Exogenous γ-Aminobutyric Acid (GABA) Application Mitigates Salinity Stress in Maize Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Preparation and Materials
2.2. Seed Germination
2.3. Salt Treatments
2.4. GABA Foliar Application
2.5. Seedlings Growth Parameters and Pigments
2.6. Determination of Cell Membrane Integrity
2.7. Determination of Methylglyoxal Content, Hydrogen Peroxide, and Malondialdehyde
2.8. Determination of Antioxidant Enzymes Activates
2.9. Determination of Na and K
2.10. Gene Expression
2.11. Statistical Analysis
3. Results
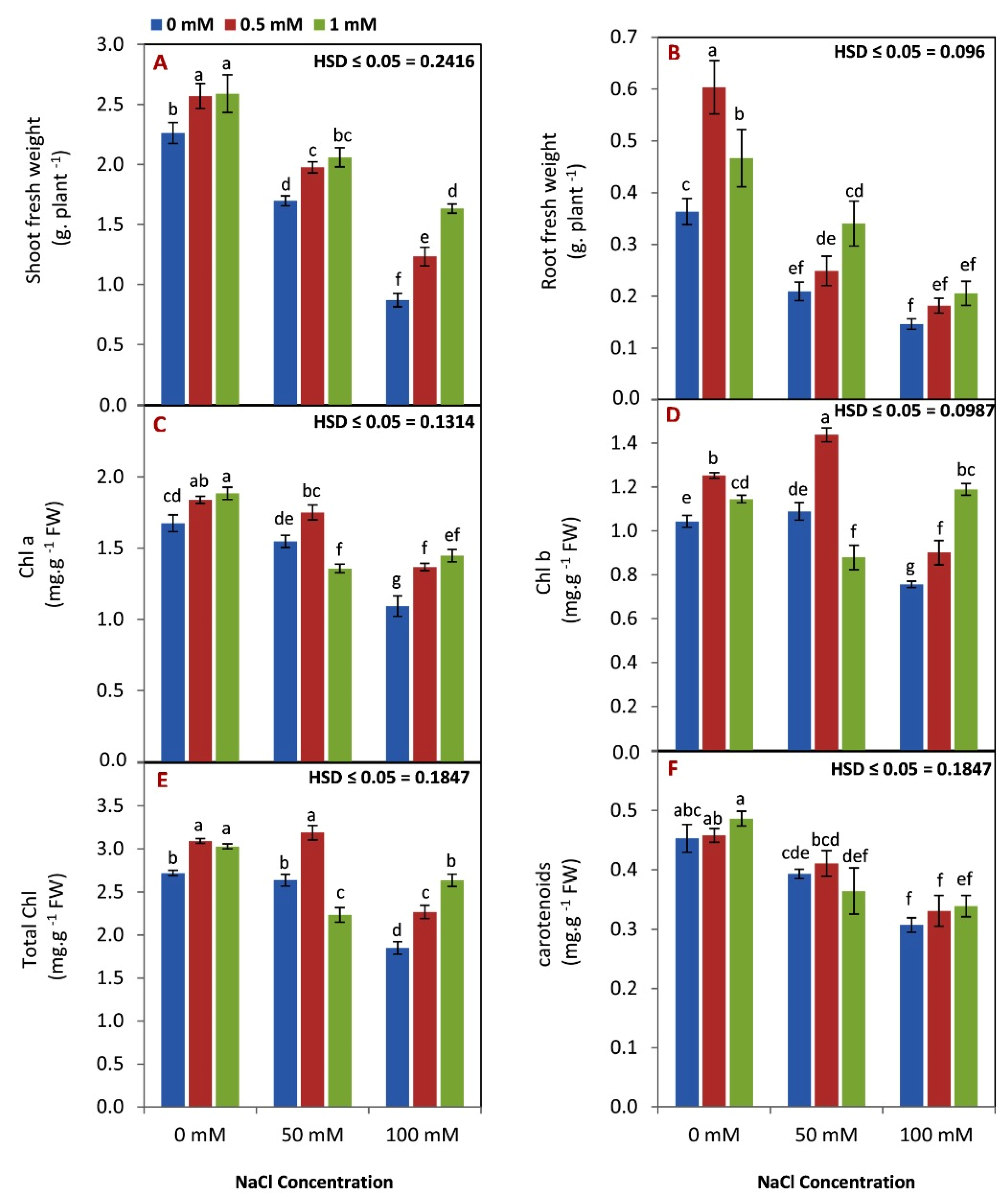
3.1. Effect of GABA on Growth and Pigments under Salinity Condition
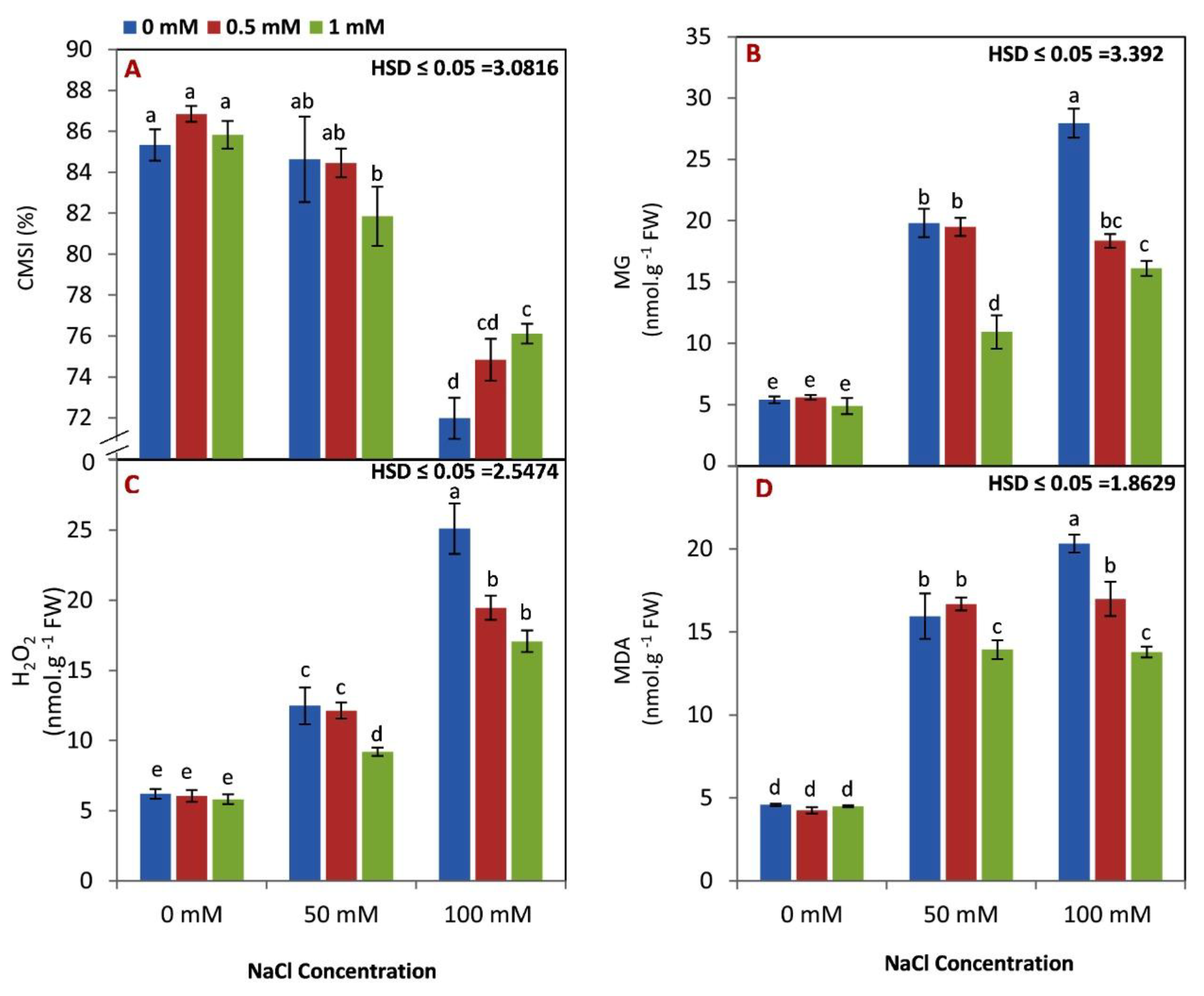
3.2. Effect of GABA on the Membrane Stability Index (CMSI), Methylglyoxal Content (MG), H2O2, and Malondialdehyde Content (MDA) under Saline Condition
3.3. Effect of the GABA Application on the Activities of Antioxidant Enzymes
3.4. Effect of GABA Application on Na, K, and Na/K Ratio
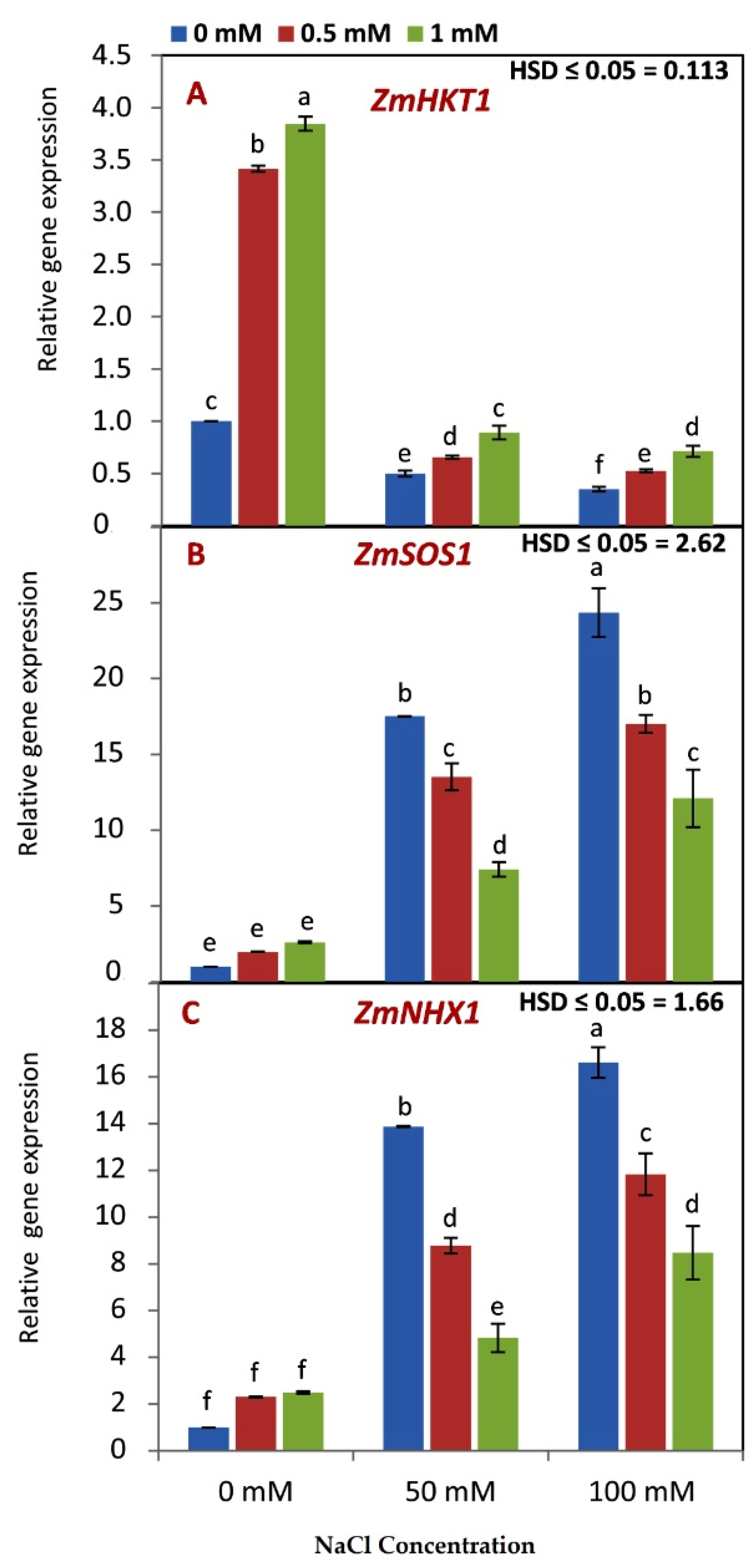
3.5. Effect of GABA Application on the Genes Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzahrani, O.; Abouseadaa, H.; Abdelmoneim, T.K.; Alshehri, M.A.; El-beltagi, H.S.; El-Mogy, M.M.; Atia, M.A.M. Agronomical, Physiological and Molecular Evaluation Reveals Superior Salt-tolerance in Bread Wheat Through Salt-induced Priming Approach. Notulae Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12310. [Google Scholar] [CrossRef]
- Aboryia, M.S.; El-Dengawy, E.-R.F.A.; El-Banna, M.F.; El-Gobba, M.H.; Kasem, M.M.; Hegazy, A.A.; Hassan, H.M.; El-Yazied, A.A.; El-Gawad, H.G.A.; Al-Qahtani, S.M.; et al. Anatomical and Physiological Performance of Jojoba Treated with Proline under Salinity Stress Condition. Horticulturae 2022, 8, 716. [Google Scholar] [CrossRef]
- Akyol, T.Y.; Yilmaz, O.; Uzilday, B.; Uzilday, R.Ö.; Türkan, İ. Plant response to salinity: An analysis of ROS formation, signaling, and antioxidant defense. Turk. J. Bot. 2020, 44, 1–13. [Google Scholar]
- Helal, N.M.; Khattab, H.I.; Emam, M.M.; Niedbała, G.; Wojciechowski, T.; Hammami, I.; Alabdallah, N.M.; Darwish, D.B.E.; El-Mogy, M.M.; Hassan, H.M. Improving Yield Components and Desirable Eating Quality of Two Wheat Genotypes Using Si and NanoSi Particles under Heat Stress. Plants 2022, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Rahman, M.A.; Skalicky, M.; Alabdallah, N.M.; Waseem, M.; Jahan, M.S.; Ahammed, G.J.; El-Mogy, M.M.; El-Yazied, A.A.; Ibrahim, M.F.M.; et al. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Garchery, C.; Stevens, R. Irrigation with salt water affects growth, yield, fruit quality, storability and marker-gene expression in cherry tomato. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 727–737. [Google Scholar] [CrossRef]
- Paz, R.C.; Rocco, R.A.; Reinoso, H.; Menéndez, A.B.; Pieckenstain, F.L.; Ruiz, O.A. Comparative study of alkaline, saline, and mixed saline–alkaline stresses with regard to their effects on growth, nutrient accumulation, and root morphology of Lotus tenuis. J. Plant Growth Regul. 2012, 31, 448–459. [Google Scholar] [CrossRef]
- Abdelgawad, F.K.; El-Mogy, M.M.; Mohamed, M.I.A.; Garchery, C.; Stevens, R.G. Increasing Ascorbic Acid Content and Salinity Tolerance of Cherry Tomato Plants by Suppressed Expression of the Ascorbate Oxidase Gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef]
- Youssef, M.H.M.; Raafat, A.; El-Yazied, A.A.; Selim, S.; Azab, E.; Khojah, E.; El Nahhas, N.; Ibrahim, M.F.M. Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes. Plants 2021, 10, 2519. [Google Scholar] [CrossRef]
- Hurkman, W.J. Effect of salt stress on plant gene expression: A review. Plant Soil 1992, 146, 145–151. [Google Scholar] [CrossRef]
- Cucci, G.; Lacolla, G.; Boari, F.; Mastro, M.A.; Cantore, V. Effect of water salinity and irrigation regime on maize (Zea mays L.) cultivated on clay loam soil and irrigated by furrow in Southern Italy. Agric. Water Manag. 2019, 222, 118–124. [Google Scholar] [CrossRef]
- Khan, A.A.; Rao, S.A.; McNeilly, T. Assessment of salinity tolerance based upon seedling root growth response functions in maize (Zea mays L.). Euphytica 2003, 131, 81–89. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007, 164, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, B.M. Maize Starch for Industrial Applications. In Industrial Crops: Breeding for BioEnergy and Bioproducts; Cruz, V.M.V., Dierig, D.A., Eds.; Springer: New York, NY, USA, 2015; pp. 171–189. [Google Scholar]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Menezes-Benavente, L.; Kernodle, S.P.; Margis-Pinheiro, M.; Scandalios, J.G. Salt-induced antioxidant metabolism defenses in maize (Zea mays L.) seedlings. Redox Rep. 2004, 9, 29–36. [Google Scholar] [CrossRef]
- Jin, X.; Liu, T.; Xu, J.; Gao, Z.; Hu, X. Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol. 2019, 19, 48. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not Just a Metabolite. Trends Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef]
- Fait, A.; Nesi, A.N.; Angelovici, R.; Lehmann, M.; Pham, P.A.; Song, L.; Haslam, R.P.; Napier, J.A.; Galili, G.; Fernie, A.R. Targeted Enhancement of Glutamate-to-γ-Aminobutyrate Conversion in Arabidopsis Seeds Affects Carbon-Nitrogen Balance and Storage Reserves in a Development-Dependent Manner. Plant Physiol. 2011, 157, 1026–1042. [Google Scholar] [CrossRef]
- Roberts, M.R. Does GABA Act as a Signal in Plants? Hints from Molecular Studies. Plant Signal. Behav. 2007, 2, 408–409. [Google Scholar] [CrossRef]
- Carroll, A.D.; Fox, G.G.; Laurie, S.; Phillips, R.; Ratcliffe, R.G.; Stewart, G.R. Ammonium Assimilation and the Role of [gamma]-Aminobutyric Acid in pH Homeostasis in Carrot Cell Suspensions. Plant Physiol. 1994, 106, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, P.; Chen, Z.; Gu, Z.; Yang, R. GABA enhances physio-biochemical metabolism and antioxidant capacity of germinated hulless barley under NaCl stress. J. Plant Physiol. 2018, 231, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, D.W.; Flügge, U.-I.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Jalil, S.U.; Ansari, M.I. Physiological Role of Gamma-Aminobutyric Acid in Salt Stress Tolerance. In Salt and Drought Stress Tolerance in Plants: Signaling Networks and Adaptive Mechanisms; Hasanuzzaman, M., Tanveer, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 337–350. [Google Scholar]
- Khan, M.I.R.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ferrante, A.; Khan, N.A.; Ansari, M.I. Role of GABA in plant growth, development and senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W.; Zhang, X.; Ma, X.; Huang, L.; Nie, G.; Liu, W.; et al. The γ-Aminobutyric Acid (GABA) Alleviates Salt Stress Damage during Seeds Germination of White Clover Associated with Na+/K+ Transportation, Dehydrins Accumulation, and Stress-Related Genes Expression in White Clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Xu, J.; Liu, T.; Yang, S.; Jin, X.; Qu, F.; Huang, N.; Hu, X. Polyamines are involved in GABA-regulated salinity-alkalinity stress tolerance in muskmelon. Environ. Exp. Bot. 2019, 164, 181–189. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.; Meng, Y.; Xie, T.; Li, L.; Li, J.; Wei, S. γ-Aminobutyric Acid Imparts Partial Protection from Salt Stress Injury to Maize Seedlings by Improving Photosynthesis and Upregulating Osmoprotectants and Antioxidants. Sci. Rep. 2017, 7, 43609. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; Elkelish, A.; Niedbała, G.; Farag, R.; Wojciechowski, T.; Mukherjee, S.; Abou-Hadid, A.F.; El-Hennawy, H.M.; Abou El-Yazied, A.; Abd El-Gawad, H.G. Protective Effect of γ-Aminobutyric Acid Against Chilling Stress during Reproductive Stage in Tomato Plants Through Modulation of Sugar Metabolism, Chloroplast Integrity, and Antioxidative Defense Systems. Front. Plant Sci. 2021, 12, 1917. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hossain, M.Z.; Fujita, M. Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust. J. Crop Sci. 2009, 3, 53. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Nasser, M.A.; El-Mogy, M.M.; Samaan, M.S.F.; Hassan, K.M.; El-Sayed, S.M.; Alsubeie, M.S.; Darwish, D.B.; Mahmoud, S.F.; Al-Harbi, N.A.; Al-Qahtani, S.M.; et al. Postharvest Exogenous Melatonin Treatment of Table Grape Berry Enhances Quality and Maintains Bioactive Compounds during Refrigerated Storage. Horticulturae 2022, 8, 860. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Doklega, S.M.A.; El-Ezz, S.F.A.; Mostafa, N.A.; Dessoky, E.S.; Abdulmajeed, A.M.; Darwish, D.B.; Alzuaibr, F.M.; El-Yazied, A.A.; El-Mogy, M.M.; Mahmoud, S.F.; et al. Effect of Titanium and Vanadium on Antioxidants Content and Productivity of Red Cabbage. Horticulturae 2022, 8, 481. [Google Scholar] [CrossRef]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Dias, M.A.; Costa, M.M. Effect of low salt concentrations on nitrate reductase and peroxidase of sugar beet leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Havre, G.N. The flame photometric determination of sodium, potassium and calcium in plant extracts with special reference to interference effects. Anal. Chim. Acta 1961, 25, 557–566. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Abdeldym, E.A.; El-Mogy, M.M.; Abdellateaf, H.R.L.; Atia, M.A.M. Genetic Characterization, Agro-Morphological and Physiological Evaluation of Grafted Tomato under Salinity Stress Conditions. Agronomy 2020, 10, 1948. [Google Scholar] [CrossRef]
- Ferjani, A.; Mustardy, L.; Sulpice, R.; Marin, K.; Suzuki, I.; Hagemann, M.; Murata, N. Glucosylglycerol, a compatible solute, sustains cell division under salt stress. Plant Physiol. 2003, 131, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Hu, L.; Xu, W.; Zhen, A.; Zhang, L.; Hu, X. Exogenous γ-Aminobutyric Acid Improves the Structure and Function of Photosystem II in Muskmelon Seedlings Exposed to Salinity-Alkalinity Stress. PLoS ONE 2016, 11, e0164847. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Chiu, G.Z.; Yu, G.; Trobacher, C.P.; Shelp, B.J. Salinity-regulated expression of genes involved in GABA metabolism and signaling. Botany 2017, 95, 621–627. [Google Scholar] [CrossRef]
- Nasrallah, A.K.; Kheder, A.A.; Kord, M.A.; Fouad, A.S.; El-Mogy, M.M.; Atia, M.A.M. Mitigation of Salinity Stress Effects on Broad Bean Productivity Using Calcium Phosphate Nanoparticles Application. Horticulturae 2022, 8, 75. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox Signal. 2006, 8, 1757–1764. [Google Scholar] [CrossRef]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of Salinity Stress on Chloroplast Structure and Function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Bouché, N.; Fait, A.; Bouchez, D.; Møller, S.G.; Fromm, H. Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 6843–6848. [Google Scholar] [CrossRef]
- Shang, H.; Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. Effect of Exogenous γ-Aminobutyric Acid Treatment on Proline Accumulation and Chilling Injury in Peach Fruit after Long-Term Cold Storage. J. Agric. Food Chem. 2011, 59, 1264–1268. [Google Scholar] [CrossRef]
- Ellis, E.M. Reactive carbonyls and oxidative stress: Potential for therapeutic intervention. Pharmacol. Ther. 2007, 115, 13–24. [Google Scholar] [CrossRef]
- Li, Z.-G.; Duan, X.-Q.; Min, X.; Zhou, Z.-H. Methylglyoxal as a novel signal molecule induces the salt tolerance of wheat by regulating the glyoxalase system, the antioxidant system, and osmolytes. Protoplasma 2017, 254, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium Supplementation Improves Na+/K+ Ratio, Antioxidant Defense and Glyoxalase Systems in Salt-Stressed Rice Seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xu, L.; Zeng, X.; Li, Z.; Qin, B.; He, N. New perspective of GABA as an inhibitor of formation of advanced lipoxidation end-products: It’s interaction with malondiadehyde. J. Biomed. Nanotechnol. 2010, 6, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Li, J.; Xie, J.; Li, N.; Bakpa, E.P.; Han, K.; Yang, Y.; Wang, C. Exogenous Zeaxanthin Alleviates Low Temperature Combined with Low Light Induced Photosynthesis Inhibition and Oxidative Stress in Pepper (Capsicum annuum L.) Plants. Curr. Issues Mol. Biol. 2022, 44, 2453–2471. [Google Scholar] [CrossRef]
- Elkelish, A.; El-Mogy, M.M.; Niedbała, G.; Piekutowska, M.; Atia, M.A.; Hamada, M.M.; Shahin, M.; Mukherjee, S.; ElYazied, A.A.; Shebl, M. Roles of Exogenous α-Lipoic Acid and Cysteine in Mitigation of Drought Stress and Restoration of Grain Quality in Wheat. Plants 2021, 10, 2318. [Google Scholar] [CrossRef]
- Jahan, M.S.; Hasan, M.M.; Alotaibi, F.S.; Alabdallah, N.M.; Alharbi, B.M.; Ramadan, K.M.; Bendary, E.S.; Alshehri, D.; Jabborova, D.; Al-Balawi, D.A. Exogenous Putrescine Increases Heat Tolerance in Tomato Seedlings by Regulating Chlorophyll Metabolism and Enhancing Antioxidant Defense Efficiency. Plants 2022, 11, 1038. [Google Scholar] [CrossRef]
- Carillo, P. GABA Shunt in Durum Wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Hossain, M.S.; Dietz, K.-J. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef]
- Türkyilmaz, B.; AktaŞ, L.Y.; Güven, A. Salinity induced differences in growth and nutrient accumulation in five barley cultivars. Turk. J. Field Crops 2011, 16, 84–92. [Google Scholar]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Alqarawi, A.A.; Hashem, A.; AbdAllah, E.F.; Al-Huqail, A.A.; Alshahrani, T.S.; Alshalawi, S.A.R.; Egamberdieva, D. Protective role of gamma amminobutyric acid on Cassia italica Mill under salt stress. Legume Res. 2016, 39, 396–404. [Google Scholar] [CrossRef]
- Ma, X.; Gu, J.; Luo, Q.; Wen, M.; Li, H.; Wang, Z.-Y. The synergistic benefits of β-aminobutyric acid and γ-aminobutyrate on salt and drought tolerance in cassava. Plant Biotechnol. Rep. 2022, 16, 29–41. [Google Scholar] [CrossRef]
- Liu, W.; Liu, K.; Chen, D.; Zhang, Z.; Li, B.; El-Mogy, M.M.; Tian, S.; Chen, T. Solanum lycopersicum, a Model Plant for the Studies in Developmental Biology, Stress Biology and Food Science. Foods 2022, 11, 2402. [Google Scholar] [CrossRef]



| Primer Name | Sequence | Tm | |
|---|---|---|---|
| ZmHKT1 | F | 5′-TGCTAATGTTTATCGTGCTG-3′ | 56 °C |
| R | 5′-AGGCTGATCCTCTTCCTAAC-3′ | ||
| ZmSOS1 | F | 5′-ACTTGCAGGAGGAATACAAC-3′ | |
| R | 5′-CGAGAAGAGAAGACCACATC-3′ | ||
| ZmNHX | F | 5′-CGTGATGTCGCATTACACCT-3′ | |
| R | 5′-CTGGCAAACTCCCACTTCTC-3′ | ||
| β-Actin | F | 5′-GTGCCCATTTACGAAGGATA-3′ | |
| R | 5′-GAAGACTCCATGCCGATCAT-3′ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljuaid, B.S.; Ashour, H. Exogenous γ-Aminobutyric Acid (GABA) Application Mitigates Salinity Stress in Maize Plants. Life 2022, 12, 1860. https://doi.org/10.3390/life12111860
Aljuaid BS, Ashour H. Exogenous γ-Aminobutyric Acid (GABA) Application Mitigates Salinity Stress in Maize Plants. Life. 2022; 12(11):1860. https://doi.org/10.3390/life12111860
Chicago/Turabian StyleAljuaid, Bandar S., and Hatem Ashour. 2022. "Exogenous γ-Aminobutyric Acid (GABA) Application Mitigates Salinity Stress in Maize Plants" Life 12, no. 11: 1860. https://doi.org/10.3390/life12111860
APA StyleAljuaid, B. S., & Ashour, H. (2022). Exogenous γ-Aminobutyric Acid (GABA) Application Mitigates Salinity Stress in Maize Plants. Life, 12(11), 1860. https://doi.org/10.3390/life12111860





