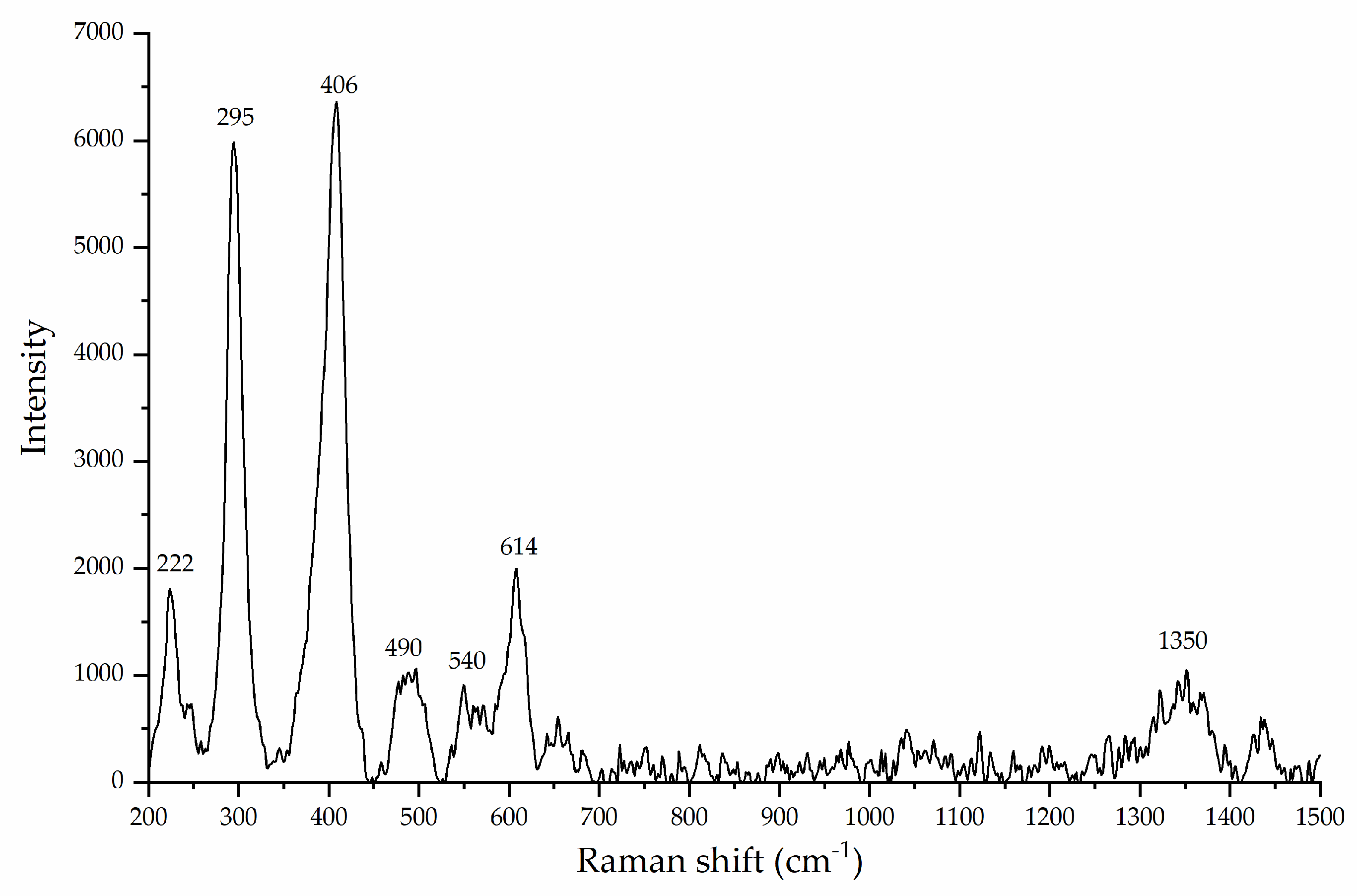
6] proposed the formation of C6 compounds via the catalytic action of goethite, which occurs through the aldol condensation of DL-glyceraldehyde with a mineral-stabilized glyceraldehyde enediolate on the surface of the iron(III) oxide hydroxide. Exhaustive analysis of the peak frequencies in goethite’s Raman spectrum after exposure to solutions of DL-glyceraldehyde 0.01 mol/L (
Figure 5) shows that two of the vibrational peaks (406 and 550 cm
−1) of goethite overlap with the vibrational peaks of DL-glyceraldehyde, which are associated with skeletal deformations of the C=C bond of the triose enol form. In particular, the 550 cm
−1 band of goethite increases in intensity, while the 406 cm
−1 band widens. These changes in the Raman spectrum suggest the adsorption of a DL-glyceraldehyde isomer (enol) on the surface of goethite. Regarding the XPS data, two binding energies are of special interest: 288.5 eV of core level C (1s) and 533.3 eV of core level O (1s). The increase in the intensity of the 288.5 eV component (associated with a carboxyl group) when the thermal exposure time is increased to 300 min can be explained by the adsorption of a carbonyl compound on the surface of goethite. The decrease exhibited when the thermal exposure time is further increased could be attributed to the decomposition of the same molecule. Due to the keto-enol equilibrium and decomposition of DL-glyceraldehyde (
Figure 15), the adsorbed molecule could be either DL-glyceraldehyde, dihydroxyacetone, or one of the aldehydes formed by its decomposition (
Figure 11). Regarding the 533.3 eV component, the increase in the intensity, proportional to the increase in thermal exposure time, can be explained by the gradual adsorption of RCOH-rich compounds on the mineral surface or by the decomposition compounds, which will continue to contribute to the increase in the same oxygen component (C-O, C-O-H). However, the exact chemical formulas of these compounds remain unknown. Considering the molecules formed in the solution due to the keto-enol tautomerism and decomposition of DL-glyceraldehyde, the adsorbed molecules could be either C5/C6 compounds, an enol isomer, an aldehyde, or hydrated glyceraldehyde/dihydroxyacetone.
According to the literature [
6], the adsorption of DL-glyceraldehyde on the surface of goethite occurs due to the formation of an Fe
3+-O
2R bond between two OH groups of the glyceraldehyde enol isomer. However, neither the Raman nor XPS spectrum shows evidence of vibrational peaks associated with the formation of an organometallic bond. Therefore, the exact mechanism by which the organic compounds are adsorbed on the surface of the goethite samples remains elusive.
The existence of Fe
3+ over the reduced Fe
2+ form in an acidic aqueous environment can be explained by aqueous corrosion mechanisms, which can occur in both oxygenic and anoxic environments [
31]. In the presence of oxygen, goethite (and Fe
3+ oxyhydroxides) can form via the corrosion of Fe/Ni deposits or iron meteorites. Fe
0 can be oxidized by two oxidizing agents, O
2 and H
+, degrading the initial material into α- and β-FeO(OH) [
32]. In acidic deaerated solutions, protons act exclusively as the oxidizing agent. Previous works provided evidence of the formation of hematite and goethite in an anaerobic acid environment [
19,
33], which confirms the ability of hot, low-pH environments to oxidize Fe
0 to Fe
3+. Goethite (and hematite) are considered the end members of many iron transformation routes due to their thermodynamic stability. Therefore, conversion to additional iron mineral phases is not expected [
32].
Implications for Prebiotic Chemistry
Under the simulated hydrothermal conditions, formaldehyde, acetaldehyde, glyoxal, and pyruvaldehyde form via the thermal decomposition of DL-glyceraldehyde. Formaldehyde is considered the cornerstone organic molecule for carbohydrate synthesis via the formose reaction [
3]. Strecker synthesis with acetaldehyde and ammonia can be used as a source for alanine [
33] while glyoxal and pyruvaldehyde are usually categorized as intermediates in formose-like reaction pathways [
4]. The results of the current work show that DL-glyceraldehyde could have been a prebiotic source of monomers in primitive hydrothermal environments, molecules that, under variable pH conditions, could act as energy sources for amino acid and carbohydrate synthesis [
34]. Nevertheless, the aldol condensation of DL-glyceraldehyde provides an additional source of C5 and C6 compounds in hydrothermal environments. The possible pathway that can lead to the synthesis of these organic compounds is the acid-catalyzed aldol condensation between DL-glyceraldehyde and enol molecules. However, the yield of these acid-catalyzed aldol condensation reactions is low.
As mentioned earlier, researchers generally believe that the prebiotic synthesis of carbohydrates and carbohydrate precursors involved the condensation of low-molecular-weight aldehydes under basic conditions (e.g., pH > 11) in the presence of inorganic catalysts [
1,
13]. The synthesis of C5 and C6 compounds under acidic conditions seems notably more plausible than the reaction pathways commonly proposed, as surface hydrothermal systems were common during the early Archean [
15,
35]. Regarding goethite, prebiotic sources of this mineral could have arisen from the photooxidation of dissolved Fe(II), [
6] and aqueous alterations of iron-rich rocks and minerals [
19,
22]. Therefore, it is highly likely that this mineral was common in the sediments of ancient hydrothermal regions [
20,
21].