Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cellular Viability Assessment
2.4. Cellular Morphology
2.5. Immunofluorescence
2.6. Chorioallantoic Membrane (CAM) Assay
2.7. Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Assay
2.8. Combination Index Calculation
2.9. Statistical Analysis
3. Results
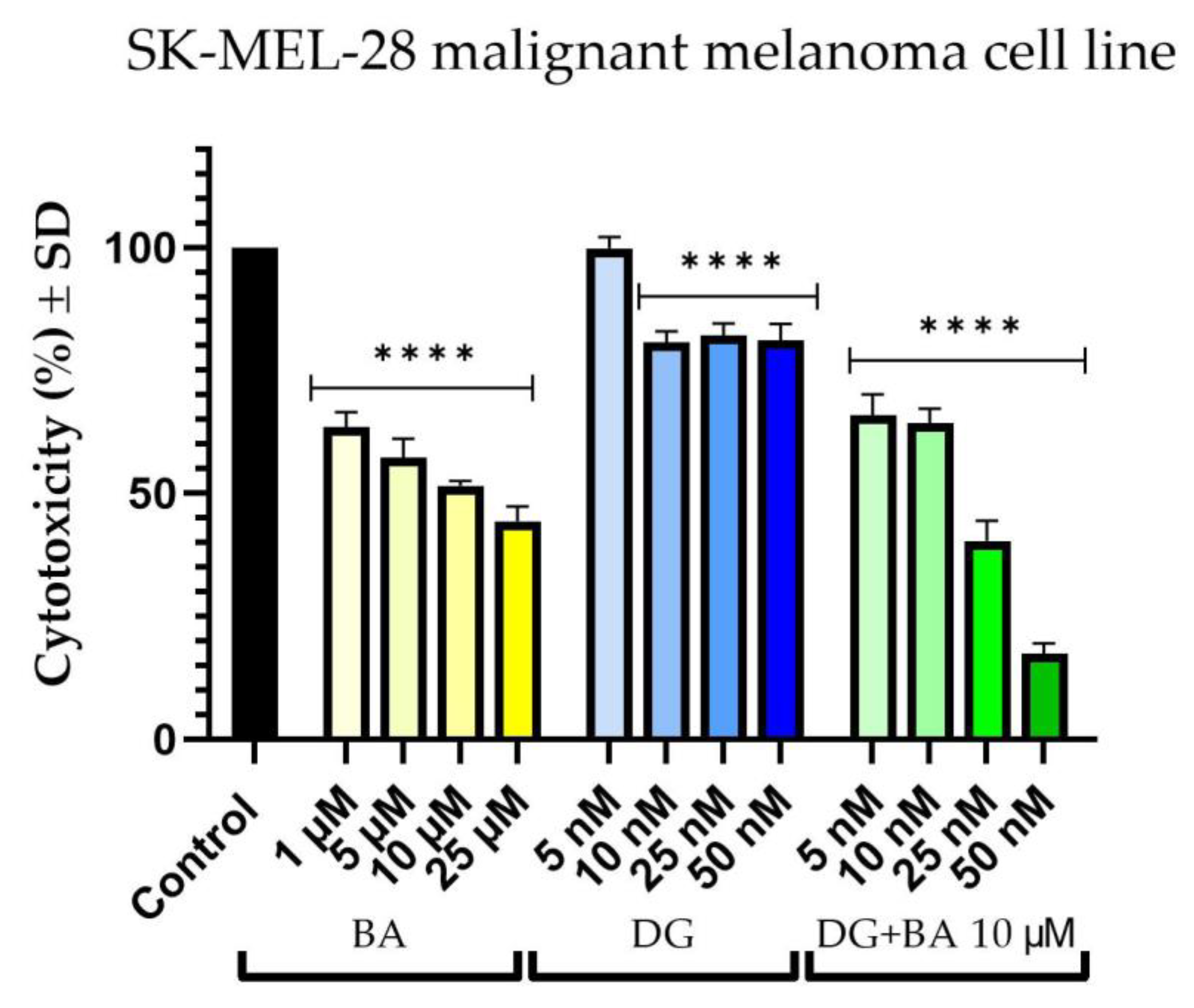
3.1. Cellular Viability Assessment
3.2. Cellular Morphology
3.3. Immunofluorescence
3.4. Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Assay
3.5. Combination Index Calculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deo, S.V.S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.H.; Mir, M.; Qian, L.; Baloch, M.; Ali Khan, M.F.; Rehman, A.; Ngowi, E.E.; Wu, D.-D.; Ji, X.-Y. Skin Cancer Biology and Barriers to Treatment: Recent Applications of Polymeric Micro/Nanostructures. J. Adv. Res. 2022, 36, 223–247. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Fijałkowska, M.; Koziej, M.; Antoszewski, B. Detailed Head Localization and Incidence of Skin Cancers. Sci. Rep. 2021, 11, 12391. [Google Scholar] [CrossRef] [PubMed]
- Domingues, B.; Lopes, J.M.; Soares, P.; Pópulo, H. Melanoma Treatment in Review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, N.; Nawaz, T.; Aziz, T. Molecular Mechanisms of Sanguinarine in Cancer Prevention and Treatment. Anticancer. Agents Med. Chem. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Isaković-Vidović, S.; Dariš, B.; Knez, Ž.; Vidović, K.; Oprić, D.; Ferk, P. Antiproliferative Activity of Selected Triterpene Acids from Rosemary on Metastatic Melanoma Cell Line WM-266-4. Open Access Maced. J. Med. Sci. 2021, 9, 515–521. [Google Scholar] [CrossRef]
- Weber, L.A.; Meißner, J.; Delarocque, J.; Kalbitz, J.; Feige, K.; Kietzmann, M.; Michaelis, A.; Paschke, R.; Michael, J.; Pratscher, B.; et al. Betulinic Acid Shows Anticancer Activity against Equine Melanoma Cells and Permeates Isolated Equine Skin in Vitro. BMC Vet. Res. 2020, 16, 44. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M. Discovery of Betulinic Acid as a Selective Inhibitor of Human Melanoma That Functions by Induction of Apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Nepovimova, E.; Wu, W.; Kuca, K. Digoxin: Pharmacology and Toxicology—A Review. Environ. Toxicol. Pharmacol. 2020, 79, 103400. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-H.; Tsai, M.-F.; Su, K.-Y.; Wu, S.-G.; Huang, C.-P.; Yu, S.-L.; Yu, Y.-L.; Lan, C.-C.; Yang, C.-H.; Lin, S.-B.; et al. Slug Confers Resistance to the Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. Am. J. Respir. Crit. Care Med. 2011, 183, 1071–1079. [Google Scholar] [CrossRef]
- Tahervand, A.; Parnian, B.; Habibi Roudkenar, M.; Mohammadi Roushandeh, A. Digoxin Effectively Inhibited Cell Growth and Induced Senescence in Cervical Cancer Cell Line. Gene Cell Tissue 2017, 4, e14216. [Google Scholar] [CrossRef] [Green Version]
- Eskiocak, U.; Ramesh, V.; Gill, J.G.; Zhao, Z.; Yuan, S.W.; Wang, M.; Vandergriff, T.; Shackleton, M.; Quintana, E.; Frankel, A.E.; et al. Erratum: Synergistic Effects of Ion Transporter and MAP Kinase Pathway Inhibitors in Melanoma. Nat. Commun. 2016, 7, 13080. [Google Scholar] [CrossRef]
- Frankel, A.E.; Eskiocak, U.; Gill, J.G.; Yuan, S.; Ramesh, V.; Froehlich, T.W.; Ahn, C.; Morrison, S.J. Digoxin Plus Trametinib Therapy Achieves Disease Control in BRAF Wild-Type Metastatic Melanoma Patients. Neoplasia 2017, 19, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.S.; Molenaar, P.; Teng, J.; Cornelissen, F.M.G.; Roelofs, I.; Menezes, R.; Dik, R.; Lagerweij, T.; Broersma, Y.; Petersen, N.; et al. A Cancer Drug Atlas Enables Synergistic Targeting of Independent Drug Vulnerabilities. Nat. Commun. 2020, 11, 2935. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.; Sorger, P.K. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell 2017, 171, 1678–1691.e13. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Xie, H.; Shen, W.; Shao, L.; Zeng, L.; Huang, X.; Zhu, Q.; Zhai, X.; Li, K.; Qiu, Z.; et al. The Synergism of Natural Compounds and Conventional Therapeutics against Colorectal Cancer Progression and Metastasis. FBL 2022, 27, 263. [Google Scholar] [CrossRef]
- Lin, S.-R.; Fu, Y.-S.; Tsai, M.-J.; Cheng, H.; Weng, C.-F. Natural Compounds from Herbs That Can Potentially Execute as Autophagy Inducers for Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 1412. [Google Scholar] [CrossRef]
- Iqbal, H.; Menaa, F.; Khan, N.U.; Razzaq, A.; Khan, Z.U.; Ullah, K.; Kamal, R.; Sohail, M.; Thiripuranathar, G.; Uzair, B.; et al. Two Promising Anti-Cancer Compounds, 2-Hydroxycinnaldehyde and 2-Benzoyloxycinnamaldehyde: Where Do We Stand? Comb. Chem. High Throughput Screen. 2022, 25, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Jorge Murillo, G.; Pérez, U.M.; Tur, E.N.; Portuondo, D.F.; Martínez, B.T.; Téllez-Martínez, D.; Betancourt, J.E.; Pérez, O. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.M.; Macasoi, I.; Paul, C.; Radulescu, M.; Buzatu, R.; Watz, C.G.; Cheveresan, A.; Berceanu, D.; Pinzaru, I.; Dinu, S.; et al. Methotrexate and Cetuximab-Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments. Medicina 2022, 58, 167. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzillo, A.; Ragone, A.; Spina, A.; Naviglio, S.; Sapio, L. Chlorogenic Acid Enhances Doxorubicin-Mediated Cytotoxic Effect in Osteosarcoma Cells. Int. J. Mol. Sci. 2021, 22, 8586. [Google Scholar] [CrossRef]
- Stenkvist, B.; Bengtsson, E.; Eriksson, O.; Holmquist, J.; Nordin, B.; Westman-Naeser, S.; Eklund, G. Cardiac Glycosides and Breast Cancer. Lancet 1979, 313, 563. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Q.; Zhang, S.; Liu, H.; Zhao, B.; Du, B.; Wang, W.; Lin, P.; Zhang, Z.; Zhong, Y.; et al. Digoxin Enhances the Anticancer Effect on Non-Small Cell Lung Cancer While Reducing the Cardiotoxicity of Adriamycin. Front. Pharmacol. 2020, 11, 186. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Li, X.; Wu, W.; Liu, S.; Shen, M.; Zhang, Z.; He, J. Digoxin Reduces the Incidence of Prostate Cancer but Increases the Cancer-Specific Mortality: A Systematic Review and Pooled Analysis. Andrologia 2021, 53, e14217. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Yan, J.Y.; Han, X.C.; Niu, F.L.; Zhang, J.H.; Hu, W.N. Anti-Proliferative Effect of Digoxin on Breast Cancer Cells via Inducing Apoptosis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5837–5842. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, R.; Pezzuto, J.M. Betulinic Acid-Induced Programmed Cell Death in Human Melanoma Cells Involves Mitogen-Activated Protein Kinase Activation. Clin. Cancer Res. 2003, 9, 2866–2875. [Google Scholar]
- Potze, L.; Mullauer, F.B.; Colak, S.; Kessler, J.H.; Medema, J.P. Betulinic Acid-Induced Mitochondria-Dependent Cell Death Is Counterbalanced by an Autophagic Salvage Response. Cell Death Dis. 2014, 5, e1169. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, A.M.; Meléndez, C.M.; Uribe, D.; Pedroza-Díaz, J. Synergistic Effects of Natural Compounds and Conventional Chemotherapeutic Agents: Recent Insights for the Development of Cancer Treatment Strategies. Heliyon 2022, 8, e09519. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.; Rotshteyn, Y.; Rabadi, L.; Parikh, R.; Bullock, P. Comparison of Alamar Blue and MTT Assays for High Through-Put Screening. Toxicol. Vitr. 2004, 18, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Zhang, A.; Franco, D.; Gupta, S. Betulinic Acid-Mediated Apoptosis in Human Prostate Cancer Cells Involves P53 and Nuclear Factor-Kappa B (NF-ΚB) Pathways. Molecules 2017, 22, 264. [Google Scholar] [CrossRef] [Green Version]
- Zeng, A.-Q.; Yu, Y.; Yao, Y.-Q.; Yang, F.-F.; Liao, M.; Song, L.-J.; Li, Y.-L.; Yu, Y.; Li, Y.-J.; Deng, Y.-L.; et al. Betulinic Acid Impairs Metastasis and Reduces Immunosuppressive Cells in Breast Cancer Models. Oncotarget 2018, 9, 3794–3804. [Google Scholar] [CrossRef] [Green Version]
- Zeng, A.; Hua, H.; Liu, L.; Zhao, J. Betulinic Acid Induces Apoptosis and Inhibits Metastasis of Human Colorectal Cancer Cells in Vitro and in Vivo. Bioorg. Med. Chem. 2019, 27, 2546–2552. [Google Scholar] [CrossRef]
- Pfarr, K.; Danciu, C.; Arlt, O.; Neske, C.; Dehelean, C.; Pfeilschifter, J.M.; Radeke, H.H. Simultaneous and Dose Dependent Melanoma Cytotoxic and Immune Stimulatory Activity of Betulin. PLoS ONE 2015, 10, e0118802. [Google Scholar] [CrossRef]
- Gheorgheosu, D.; Jung, M.; Bilge, Ö.; Schmid, T.; Dehelean, C. Betulinic Acid Suppresses NGAL-Induced Epithelial-to-Mesenchymal Transition in Melanoma. Biol. Chem. 2013, 394, 773–781. [Google Scholar] [CrossRef]
- Coricovac, D.; Dehelean, C.A.; Pinzaru, I.; Mioc, A.; Aburel, O.-M.; Macasoi, I.; Draghici, G.A.; Petean, C.; Soica, C.; Boruga, M.; et al. Assessment of Betulinic Acid Cytotoxicity and Mitochondrial Metabolism Impairment in a Human Melanoma Cell Line. Int. J. Mol. Sci. 2021, 22, 4870. [Google Scholar] [CrossRef]
- Selzer, E.; Pimentel, E.; Wacheck, V.; Schlegel, W.; Pehamberger, H.; Jansen, B.; Kodym, R. Effects of Betulinic Acid Alone and in Combination with Irradiation in Human Melanoma Cells. J. Investig. Dermatol. 2000, 114, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective Cytotoxicity of Betulinic Acid on Tumor Cell Lines, but Not on Normal Cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Fulda, S.; Jeremias, I.; Pietsch, T.; Debatin, K.M. Betulinic Acid: A New Chemotherapeutic Agent in the Treatment of Neuroectodermal Tumors. Klin. Padiatr. 1999, 211, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Jeremias, I.; Steiner, H.H.; Pietsch, T.; Debatin, K.M. Betulinic Acid: A New Cytotoxic Agent against Malignant Brain-Tumor Cells. Int. J. Cancer 1999, 82, 435–441. [Google Scholar] [CrossRef]
- Lines, C.; Wr, P.; Cabaj, J.; Weronika, B. Additive Interactions between Betulinic Acid and Two Taxanes in In Vitro Tests against Four Human Malignant Melanoma. Int. J. Mol. Sci. 2022, 23, 9641. [Google Scholar]
- Adams, K.F.J.; Patterson, J.H.; Gattis, W.A.; O’Connor, C.M.; Lee, C.R.; Schwartz, T.A.; Gheorghiade, M. Relationship of Serum Digoxin Concentration to Mortality and Morbidity in Women in the Digitalis Investigation Group Trial: A Retrospective Analysis. J. Am. Coll. Cardiol. 2005, 46, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Chou, J.-C.; Li, J.-H.; Chen, C.-C.; Chen, C.-W.; Lin, H.; Wang, P.S. Inhibitory Effects of Digoxin and Digitoxin on Cell Growth in Human Ovarian Cancer Cell Line SKOV-3. Integr. Cancer Ther. 2021, 20, 15347354211002662. [Google Scholar] [CrossRef]
- Syahputra, R.A.; Harahap, U.; Dalimunthe, A.; Nasution, M.P.; Satria, D. Drug Therapy Monitoring (TDM) of Digoxin: Safety and Efficacy Review. Pharmacia 2022, 69, 261–264. [Google Scholar] [CrossRef]
- Levy, A. Correlation between In-Vitro and In-Vivo Studies Based on Pharmacokinetic Considerations. Am. J. Biomed. Sci. Res. 2020, 8, 48–50. [Google Scholar] [CrossRef]
- Clarke, P.G.; Clarke, S. Nineteenth Century Research on Naturally Occurring Cell Death and Related Phenomena. Anat. Embryol. 1996, 193, 81–99. [Google Scholar] [CrossRef]
- Thuret, G.; Chiquet, C.; Herrag, S.; Dumollard, J.M.; Boudard, D.; Bednarz, J.; Campos, L.; Gain, P. Mechanisms of Staurosporine Induced Apoptosis in a Human Corneal Endothelial Cell Line. Br. J. Ophthalmol. 2003, 87, 346–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, U.; Groscurth, P. Morphological Features of Cell Death. News Physiol. Sci. 2004, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective Assessment of Changes in Nuclear Morphology and Cell Distribution Following Induction of Apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toné, S.; Sugimoto, K.; Tanda, K.; Suda, T.; Uehira, K.; Kanouchi, H.; Samejima, K.; Minatogawa, Y.; Earnshaw, W.C. Three Distinct Stages of Apoptotic Nuclear Condensation Revealed by Time-Lapse Imaging, Biochemical and Electron Microscopy Analysis of Cell-Free Apoptosis. Exp. Cell Res. 2007, 313, 3635–3644. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Zhao, W.; Cao, L.; Huang, J. Involvement of the Actin Machinery in Programmed Cell Death. Front. Cell Dev. Biol. 2021, 8, 634849. [Google Scholar] [CrossRef] [PubMed]
- Budai, P.; Kormos, É.; Buda, I.; Somody, G.; Lehel, J. Comparative Evaluation of HET-CAM and ICE Methods for Objective Assessment of Ocular Irritation Caused by Selected Pesticide Products. Toxicol. Vitr. 2021, 74, 105150. [Google Scholar] [CrossRef]
- Qi, X.; Gao, C.; Yin, C.; Fan, J.; Wu, X.; Guo, C. Improved Anticancer Activity of Betulinic Acid on Breast Cancer through a Grafted Copolymer-Based Micelles System. Drug Deliv. 2021, 28, 1962–1971. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Feflea, S.; Ganta, S.; Amiji, M. Anti-Angiogenic Effects of Betulinic Acid Administered in Nanoemulsion Formulation Using Chorioallantoic Membrane Assay. J. Biomed. Nanotechnol. 2011, 7, 317–324. [Google Scholar] [CrossRef]
- Coricovac, D.; Pînzaru, I.; Avram, Ș.; Macașoi, I.; Șoica, C.; Dehelean, C. In Vitro and In Ovo Assessment of Betulinic Acid Antimelanoma Effect. Timisoara Med. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Articles, O. Implants Composed of Digoxin and Poly (ε-Caprolactone): Development, Characterization, Anti-Proliferative and Anti-Angiogenic Activities. Die Pharm. Int. J. Pharm. Sci. 2017, 72, 383–388. [Google Scholar] [CrossRef]
- Svensson, A.; Azarbayjani, F.; Bäckman, U.; Matsumoto, T.; Christofferson, R. Digoxin Inhibits Neuroblastoma Tumor Growth in Mice. Anticancer Res. 2005, 25, 207–212. [Google Scholar] [PubMed]






| H2O | SDS 1% | BA 10 μM | DG 50 nM | DG 50 nM + BA 10 μM | |
|---|---|---|---|---|---|
| IS | 0.10 | 19.86 | 0.75 | 1.09 | 0.52 |
| tH | 300 | 20 s | 300 | 296 | 300 |
| tL | 300 | 18 s | 290 | 285 | 295 |
| tC | 299 | 15 s | 285 | 280 | 289 |
| Inhibitori Effect (Fa) | Combination Index (CI) | Dose BA | Dose DG | Dose Reduction Index (DRI) BA | Dose Reduction Index (DRI) DG |
|---|---|---|---|---|---|
| 0.65 | 11.9064 | 10 μM | 5 nM | 0.08454 | 12.7600 |
| 0.64 | 10.0020 | 10 μM | 10 nM | 0.10151 | 6.62483 |
| 0.4 | 0.32406 | 10 μM | 25 nM | 6.17001 | 6.17336 |
| 0.17 | 0.11826 | 10 μM | 50 nM | 864.114 | 8.53929 |
| Inhibitori Effect (Fa) | Combination Index (CI) | Dose BA | Dose DG | Dose Reduction Index (DRI) BA | Dose Reduction Index (DRI) DG |
|---|---|---|---|---|---|
| 0.74 | 0.24457 | 10 μM | 5 nM | 5.77905 | 13.9796 |
| 0.68 | 0.20759 | 10 μM | 10 nM | 9.93477 | 9.35133 |
| 0.55 | 0.19019 | 10 μM | 25 nM | 27.7052 | 6.48955 |
| 0.23 | 0.07838 | 10 μM | 50 nM | 377.738 | 13.2049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rednic, R.; Macasoi, I.; Pinzaru, I.; Dehelean, C.A.; Tomescu, M.-C.; Susan, M.; Feier, H. Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life 2022, 12, 1855. https://doi.org/10.3390/life12111855
Rednic R, Macasoi I, Pinzaru I, Dehelean CA, Tomescu M-C, Susan M, Feier H. Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life. 2022; 12(11):1855. https://doi.org/10.3390/life12111855
Chicago/Turabian StyleRednic, Robert, Ioana Macasoi, Iulia Pinzaru, Cristina Adriana Dehelean, Mirela-Cleopatra Tomescu, Monica Susan, and Horea Feier. 2022. "Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells" Life 12, no. 11: 1855. https://doi.org/10.3390/life12111855
APA StyleRednic, R., Macasoi, I., Pinzaru, I., Dehelean, C. A., Tomescu, M.-C., Susan, M., & Feier, H. (2022). Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life, 12(11), 1855. https://doi.org/10.3390/life12111855








