Concomitant Intra-Aortic Balloon Pumping Significantly Reduces Left Ventricular Pressure during Central Veno-Arterial Extracorporeal Membrane Oxygenation—Results from a Large Animal Model
Abstract
1. Introduction
2. Materials and Methods
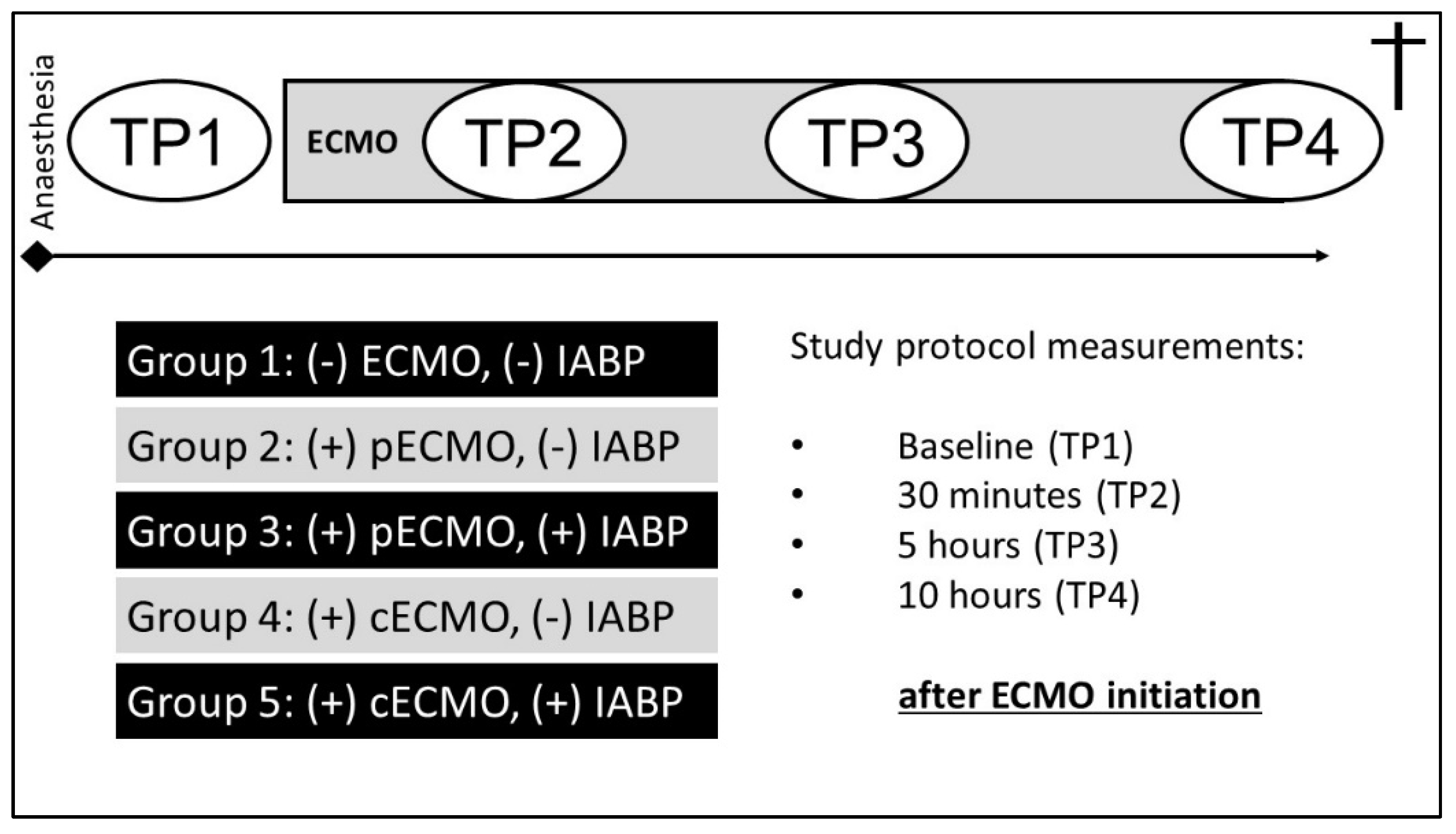
2.1. Experimental Protocol
2.1.1. Groups
2.1.2. Instrumentation and Operative Technique
2.1.3. MCS Implantation
2.1.4. Haemodynamic Measurements and Data Analysis
2.2. Biochemical Analysis
2.2.1. Quantification of Systemically Circulating Cytokines and Endothelin-1
2.2.2. Quantification of cfDNA and NETs
2.2.3. Measurement of Reactive Oxygens Species (ROS) in Plasma
2.3. Statistical Analysis
3. Results
3.1. Haemodynamics and Blood Flow Data
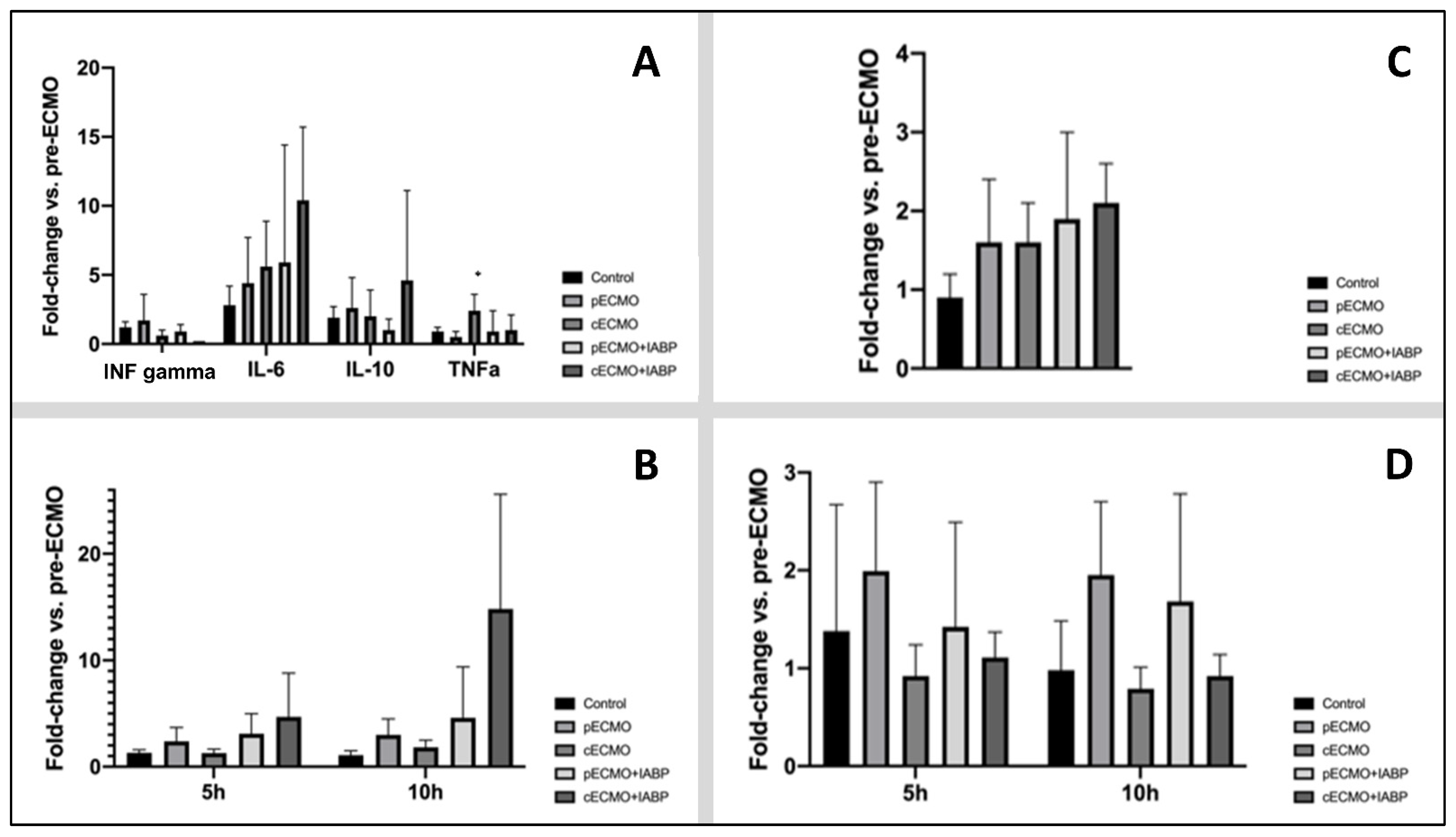
3.2. Quantification of Plasma Levels of Circulating Cytokines, ET-1, NETs, and ROS
4. Discussion
- (1)
- Extracorporeal life support decreased right and left ventricular pressure (RVPsys and LVPsys). The antegrade flow of the central ECMO cannulation was associated with a greater reduction of left ventricular pressure and was enhanced by additional IABP support.
- (2)
- Extracorporeal life support decreased the contractility index (LVdp/dtmax). The antegrade ECMO flow was associated with a better LV unloading. Furthermore, our data suggest that in the central ECMO group, the IABP may have an optimising effect.
- (3)
- IABP support neither affected coronary nor carotidal blood flow during ECMO circulation, independent of antegrade or central ECMO flow.
- (4)
- Utilisation of extracorporeal circulation did not promote inflammatory cascade.
4.1. Haemodynamic Effects of Simultaneous IABP Support during ECMO Circulation
4.2. Inflammatory Processes Induced by ECMO
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukuhara, S.; Takeda, K.; Garan, A.R.; Kurlansky, P.; Hastie, J.; Naka, Y.; Takayama, H. Contemporary mechanical circulatory support therapy for postcardiotomy shock. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 183–191. [Google Scholar] [CrossRef]
- Acharya, D.; Loyaga-Rendon, R.Y.; Tallaj, J.A.; Pamboukian, S.V.; Sasse, M.F. Circulatory support for shock complicating myocardial infarction. J. Invasive Cardiol. 2014, 26, E109–E114. [Google Scholar]
- Mebazaa, A.; Pitsis, A.A.; Rudiger, A.; Toller, W.; Longrois, D.; Ricksten, S.-E.; Bobek, I.; De Hert, S.; Wieselthaler, G.; Schirmer, U.; et al. Clinical review: Practical recom-mendations on the management of perioperative heart failure in cardiac surgery. Crit. Care. 2010, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.J.; Aleksova, N.; Pitcher, I.; Couture, E.; Parlow, S.; Faraz, M.; Visintini, S.; Simard, T.; Di Santo, P.; Mathew, R.; et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients with Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; O’Horo, J.C.; Antharam, P.; Ananthaneni, S.; Vallabhajosyula, S.; Stulak, J.M.; Dunlay, S.M.; Holmes, D.R., Jr.; Barsness, G.W. Venoarterial Extracorporeal Membrane Oxygenation with Concomitant Impella Versus Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. ASAIO J. 2020, 66, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Deppe, A.-C.; Weber, C.; Liakopoulos, O.J.; Zeriouh, M.; Slottosch, I.; Scherner, M.; Kuhn, E.W.; Choi, Y.-H.; Wahlers, T. Preoperative intra-aortic balloon pump use in high-risk patients prior to coronary artery bypass graft surgery decreases the risk for morbidity and mortality-A meta-analysis of 9,212 patients. J. Card. Surg. 2017, 32, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Hachamovitch, R.; Makkar, R.; Ramzy, D.; Moriguchi, J.D.; Arabia, F.A.; Esmailian, F.; Azarbal, B. Lack of Survival Benefit Found with Use of Intraaortic Balloon Pump in Extracorporeal Membrane Oxygenation: A Pooled Experience of 1517 Patients. J. Invasive Cardiol. 2015, 27, 453–458. [Google Scholar] [PubMed]
- Vallabhajosyula, S.; O’Horo, J.C.; Antharam, P.; Ananthaneni, S.; Vallabhajosyula, S.; Stulak, J.M.; Eleid, M.F.; Dunlay, S.M.; Gersh, B.J.; Rihal, C.S.; et al. Concomitant Intra-Aortic Balloon Pump Use in Cardiogenic Shock Requiring Veno-Arterial Extracorporeal Membrane Oxygenation. Circ. Cardiovasc. Interv. 2018, 11, e006930. [Google Scholar] [CrossRef]
- Li, Y.; Yan, S.; Gao, S.; Liu, M.; Lou, S.; Liu, G.; Ji, B.; Gao, B. Effect of an intra-aortic balloon pump with venoarterial extracorporeal membrane oxygenation on mortality of patients with cardiogenic shock: A systematic review and meta-analysis dagger. Eur. J. Cardiothorac. Surg. 2019, 55, 395–404. [Google Scholar] [CrossRef]
- Rupprecht, L.; Florchinger, B.; Schopka, S.; Schmid, C.; Philipp, A.; Lunz, D.; Müller, T.; Camboni, D. Cardiac decompression on extra-corporeal life support: A review and discussion of the literature. ASAIO J. 2013, 59, 547–553. [Google Scholar] [CrossRef]
- Meani, P.; Gelsomino, S.; Natour, E.; Johnson, D.M.; La Rocca, H.-P.B.; Pappalardo, F.; Bidar, E.; Makhoul, M.; Raffa, G.; Heuts, S.; et al. Modalities and Effects of Left Ventricle Unloading on Extracorporeal Life support: A Review of the Current Literature. Eur. J. Heart Fail. 2017, 19 (Suppl. 2), 84–91. [Google Scholar] [CrossRef]
- O’Neil, M.P.; Fleming, J.C.; Badhwar, A.; Guo, L.R. Pulsatile versus nonpulsatile flow during cardiopulmonary bypass: Microcirculatory and systemic effects. Ann. Thorac. Surg. 2012, 94, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Bréchot, N.; Demondion, P.; Santi, F.; Lebreton, G.; Pham, T.; Dalakidis, A.; Gambotti, L.; Luyt, C.-E.; Schmidt, M.; Hekimian, G.; et al. Intra-aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial-extracorporeal membrane oxygenation. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Verghese, D.; Vallabhajosyula, S. Intra-Aortic Balloon Pump for Left Ventricular Unloading in Veno-Arterial Extracorporeal Membrane Oxygenation: The Last Remaining Indication in Cardiogenic Shock. J. Am. Heart Assoc. 2022, 11, e025274. [Google Scholar] [CrossRef] [PubMed]
- Bělohlávek, J.; Mlček, M.; Huptych, M.; Svoboda, T.; Havránek, S.; Ošt’ádal, P.; Bouček, T.; Kovárník, T.; Mlejnský, F.; Mrázek, V.; et al. Coronary versus carotid blood flow and coronary perfusion pressure in a pig model of prolonged cardiac arrest treated by different modes of venoarterial ECMO and intraaortic balloon counterpulsation. Crit. Care 2012, 16, R50. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, I.; Eghbalzadeh, K.; Sabashnikov, A.; Deppe, A.; Kuhn, E.; Merkle, J.; Weber, C.; Ivanov, B.; Ghodsizad, A.; Rustenbach, C.; et al. Central vs peripheral venoarterial ECMO in postcardiotomy cardiogenic shock. J. Card. Surg. 2020, 35, 1037–1042. [Google Scholar] [CrossRef]
- Schroeter, T.; Vollroth, M.; Hobartner, M.; Sauer, M.; Mende, M.; Mohr, F.W.; Misfeld, M. Influence of ECMO and IABP on coronary blood flow. Valuable combination or waste of resources? Med. Klin. Intensivmed. Notfmed. 2015, 110, 210–216. [Google Scholar] [CrossRef]
- Thangappan, K.; Cavarocchi, N.C.; Baram, M.; Thoma, B.; Hirose, H. Systemic inflammatory response syndrome (SIRS) after extracorporeal membrane oxygenation (ECMO): Incidence, risks and survivals. Heart Lung 2016, 45, 449–453. [Google Scholar] [CrossRef]
- Edinger, F.; Schneck, E.; Schulte, C.; Gehron, J.; Mueller, S.; Sander, M.; Koch, C. Comparison of the effect of membrane sizes and fibre arrangements of two membrane oxygenators on the inflammatory response, oxygenation and decarboxylation in a rat model of extracorporeal membrane oxygenation. BMC Cardiovasc. Disord. 2020, 20, 294. [Google Scholar] [CrossRef]
- Paunel-Görgülü, A.; Wacker, M.; El Aita, M.; Hassan, S.; Schlachtenberger, G.; Deppe, A.; Choi, Y.-H.; Kuhn, E.; Mehler, T.O.; Wahlers, T. cfDNA correlates with endothelial damage after cardiac surgery with prolonged cardiopulmonary bypass and amplifies NETosis in an intracellular TLR9-independent manner. Sci. Rep. 2017, 7, 17421. [Google Scholar] [CrossRef]
- Wacker, M.; Kießwetter, V.; Slottosch, I.; Awad, G.; Paunel-Görgülü, A.; Varghese, S.; Klopfleisch, M.; Kupitz, D.; Klemm, D.; Nietzsche, S.; et al. In vitro hemo- and cytocompatibility of bacterial nanocelluose small diameter vascular grafts: Impact of fabrication and surface characteristics. PLoS ONE 2020, 15, e0235168. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, F.S.; Zivelonghi, C.; Vossenberg, T.N.; Bleeker, G.B.; Winia, V.L.; Sjauw, K.D.; Ten Berg, J.M. VA-ECMO With IABP is Associated with Better Outcome Than VA-ECMO Alone in the Treatment of Cardiogenic Shock in ST-Elevation Myocardial Infarction. J. Invasive Cardiol. 2021, 33, E387–E392. [Google Scholar] [PubMed]
- Djordjevic, I.; Deppe, A.-C.; Sabashnikov, A.; Kuhn, E.; Eghbalzadeh, K.; Merkle, J.; Gerfer, S.; Gaisendrees, C.; Ivanov, B.; Moellenbeck, L.; et al. Concomitant ECMO And IABP Support in Postcardiotomy Cardiogenic Shock Patients. Hear. Lung Circ. 2021, 30, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Ishii, M.; Tsujita, K.; Okamoto, H.; Koto, S.; Nakai, M.; Sumita, Y.; Iwanaga, Y.; Matoba, S.; Kobayashi, Y.; et al. Outcomes of Venoarterial Extracorporeal Membrane Oxygenation Plus Intra-Aortic Balloon Pumping for Treatment of Acute Myocardial Infarction Complicated by Cardiogenic Shock. J. Am. Heart Assoc. 2022, 11, e023713. [Google Scholar] [CrossRef]
- Zeng, P.; Yang, C.; Chen, J.; Fan, Z.; Cai, W.; Huang, Y.; Xiang, Z.; Yang, J.; Zhang, J.; Yang, J. Comparison of the Efficacy of ECMO With or Without IABP in Patients with Cardiogenic Shock: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 917610. [Google Scholar] [CrossRef]
- Madershahian, N.; Liakopoulos, O.J.; Wippermann, J.; Salehi-Gilani, S.; Wittwer, T.; Choi, Y.-H.; Naraghi, H.; Wahlers, T. The impact of intraaortic balloon counterpulsation on bypass graft flow in patients with peripheral ECMO. J. Card. Surg. 2009, 24, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Madershahian, N.; Wippermann, J.; Liakopoulos, O.; Wittwer, T.; Kuhn, E.; Er, F.; Hoppe, U.; Wahlers, T. The acute effect of IABP-induced pulsatility on coronary vascular resistance and graft flow in critical ill patients during ECMO. J. Cardiovasc. Surg. 2011, 52, 411–418. [Google Scholar]
- Sauren, L.D.; Reesink, K.D.; Selder, J.L.; Beghi, C.; van der Veen, F.H.; Maessen, J.G. The acute effect of intra-aortic balloon counterpulsation during extracorporeal life support: An experimental study. Artif. Organs. 2007, 31, 31–38. [Google Scholar] [CrossRef]
- Davies, M.G.; Hagen, P.O. Systemic inflammatory response syndrome. Br. J. Surg. 1997, 84, 920–935. [Google Scholar] [CrossRef] [PubMed]
- Adrian, K.; Mellgren, K.; Skogby, M.; Friberg, L.G.; Mellgren, G.; Wadenvik, H. Cytokine release during long-term extracorporeal circulation in an experimental model. Artif. Organs. 1998, 22, 859–863. [Google Scholar] [CrossRef] [PubMed]
- McILwain, R.B.; Timpa, J.G.; Kurundkar, A.R.; Holt, D.W.; Kelly, D.R.; Hartman, Y.E.; Neel, M.L.; Karnatak, R.K.; Schelonka, R.L.; Anantharamaiah, G.M.; et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab. Investig. 2010, 90, 128–139. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Group | Before ECMO | 10 h on ECMO |
|---|---|---|---|
| HR (bpm) | (-)ECMO(-)IABP | 85.6 ± 5.5 | 90.6 ± 10.2 |
| (p)ECMO(-)IABP | 92.1 ± 9.8 | 98.4 ± 22.2 | |
| (c)ECMO(-)IABP | 96.5 ± 9.7 | 86.7 ± 6.7 | |
| (p)ECMO(+)IABP | 99.6 ± 12.2 | 91.0 ± 12.1 | |
| (c)ECMO(+)IABP | 97.3 ± 5.9 | 98.0 ± 11.0 | |
| MAP (mmHg) | (-)ECMO(-)IABP | 68.4 ± 12.7 | 61.6 ± 10.4 * |
| (p)ECMO(-)IABP | 71.0 ± 7.4 | 65.7 ± 9.9 * | |
| (c)ECMO(-)IABP | 77.8 ± 13.3 | 57.7 ± 3.1 * | |
| (p)ECMO(+)IABP | 73.1 ± 16.3 | 57.3 ± 5.4 * | |
| (c)ECMO(+)IABP | 74.9 ± 17.0 | 63.4 ± 7.5 * | |
| CI (L/min/m2) | (-)ECMO(-)IABP | 3.0 ± 0.2 | 3.0 ± 0.3 |
| (p)ECMO(-)IABP | 3.0 ± 0.03 | 3.3 ± 0.4 | |
| (c)ECMO(-)IABP | 3.3 ± 0.3 | 3.5 ± 0.4 | |
| (p)ECMO(+)IABP | 3.1 ± 0.4 | 3.5 ± 0.2 | |
| (c)ECMO(+)IABP | 3.3 ± 0.4 | 3.7 ± 0.6 | |
| Hb (mg/dL) | (-)ECMO(-)IABP | 8.9 ± 0.8 | 8.0 ± 1.0 * |
| (p)ECMO(-)IABP | 8.9 ± 0.6 | 7.2 ± 1.0 * | |
| (c)ECMO(-)IABP | 8.3 ± 0.8 | 6.2 ± 0.6 *;## | |
| (p)ECMO(+)IABP | 9.1 ± 0.6 | 6.9 ± 0.8 * | |
| (c)ECMO(+)IABP | 8.2 ± 0.8 | 6.0 ± 0.8 *;##;§ | |
| pO2, (mmHg) | (-)ECMO(-)IABP | 158 ± 38 | 138 ± 5 |
| (p)ECMO(-)IABP | 142 ± 33 | 145 ± 36 | |
| (c)ECMO(-)IABP | 147 ± 28 | 150 ± 27 | |
| (p)ECMO(+)IABP | 159 ± 24 | 138 ± 25 | |
| (c)ECMO(+)IABP | 148 ± 37 | 150 ± 37 | |
| CVP (mmHg) | (-)ECMO(-)IABP | 11.8 ± 1.6 | 14.1 ± 0.9 |
| (p)ECMO(-)IABP | 12.1 ± 3.2 | 10.8 ± 1.4 | |
| (c)ECMO(-)IABP | 13.1 ± 1.0 | 12.9 ± 1.3 | |
| (p)ECMO(+)IABP | 10.6 ± 3.1 | 10.5 ± 3.4 | |
| (c)ECMO(+)IABP | 11.7 ± 0.6 | 10.8 ± 3.1 | |
| RVPsys (mmHg) | (-)ECMO(-)IABP | 29.9 ± 9.5 | 29.8 ± 6.1 |
| (p)ECMO(-)IABP | 28.1 ± 5.2 | 15.0 ± 6.8 *,## | |
| (c)ECMO(-)IABP | 25.2 ± 2.9 | 8.7 ± 5.1 *;### | |
| (p)ECMO(+)IABP | 23.2 ± 2.8 | 18.4 ± 4.5 *;## | |
| (c)ECMO(+)IABP | 25.2 ± 2.9 | 11.5 ± 8.7 *;### | |
| LVPsys (mmHg) | (-)ECMO(-)IABP | 82.4 ± 9.9 | 79.5 ± 8.6 |
| (p)ECMO(-)IABP | 81.7 ± 7.6 | 66.4 ± 19.4 * | |
| (c)ECMO(-)IABP | 76.3 ± 13.0 | 44.3 ± 8.4 *;## | |
| (p)ECMO(+)IABP | 87.3 ± 19.0 | 55.0 ± 14.4 * | |
| (c)ECMO(+)IABP | 75.5 ± 8.1 | 33.6 ± 2.7 *;###;§§;+ | |
| LVdp/dtmax (mmHg) | (-)ECMO(-)IABP | 1729 ± 368 | 1195 ± 128 |
| (p)ECMO(-)IABP | 1434 ± 429 | 582 ± 147 *;## | |
| (c)ECMO(-)IABP | 1497 ± 162 | 378 ± 164 *;## | |
| (p)ECMO(+)IABP | 1678 ± 628 | 604 ± 151 * | |
| (c)ECMO(+)IABP | 1484 ± 56 | 266 ± 114 *;### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djordjevic, I.; Liakopoulos, O.; Elskamp, M.; Maier-Trauth, J.; Gerfer, S.; Mühlbauer, T.; Slottosch, I.; Kuhn, E.; Sabashnikov, A.; Rademann, P.; et al. Concomitant Intra-Aortic Balloon Pumping Significantly Reduces Left Ventricular Pressure during Central Veno-Arterial Extracorporeal Membrane Oxygenation—Results from a Large Animal Model. Life 2022, 12, 1859. https://doi.org/10.3390/life12111859
Djordjevic I, Liakopoulos O, Elskamp M, Maier-Trauth J, Gerfer S, Mühlbauer T, Slottosch I, Kuhn E, Sabashnikov A, Rademann P, et al. Concomitant Intra-Aortic Balloon Pumping Significantly Reduces Left Ventricular Pressure during Central Veno-Arterial Extracorporeal Membrane Oxygenation—Results from a Large Animal Model. Life. 2022; 12(11):1859. https://doi.org/10.3390/life12111859
Chicago/Turabian StyleDjordjevic, Ilija, Oliver Liakopoulos, Mara Elskamp, Johanna Maier-Trauth, Stephen Gerfer, Thomas Mühlbauer, Ingo Slottosch, Elmar Kuhn, Anton Sabashnikov, Pia Rademann, and et al. 2022. "Concomitant Intra-Aortic Balloon Pumping Significantly Reduces Left Ventricular Pressure during Central Veno-Arterial Extracorporeal Membrane Oxygenation—Results from a Large Animal Model" Life 12, no. 11: 1859. https://doi.org/10.3390/life12111859
APA StyleDjordjevic, I., Liakopoulos, O., Elskamp, M., Maier-Trauth, J., Gerfer, S., Mühlbauer, T., Slottosch, I., Kuhn, E., Sabashnikov, A., Rademann, P., Maul, A., Paunel-Görgülü, A., Wahlers, T., & Deppe, A. C. (2022). Concomitant Intra-Aortic Balloon Pumping Significantly Reduces Left Ventricular Pressure during Central Veno-Arterial Extracorporeal Membrane Oxygenation—Results from a Large Animal Model. Life, 12(11), 1859. https://doi.org/10.3390/life12111859








