Comprehensive Phytochemical Analysis of Various Solvent Extracts of Artemisia judaica and Their Potential Anticancer and Antimicrobial Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Preparation of A. judaica Extracts
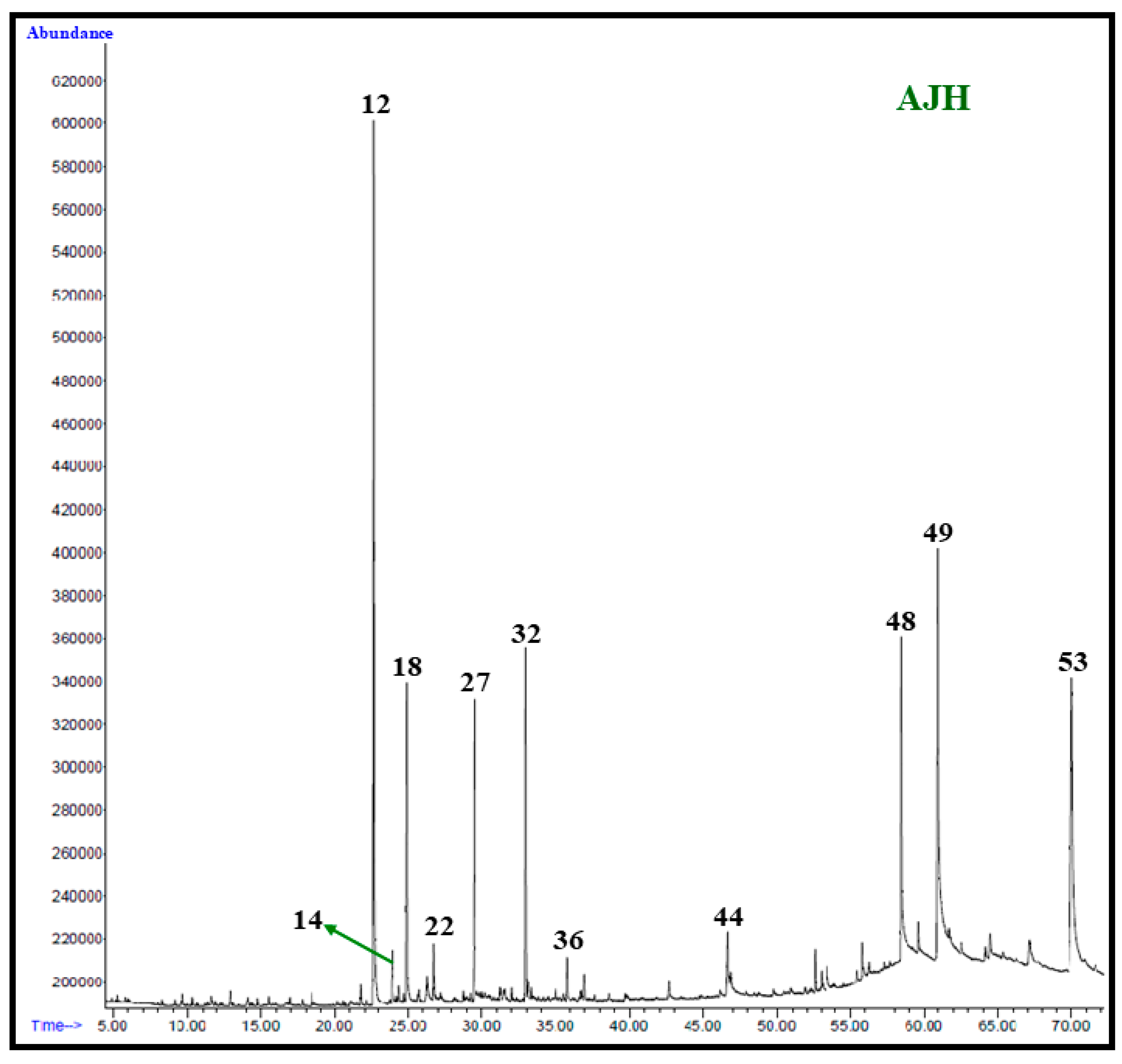
2.4. GC and GC–MS Analysis of A. judaica Extracts
2.5. Calculation of Linear Retention Indices (LRIs)
2.6. Identification of Volatile Components
2.7. Evaluation of Antimicrobial and Anticancer Activity
2.7.1. Antimicrobial Activity
2.7.2. Anticancer Activity
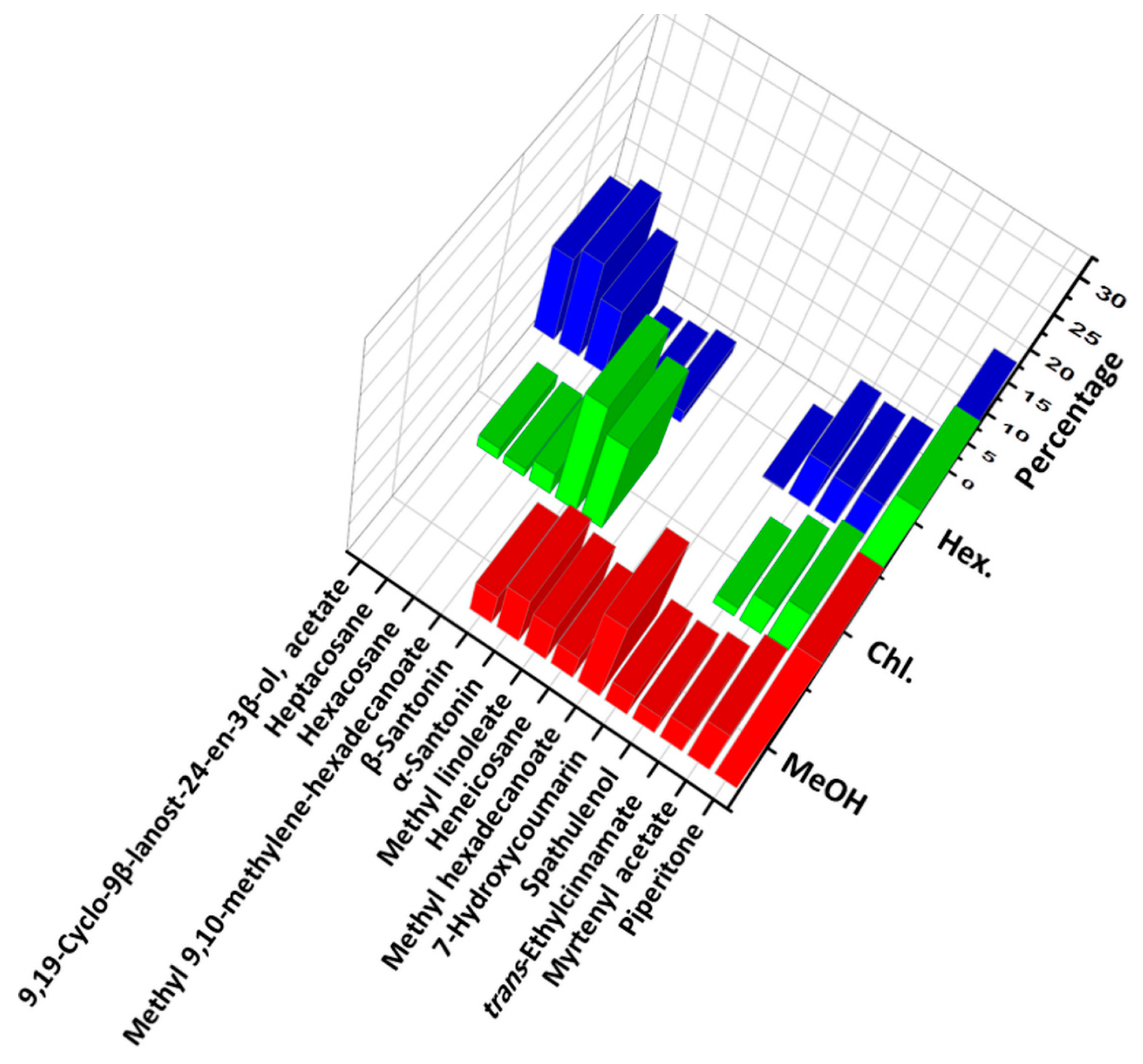
3. Results and Discussion
| Peak | Compound * | M.F. | CAS No. | R.T. (min) | LRILit | LRIExp | Hex % | Chl % | MeOH % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Camphene | C10H16 | 79-92-5 | 11.501 | 946 | 953 | 0.356 | 1.632 | - |
| 2 | Mesitylene | C9H12 | 108-67-8 | 13.051 | 994 | 994 | 0.17 | - | - |
| 3 | Undecane | C11H24 | 1120-21-4 | 17.083 | 1100 | 1100 | - | - | 1.223 |
| 4 | Lavender lactone | C7H10O2 | 1073-11-6 | 14.854 | 1034 | 1041 | 0.492 | 1.138 | - |
| 5 | Artemisia ketone | C10H16O | 546-49-6 | 15.677 | 1056 | 1062 | 0.254 | - | - |
| 6 | p-Cymenene | C10H12 | 1195-32-0 | 16.722 | 1089 | 1089 | 0.265 | - | - |
| 7 | Isophorone | C9H14O | 78-59-1 | 17.92 | 1118 | 1122 | 0.731 | 1.702 | - |
| 8 | p-Menth-2-en-1-ol | C10H18O | 29803-81-4 | 18.526 | 1136 | 1138 | 0.419 | 2.01 | - |
| 9 | 4-Oxoisophorone | C9H12O2 | 1125-21-9 | 18.764 | 1142 | 1144 | 0.297 | - | - |
| 10 | Nordavanone | C11H18O2 | 54933-91-4 | 21.902 | 1231 | 1232 | 0.343 | - | - |
| 11 | Cuminaldehyde | C10H12O | 122-03-2 | 22.325 | 1242 | 1244 | 0.324 | - | - |
| 12 | Piperitone | C10H16O | 89-81-6 | 22.797 | 1249 | 1258 | 20.154 | 28.846 | 26.154 |
| 13 | (2E)-Decenal | C10H18O | 3913-81-3 | 22.968 | 1260 | 1263 | - | - | 3.183 |
| 14 | Thymol | C10H14O | 89-83-8 | 24.003 | 1289 | 1293 | 2.194 | 3.507 | 2.889 |
| 15 | Carvacrol | C10H14O | 499-75-2 | 24.328 | 1298 | 1303 | 0.437 | - | - |
| 16 | cis-Methyl cinnamate | C10H10O2 | 19713-73-6 | 24.486 | 1299 | 1307 | 0.714 | - | - |
| 17 | Filifolide-A | C10H14O2 | 50585-61-0 | 24.806 | 1318 | 1317 | 0.156 | - | - |
| 18 | Myrtenyl acetate | C12H18O2 | 1079-01-2 | 25.011 | 1324 | 1324 | 6.722 | 7.536 | 7.83 |
| 19 | Piperitenone | C10H14O | 491-09-8 | 25.711 | 1340 | 1345 | 0.166 | - | - |
| 20 | Ethyldihydrocinnamate | C11H14O2 | 2021-28-5 | 25.792 | 1347 | 1348 | 0.527 | - | - |
| 21 | cis-Carvyl acetate | C12H18O2 | 1205-42-1 | 26.389 | 1365 | 1366 | 0.235 | - | 1.132 |
| 22 | cis-Ethylcinnamate | C11H12O2 | 4610-69-9 | 26.811 | 1376 | 1379 | 2.402 | 1.331 | - |
| 23 | trans-Methylcinnamate | C10H10O2 | 1754-62-7 | 27.038 | 1376 | 1386 | 0.12 | - | - |
| 24 | β-caryophyllene | C15H24 | 87-44-5 | 28.368 | 1417 | 1428 | 0.115 | - | - |
| 25 | Aromadendrene | C15H24 | 109119-91-7 | 28.889 | 1439 | 1445 | 0.103 | - | - |
| 26 | Seychellene | C15H24 | 20085-93-2 | 29.07 | 1444 | 1451 | 0.431 | 1.101 | - |
| 27 | trans-Ethylcinnamate | C11H12O2 | 103-36-6 | 29.606 | 1465 | 1469 | 6.325 | 5.214 | 4.629 |
| 28 | γ-Gurjunene | C15H24 | 22567-17-5 | 29.824 | 1475 | 1476 | - | 1.978 | 2.859 |
| 29 | Myristicin | C11H12O3 | 607-91-0 | 31.308 | 1517 | 1526 | 0.706 | - | - |
| 30 | 5,6,7,7a-Tetrahydro-4,4,7a-trimethyl-2(4H)-benzofuranone | C11H16O2 | 15356-74-8 | 31.616 | 1535 | 1536 | 0.248 | - | - |
| 31 | Artedouglasia oxide-A | C15H22O3 | 115403-96-8 | 31.72 | 1534 | 1540 | 0.169 | - | - |
| 32 | Spathulenol | C15H24O | 6750-60-3 | 33.034 | 1577 | 1585 | 5.09 | 1.632 | 3.361 |
| 33 | Caryophyllene oxide | C15H24O | 1139-30-6 | 33.224 | 1582 | 1592 | 0.403 | - | - |
| 34 | Allyltetramethoxybenzene | C13H18O4 | 15361-99-6 | 33.483 | 1603 | 1600 | 0.48 | - | - |
| 35 | γ-Dodecalactone | C12H22O2 | 2305-05-7 | 35.606 | 1676 | 1678 | 0.184 | - | - |
| 36 | Apiol | C12H14O4 | 523-80-8 | 35.863 | 1677 | 1687 | 1.3 | - | - |
| 37 | Nonyl phenol | C15H24O | 25154-52-3 | 36.911 | 1727 | 1726 | 0.188 | - | - |
| 38 | (1E)-1-Ethylidene-7a-methyloc tahydro-1H-indene a | C12H20 | 56324-69-7 | 37.122 | - | 1734 | 1.123 | 1.696 | 2.013 |
| 39 | 7-Hydroxycoumarin | C9H6O3 | 93-35-6 | 39.844 | 1836 | 1840 | 0.203 | - | 3.875 |
| 40 | Methyl hexadecanoate | C17H34O2 | 112-39-0 | 41.949 | 1921 | 1925 | - | - | 13.522 |
| 41 | 2-[(1,3-Dimethyl-1H-pyrazol-4-yl)methylene]-3,4-dihydro-1-(2H)naphthalenone a | C16H16N2O | 999476-23-5 | 45.88 | - | 2090 | - | - | 2.444 |
| 42 | Heneicosane | C21H44 | 629-94-7 | 46.029 | 2100 | 2100 | - | - | 3.975 |
| 43 | Methyl linoleate | C19H34O2 | 112-63-0 | 46.291 | 2095 | 2107 | - | - | 6.13 |
| 44 | α-Santonin | C15H18O3 | 481-06-1 | 46.82 | 2117 | 2129 | 1.758 | 13.715 | 7.769 |
| 45 | β-Santonin | C15H18O3 | 481-07-2 | 47.022 | - | 2138 | 0.559 | 17.157 | 5.011 |
| 46 | Methyl 9,10-methylene-hexadecanoate a | C18H34O2 | 1000336-51-3 | 53.607 | - | 2413 | 0.299 | 3.415 | - |
| 47 | Pentacosane | C25H52 | 629-99-2 | 55.946 | 2500 | 2500 | 0.243 | - | - |
| 48 | Hexacosane | C26H54 | 630-01-3 | 58.529 | 2600 | 2600 | 9.52 | 1.37 | - |
| 49 | Heptacosane | C27H56 | 593-49-7 | 61.123 | 2700 | 2700 | 13.973 | 1.825 | - |
| 50 | Octacosane | C28H58 | 630-02-4 | 62.711 | 2800 | 2800 | 0.355 | - | - |
| 51 | Nonacosane | C29H60 | 630-03-5 | 64.648 | 2900 | 2900 | 0.91 | - | - |
| 52 | Triacontane | C30H62 | 638-68-6 | 67.233 | 3000 | 3000 | 0.536 | - | - |
| 53 | 9,19-Cyclo-9β-lanost-24-en-3β-ol, acetate a | C32H52O2 | 1259-10-5 | 70.165 | - | 3106 | 12.106 | - | - |
| Monoterpenes hydrocarbons | 0.621 | 1.632 | - | ||||||
| Oxygenated monoterpenes | 29.004 | 42.899 | 39.005 | ||||||
| Sesquiterpene hydrocarbons | 0.649 | 3.079 | 2.859 | ||||||
| Oxygenated sesquiterpenes | 7.979 | 31.504 | 15.141 | ||||||
| Aliphatic hydrocarbons | 26.66 | 4.891 | 10.394 | ||||||
| Oxygenated aliphatic hydrocarbons | 14.109 | 6.255 | 19.652 | ||||||
| Aromatics | 18.3 | 6.545 | 10.948 | ||||||
| Total identified | 97.322 | 96.805 | 97.999 | ||||||
3.1. Antibacterial Properties
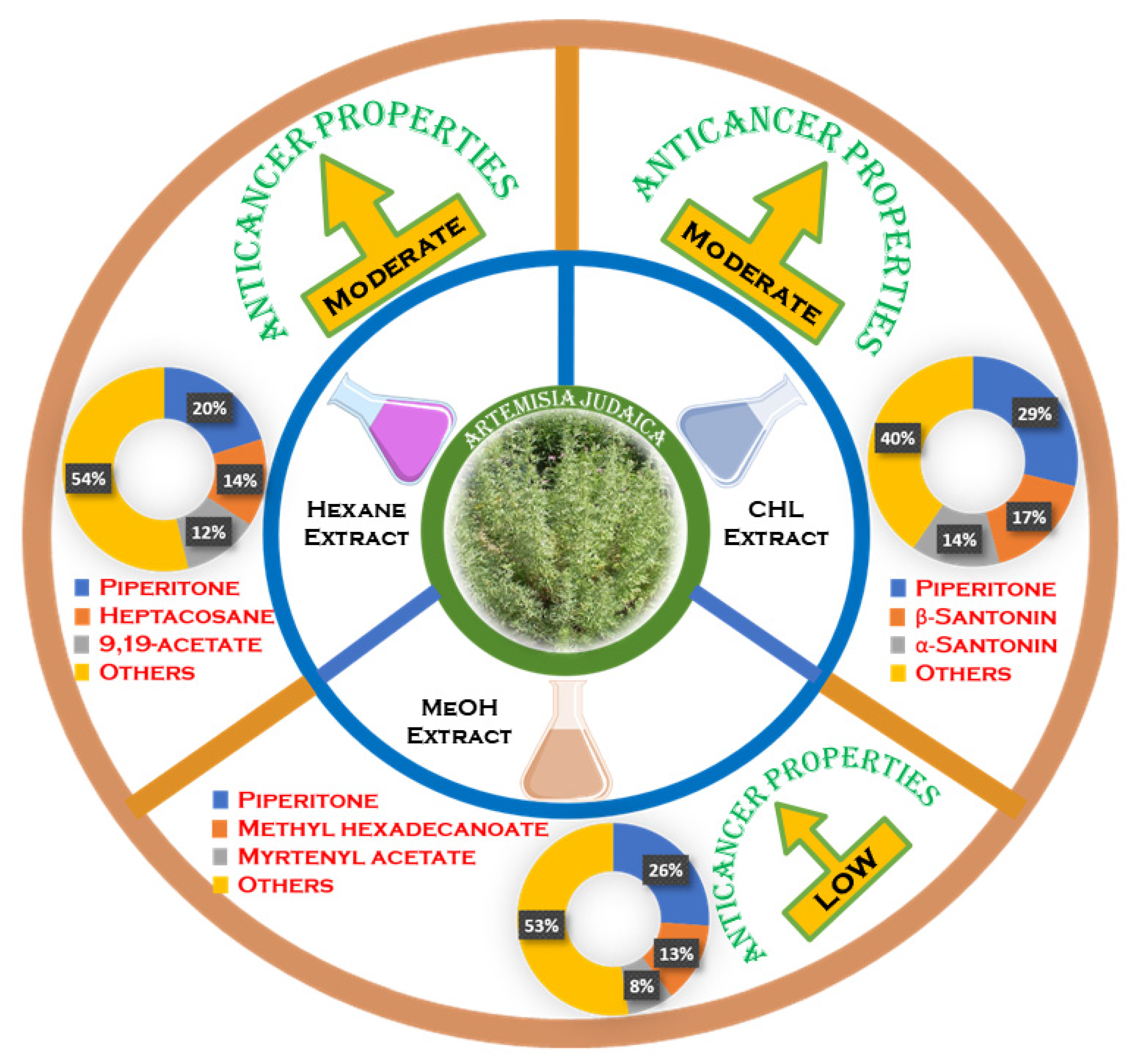
3.2. Anticancer Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howes, M.J.R.; Quave, C.L.; Collemare, J.; Tatsis, E.C.; Twilley, D.; Lulekal, E.; Farlow, A.; Li, L.; Cazar, M.E.; Leaman, D.J. Molecules from nature: Reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants People Planet 2020, 2, 463–481. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lautie, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling plant natural chemical diversity for drug discovery purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef]
- Williamson, E.M.; Liu, X.; Izzo, A.A. Trends in use, pharmacology, and clinical applications of emerging herbal nutraceuticals. Br. J. Pharmacol. 2020, 177, 1227–1240. [Google Scholar] [CrossRef] [Green Version]
- Royal Botanic Gardens, Kew. WCVP, World Checklist of Vascular Plants, Version 2.0; Royal Botanic Gardens, Kew: Richmond, UK, 2020. [Google Scholar]
- Astutik, S.; Pretzsch, J.; Ndzifon Kimengsi, J. Asian medicinal plants’ production and utilization potentials: A review. Sustainability 2019, 11, 5483. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.P.; Chin, Y.-W.; Kinghorn, A.D. The role of pharmacognosy in modern medicine and pharmacy. Curr. Drug Targets 2006, 7, 247–264. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Srivastava, N.; Singh, A.; Kumari, P.; Nishad, J.H.; Gautam, V.S.; Yadav, M.; Bharti, R.; Kumar, D.; Kharwar, R.N. Advances in extraction technologies: Isolation and purification of bioactive compounds from biological materials. In Natural Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2021; pp. 409–433. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Rafińska, K.; Pomastowski, P.; Rudnicka, J.; Krakowska, A.; Maruśka, A.; Narkute, M.; Buszewski, B. Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem. 2019, 289, 16–25. [Google Scholar] [CrossRef]
- Dirar, A.; Alsaadi, D.; Wada, M.; Mohamed, M.; Watanabe, T.; Devkota, H. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S. Afr. J. Bot. 2019, 120, 261–267. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Žutić, I.; Radman, S.; Pleša, M.; Brnčić, M.; Barba, F.J.; Rocchetti, G.; Lucini, L.; Lorenzo, J.M.; Domínguez, R. Effect of different green extraction methods and solvents on bioactive components of chamomile (Matricaria chamomilla L.) flowers. Molecules 2020, 25, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syukriah, A.N.; Liza, M.; Harisun, Y.; Fadzillah, A. Effect of solvent extraction on antioxidant and antibacterial activities from Quercus infectoria (Manjakani). Int. Food Res. J. 2014, 21, 1031. [Google Scholar]
- Trendafilova, A.; Moujir, L.; Sousa, P.; Seca, A. Research advances on health effects of edible artemisia species and some sesquiterpene lactones constituents. Foods 2021, 10, 65. [Google Scholar] [CrossRef]
- Fahd, A.N.; Omar, M.N.; Ramzi, A.M.; Ali, S.A.; Abdullah, A.A.-M. Cytotoxic, antimicrobial and antioxidant activities and phytochemical analysis of Artemisia judaica and A. sieberi in Saudi Arabia. Afr. J. Pharm. Pharmacol. 2020, 14, 278–284. [Google Scholar] [CrossRef]
- Guetat, A.; Al-Ghamdi, F.A.; Osman, A.K. The genus Artemisia L. in the northern region of Saudi Arabia: Essential oil variability and antibacterial activities. Nat. Prod. Res. 2017, 31, 598–603. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.N.; Mahmood, A.; Khan, M.; Alkhathlan, H.Z. Comparative study on the essential oils of Artemisia judaica and A. herba-alba from Saudi Arabia. Arab. J. Chem. 2020, 13, 2053–2065. [Google Scholar] [CrossRef]
- Liu, C.; Murch, S.; El-Demerdash, M.; Saxena, P. Artemisia judaica L.: Micropropagation and antioxidant activity. J. Biotechnol. 2004, 110, 63–71. [Google Scholar] [CrossRef]
- Khan, M.; Al-Saleem, M.S.; Alkhathlan, H.Z. A detailed study on chemical characterization of essential oil components of two Plectranthus species grown in Saudi Arabia. J. Saudi Chem. Soc. 2016, 20, 711–721. [Google Scholar] [CrossRef]
- Acree, T.; Arn, H.; Gas chromatography-olfactometry (GCO) of natural products. Flavornet and human odor space, Sponsored by DATU Inc. 2004. Available online: http://www.flavornet.org (accessed on 27 October 2022).
- Wallace, W.E. Director "Retention Indices" in NIST Chemistry WebBook; NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2022. [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Swapnaja, K.J.M.; Yennam, S.; Chavali, M.; Poornachandra, Y.; Kumar, C.G.; Muthusamy, K.; Jayaraman, V.B.; Arumugam, P.; Balasubramanian, S.; Sriram, K.K. Design, synthesis and biological evaluation of diaziridinyl quinone isoxazole hybrids. Eur. J. Med. Chem. 2016, 117, 85–98. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.; Abdullah, M.M.; Al-Wahaibi, L.H.; Alkhathlan, H.Z. Characterization of secondary metabolites of leaf and stem essential oils of Achillea fragrantissima from central region of Saudi Arabia. Arab. J. Chem. 2020, 13, 5254–5261. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical diversity in leaf and stem essential oils of Origanum vulgare L. and their effects on microbicidal activities. AMB Express 2019, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Abdullah, M.M.S.; Mousa, A.A.; Hamad, Z.A. Chemical composition of vegetative parts and flowers essential oils of wild Anvillea garcinii grown in Saudi Arabia. Records Natl. Prod. 2016, 10, 251. [Google Scholar]
- Ramdane, F.; Hammami, D.E.O.M.; Essid, R.; Sobti, A.; Hrizat, N.; Amara, S.B.; Fares, N.; Mahammed, M.H.; Mohamed, D.O.H.; Limam, F. Chemical Composition and Biological Effects of Essential oil of Artemisia judaica an endemic plant from central Sahara of Algeria Hoggar. Int. J. Biosci. 2017, 10, 16–23. [Google Scholar]
- Al-Qudah, M.A.; Onizat, M.A.; Alshamari, A.K.; Al-Jaber, H.I.; Bdair, O.M.; Muhaidat, R.; Al Zoubi, M.; Al-Bataineh, N. Chemical composition and antioxidant activity of Jordanian Artemisia judaica L. as affected by different drying methods. Int. J. Food Prop. 2021, 24, 482–492. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Qureshi, K.A.; Ali, H.M.; Al-Omar, M.S.; Khan, O.; Mohammed, S.A. Bio-Evaluation of the Wound Healing Activity of Artemisia judaica L. as Part of the Plant’s Use in Traditional Medicine; Phytochemical, Antioxidant, Anti-Inflammatory, and Antibiofilm Properties of the Plant’s Essential Oils. Antioxidants 2022, 11, 332. [Google Scholar] [CrossRef]
- Abu-Darwish, M.; Cabral, C.; Gonçalves, M.; Cavaleiro, C.; Cruz, M.; Zulfiqar, A.; Khan, I.; Efferth, T.; Salgueiro, L. Chemical composition and biological activities of Artemisia judaica essential oil from southern desert of Jordan. J. Ethnopharmacol. 2016, 191, 161–168. [Google Scholar] [CrossRef]
- Abdallah Sallam, S.M.; Mohamed Abdelgaleil, S.A.; da Silva Bueno, I.C.; Abdelwahab Nasser, M.E.; Araujo, R.C.; Abdalla, A.L. Effect of some essential oils on in vitro methane emission. Arch. Anim. Nutr. 2011, 65, 203–214. [Google Scholar] [CrossRef]
- Fleisher, Z.; Fleisher, A. The essential oil of Artemisia judaica L. from the Sinai and Negev deserts. aromatic plants of the Holy Land and the Sinai, Part II. J. Essent. Oil Res. 1990, 2, 271–273. [Google Scholar] [CrossRef]
- Telci, I.; Demirtas, I.; Bayram, E.; Arabaci, O.; Kacar, O. Environmental variation on aroma components of pulegone/piperitone rich spearmint (Mentha spicata L.). Ind. Crops Prod. 2010, 32, 588–592. [Google Scholar] [CrossRef]
- Hellali, N.; Mahammed, M.H.; Ramdane, F.; Talli, A. Antimicrobial and antioxidant activities of Cymbopogon schoenanthus (L.) spreng. essential oil, growing in Illizi-Algeria. J. Med. Plants Res. 2016, 10, 188–194. [Google Scholar]
- Kpadonou, D.; Kpadonou-Kpoviessi, B.; Glinma, B.; Orou, A.-A.S.; Agbani, P.; Gbaguidi, F.; Gbenou, J.; Baba-Moussa, L.; Kpoviessi, S. Effects of the chemical composition of essential oils from seven plants used in traditional medicine in Benin on the growth of eleven pathogenic bacteria in antimicrobial control. J. Pharmacogn. Phytochem. 2022, 11, 23–31. [Google Scholar] [CrossRef]
- Wang, J.; Su, S.; Zhang, S.; Zhai, S.; Sheng, R.; Wu, W.; Guo, R. Structure-activity relationship and synthetic methodologies of α-santonin derivatives with diverse bioactivities: A mini-review. Eur. J. Med. Chem. 2019, 175, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Arantes, F.F.; Barbosa, L.C.; Maltha, C.R.; Demuner, A.J.; da Costa, P.M.; Ferreira, J.R.; Costa-Lotufo, L.V.; Moraes, M.O.; Pessoa, C. Synthesis of novel α-santonin derivatives as potential cytotoxic agents. Eur. J. Med. Chem. 2010, 45, 6045–6051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeragui, B.; Hachem, K.; Halla, N.; Kahloula, K. Essential oil from Artemisia judaica L.(ssp. sahariensis) flowers as a natural cosmetic preservative: Chemical composition, and antioxidant and antibacterial activities. J. Essent. Oil-Bear. Plants 2019, 22, 685–694. [Google Scholar] [CrossRef]
- Dob, T.; Chelghoum, C. Chemical composition of the essential oil of Artemisia judaica L. from Algeria. Flavour Fragr. J. 2006, 21, 343–347. [Google Scholar] [CrossRef]
- AL-Hmadi, H.; El Mokni, R.; Joshi, R.K.; Ashour, M.L.; Hammami, S. The impact of geographical location on the chemical compositions of Pimpinella lutea Desf. growing in Tunisia. Appl. Sci. 2021, 11, 7739. [Google Scholar] [CrossRef]
- Goda, M.S.; Nafie, M.S.; Awad, B.M.; Abdel-Kader, M.S.; Ibrahim, A.K.; Badr, J.M.; Eltamany, E.E. In vitro and in vivo studies of anti-lung cancer activity of Artemesia judaica L. crude extract combined with LC-MS/MS metabolic profiling, docking simulation and HPLC-DAD quantification. Antioxidants 2021, 11, 17. [Google Scholar] [CrossRef]
- Elansary, H.O.; Abdelgaleil, S.A.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. BMC Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef]



| S. No. | Country | City | Major Components (%) | Reference |
|---|---|---|---|---|
| 1. | Jordan | Irbid | (E)-Ethyl cinnamate (21.46), artemisia ketone (20.76), davanone (16.78), (Z)-ethyl cinnamate (12.13), yomogi alcohol (5.15), artemisyl acetate (4.70), and chrysanthenone (4.60). | [31] |
| Al-Mudawarh | Piperitone (30.4), camphor (16.1) and ethyl cinnamate (11.0) and chrysanthenone (6.7) and piperitenone oxide (3.9). | [33] | ||
| 2. | Algeria | Tassili n’Ajjer | Piperitone (71.1), 3-methyl-ethylbutanoate (12.3) and 1-butanol (3.5). | [41] |
| Ilizi | Piperitone (61.9), terpinen-4-ol (4.6) and bornyl acetate (3.0). | [42] | ||
| 3. | Saudi Arabia | Madinah | Piperitone (20–29), myrtenyl acetate (6.7–8.0), α-santonin (1.7–14.0), β-santonin (0.5–17%) and trans-ethyl cinnamate (4.6–6.3), methyl hexadecanoate (0–13.5), 9,19-cyclo-9β-lanost-24-en-3β-ol, acetate (0–12.1), heptacosane (0–14) and hexacosane (0–10). | Present study |
| Tested Extracts of A. judaica | Minimum Inhibitory Concentration (µg/mL) | |||
|---|---|---|---|---|
| Gram-Positive | Gram-Negative | |||
| S. aureus MTCC 96 | M. luteus MTCC 2470 | K. planticola MTCC 530 | E. coli MTCC 739 | |
| MeOH | 3.9 | >250 | 1.9 | >250 |
| Hex | 0.9 | 0.9 | 0.9 | >250 |
| Chl | 0.9 | 0.9 | 0.9 | >250 |
| Ciprofloxacin * | 0.9 | 0.9 | 0.9 | 0.9 |
| Tested Extracts of A. judaica | IC50 (µg/mL) | |||
|---|---|---|---|---|
| HepG2 | DU145 | Hela | A549 | |
| MeOH | 99.95 ± 4.13 | 51.97 ± 0.19 | 67.12 ± 1.75 | 168.54 ± 5.13 |
| Hex | 54.30 ± 0.66 | 48.49 ± 0.16 | 54.40 ± 1.11 | 67.36 ± 0.41 |
| Chl | 56.89 ± 0.37 | 35.41 ± 1.78 | 61.85 ± 0.18 | 76.48 ± 4.7 |
| Doxorubicin | 0.72 ± 0.012 (µM) | 0.36 ± 0.01 (µM) | 0.8 ± 0.71 (µM) | 0.55 ± 0.16 (µM) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; Khan, M.; Al-hamoud, K.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z. Comprehensive Phytochemical Analysis of Various Solvent Extracts of Artemisia judaica and Their Potential Anticancer and Antimicrobial Activities. Life 2022, 12, 1885. https://doi.org/10.3390/life12111885
Khan M, Khan M, Al-hamoud K, Adil SF, Shaik MR, Alkhathlan HZ. Comprehensive Phytochemical Analysis of Various Solvent Extracts of Artemisia judaica and Their Potential Anticancer and Antimicrobial Activities. Life. 2022; 12(11):1885. https://doi.org/10.3390/life12111885
Chicago/Turabian StyleKhan, Merajuddin, Mujeeb Khan, Khaleel Al-hamoud, Syed Farooq Adil, Mohammed Rafi Shaik, and Hamad Z. Alkhathlan. 2022. "Comprehensive Phytochemical Analysis of Various Solvent Extracts of Artemisia judaica and Their Potential Anticancer and Antimicrobial Activities" Life 12, no. 11: 1885. https://doi.org/10.3390/life12111885
APA StyleKhan, M., Khan, M., Al-hamoud, K., Adil, S. F., Shaik, M. R., & Alkhathlan, H. Z. (2022). Comprehensive Phytochemical Analysis of Various Solvent Extracts of Artemisia judaica and Their Potential Anticancer and Antimicrobial Activities. Life, 12(11), 1885. https://doi.org/10.3390/life12111885











