Non-Aerated Common Nettle (Urtica dioica L.) Extract Enhances Green Beans (Phaseolus vulgaris L.) Growth and Soil Enzyme Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Experiment Design
2.2. Plant Sampling
2.3. Soil Chemical Analysis
Soil Enzymatic Activity and Soil Respiration
2.4. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Soil Nutrients
3.3. Plant Functional Traits
3.4. Plant Growth and Production
3.5. Soil Enzymatic Activity
3.6. Soil Respiration
3.7. Correlation Analyses
4. Discussion
4.1. Effects of Soil Type
4.2. Effects of Soil Disinfection
4.3. Effects of Nettle Extract Application on Green Bean Growth
4.4. Effects of Soil Type, Soil Disinfection and Nettle Extract Application Interaction on Green Bean Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kuepper, G. A Brief Overview of the History and Philosophy of Organic Agriculture; Kerr Center for Sustainable Agriculture: Poteau, OK, USA, 2010. [Google Scholar]
- Godlewska, K.; Biesiada, A.; Michalak, I.; Pacyga, P. The Effect of Plant-Derived Biostimulants on White Head Cabbage Seedlings Grown under Controlled Conditions. Sustainability 2019, 11, 5317. [Google Scholar] [CrossRef] [Green Version]
- Soil Health. NRCS Soils. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/main/soils/health/ (accessed on 11 November 2020).
- Palčić, I.; Jagatić Korenika, A.M.; Jakobović, S.; Pasković, I.; Major, N.; Ban, D.; Ban, S.G.; Karoglan, M.; Petek, M.; Herak Ćustić, M.; et al. Soil type affects grape juice free amino acids profile during ripening of cv. Malvasia Istriana (Vitis vinifera L.). N. Z. J. Crop Hortic. Sci. 2020, 48, 22–33. [Google Scholar] [CrossRef]
- Yim, B.; Smalla, K.; Winkelmann, T. Evaluation of apple replant problems based on different soil disinfection treatments—links to soil microbial community structure? Plant Soil 2013, 366, 617–631. [Google Scholar] [CrossRef]
- Peterson, R.; Jensen, P. Effects of nettle water on growth and mineral nutrition of plants. I. composition and properties of nettle water. Biol. Agric. Hortic. 1985, 2, 303–314. [Google Scholar] [CrossRef]
- Pane, C.; Palese, A.M.; Celano, G.; Zaccardelli, M. Effects of compost tea treatments on productivity of lettuce and kohlrabi systems under organic cropping management. Ital. J. Agron. 2014, 9, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Mohd Din, A.R.J.; Cheng, K.K.; Sarmidi, M.R. Assessment of compost extract on yield and phytochemical contents of Pak Choi (Brassica rapa cv. chinensis) grown under different fertilizer strategies. Commun. Soil Sci. Plant Anal. 2017, 48, 274–284. [Google Scholar] [CrossRef]
- Ji, R.; Dong, G.; Shi, W.; Min, J. Effects of liquid organic fertilizers on plant growth and rhizosphere soil characteristics of chrysanthemum. Sustainability 2017, 9, 841. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.; Jensen, P. Effects of nettle (Urtica dioica) water on growth and mineral nutrition of plants: II. Pot-culture and water-culture experiments. Biol. Agric. Hortic. 1986, 4, 7–18. [Google Scholar] [CrossRef]
- Kandeler, E.; Tscherko, D.; Spiegel, H. Long-term monitoring of microbial biomass, N mineralisation and enzyme activities of a Chernozem under different tillage management. Biol. Fertil. Soils 1999, 28, 343–351. [Google Scholar] [CrossRef]
- Chang, E.H.; Chung, R.S.; Tsai, Y.H. Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Sci. Plant Nutr. 2007, 53, 132–140. [Google Scholar] [CrossRef]
- Adeyeye, A.S.; Togun, A.O.; Olaniyan, A.B.; Akanbi, W.B. Effect of fertilizer and rhizobium inoculation on growth and yield of soyabean variety (Glycine max L. Merrill). Adv. Crop Sci. Technol. 2017, 5, 255. [Google Scholar] [CrossRef] [Green Version]
- Canfora, L.; Malusà, E.; Salvati, L.; Renzi, G.; Petrarulo, M.; Benedetti, A. Short-term impact of two liquid organic fertilizers on Solanum lycopersicum L. rhizosphere Eubacteria and Archaea diversity. Appl. Soil Ecol. 2015, 88, 50–59. [Google Scholar] [CrossRef]
- Bulgari, R.; Morgutti, S.; Cocetta, G.; Negrini, N.; Farris, S.; Calcante, A.; Negrini, N.; Farris, S.; Calcante, A.; Spinardi, A.; et al. Evaluation of borage extracts as potential biostimulant using a phenomic, agronomic, physiological, and biochemical approach. Front. Plant Sci. 2017, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Godlewska, K.; Biesiada, A.; Michalak, I.; Pacyga, P. The Effect of Botanical Extracts Obtained through Ultrasound-Assisted Extraction on White Head Cabbage (Brassica Oleracea L. var. Capitata L.) Seedlings Grown under Controlled Conditions. Sustainability 2020, 12, 1871. [Google Scholar] [CrossRef] [Green Version]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Tinti, A.; Nardi, S. Biological activity of vegetal extracts containing phenols on plant metabolism. Molecules 2016, 21, 205. [Google Scholar] [CrossRef]
- Merwad, A.R.M. Using Moringa oleifera extract as biostimulant enhancing the growth, yield and nutrients accumulation of pea plants. J. Plant Nutr. 2018, 41, 425–431. [Google Scholar] [CrossRef]
- Gülçin, I.; Küfrevioǧlu, Ö.İ.; Oktay, M.; Büyükokuroǧlu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef]
- Yıldız, L.; Başkan, K.S.; Tütem, E.; Apak, R. Combined HPLC-CUPRAC (cupric ion reducing antioxidant capacity) assay of parsley, celery leaves, and nettle. Talanta 2008, 77, 304–313. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Mohammadian, M.; Dianat, M. Antidiabetic effect of hydroalcholic Urtica dioica leaf extract in male rats with fructose-induced insulin resistance. Iran J. Med. Sci. 2012, 37, 181. [Google Scholar] [CrossRef] [PubMed]
- Upton, R. Stinging nettles leaf (Urtica dioica L.): Extraordinary vegetable medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Bisht, S.; Bhandari, S.; Bisht, N.S. Urtica dioica (L): An undervalued, economically important plant. Agric. Sci. Res. J. 2012, 2, 250–252. [Google Scholar]
- Bacci, L.; Baronti, S.; Predieri, S.; di Virgilio, N. Fiber yield and quality of fiber nettle (Urtica dioica L.) cultivated in Italy. Ind. Crops Prod. 2009, 29, 480–484. [Google Scholar] [CrossRef]
- Di Virgilio, N.; Papazoglou, E.G.; Jankauskiene, Z.; Di Lonardo, S.; Praczyk, M.; Wielgusz, K. The potential of stinging nettle (Urtica dioica L.) as a crop with multiple uses. Ind. Crops Prod. 2015, 68, 42–49. [Google Scholar] [CrossRef]
- Amini, S.; Azizi, M.; Joharchi, M.R.; Shafei, M.N.; Moradinezhad, F.; Fujii, Y. Determination of allelopathic potential in some medicinal and wild plant species of Iran by dish pack method. Theor. Exp. Plant Physiol. 2014, 26, 189–199. [Google Scholar] [CrossRef]
- Maričić, B.; Radman, S.; Romić, M.; Perković, J.; Major, N.; Urlić, B.; Palčić, I.; Ban, D.; Zorić, Z.; Ban, S.G. Stinging Nettle (Urtica dioica L.) as an Aqueous Plant-Based Extract Fertilizer in Green Bean (Phaseolus vulgaris L.) Sustainable Agriculture. Sustainability 2021, 13, 4042. [Google Scholar] [CrossRef]
- Bozsik, A. Studies on aphicidal efficiency of different stinging nettle extracts. Anz. Schädlingskd. Pfl. Umwelt. 1996, 69, 21–22. [Google Scholar] [CrossRef]
- Kaberia, D.K. Participatory Action Research and Testing the Effectiveness of Stinging Nettle as a Biopesticide in Kenya. Ph.D. Thesis, University of Wisconsin, College of Natural Resources, Stevens Point, WI, USA, 2007. [Google Scholar]
- Hadizadeh, I.; Peivastegan, B.; Kolahi, M. Antifungal activity of nettle (Urtica dioica L.), colocynth (Citrullus colocynthis L. Schrad), oleander (Nerium oleander L.) and konar (Ziziphus spina-christi L.) extracts on plants pathogenic fungi. Pak. J. Biol. Sci. 2009, 12, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Nygaard Sørensen, J.; Thorup-Kristensen, K. Plant-based fertilizers for organic vegetable production. J. Plant. Nutr. Soil Sci. 2011, 174, 321–332. [Google Scholar] [CrossRef]
- Rivera, M.C.; Wright, E.R.; Salice, S.; Fabrizio, M.C. Effect of plant preparations on lettuce yield. Acta Hortic. 2012, 933, 173–179. [Google Scholar] [CrossRef]
- Otles, S.; Yalcin, B. Phenolic compounds analysis of root, stalk, and leaves of nettle. Sci. World J. 2012, 2012, 564367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeipiņa, S.; Alsiņa, I.; Lepse, L. Stinging nettle–the source of biologically active compounds as sustainable daily diet supplement. Res. Rural Dev. 2014, 20, 34–38. [Google Scholar]
- Peterson, R.; Jensen, P. Uptake and transport of nitrogen, phosphorus and potassium in tomato supplied with nettle water and nutrient solution. Plant Soil 1988, 107, 189–196. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [Green Version]
- Chávez-Mendoza, C.; Sánchez, E. Bioactive compounds from Mexican varieties of the common bean (Phaseolus vulgaris): Implications for health. Molecules 2017, 22, 1360. [Google Scholar] [CrossRef] [Green Version]
- Lešić, R.; Borošić, J.; Buturac, I.; Herak Ćustić, M.; Poljak, M.; Romić, D. Povrćarstvo, 3rd ed.; Zrinski d.d.: Čakovec, Croatia, 2016; pp. 552–564. [Google Scholar]
- Bogunović, M.; Vidaček, Ž.; Racz, Z.; Husnjak, S.; Špoljar, A.; Sraka, M. FAO/Unesco. In Pedološka Karta 1:1.000.000; Agronomski Fakultet Sveučilišta u Zagrebu: Zagreb, Croatia, 1998. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Al-Busaidi, A.; Cookson, P.; Yamamoto, T. Methods of pH determination in calcareous soils: Use of electrolytes and suspension effect. Aust. J. Soil Res. 2005, 43, 541–545. [Google Scholar] [CrossRef]
- FAO. Standard Operating Procedure for Soil Organic Carbon. Walkley-Black Method. Titration and Colorimetric Method, GLOSOLAN-SOP-02, Food and Agriculture Organization of the United Nations. 2019. Available online: http://www.fao.org/3/ca7471en/CA7471EN.pdf (accessed on 11 November 2021).
- Zebec, V.; Rastija, D.; Lončarić, Z.; Bensa, A.; Popović, B.; Ivezić, V. Comparison of chemical extraction methods for determination of soil potassium in different soil types. Eurasian Soil Sci. 2017, 50, 1420–1427. [Google Scholar] [CrossRef]
- Kargas, G.; Chatzigiakoumis, I.; Kollias, A.; Spiliotis, D.; Kerkides, P. An Investigation of the relationship between the electrical conductivity of the soil saturated paste extract ECe with the respective values of the mass soil/water ratios 1: 1 and 1: 5 (EC1: 1 and EC1: 5). Proceedings 2018, 2, 661. [Google Scholar] [CrossRef] [Green Version]
- Loeppert, R.H.; Suarez, D.L. Carbonate and gypsum. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1996; Volume 5, pp. 437–474. [Google Scholar]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; Bottomley, P.J., Angle, J.S., Weaver, R.W., Eds.; Wiley: Hoboken, NJ, USA, 1994; Volume 5, pp. 775–833. [Google Scholar]
- Wolinska, A.; Stępniewska, Z.; Szymańska, E. Dehydrogenase activity of soil microorganisms and the total DNA level in soil of different use. J. Agric. Sci. Technol. B 2013, 3, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Rubio, V.E.; Detto, M. Spatiotemporal variability of soil respiration in a seasonal tropical forest. Ecol. Evol. 2017, 7, 7104–7116. [Google Scholar] [CrossRef] [PubMed]
- Green Bean Production Guideline. Available online: https://www.starkeayres.com/uploads/files/Bean-Production-Guideline-2019.pdf (accessed on 14 November 2020).
- Lemanowicz, J. Phosphatases activity and plant available phosphorus in soil under winter wheat (Triticum aestivum L.) fertilized minerally. Pol. J. Agron. 2011, 4, 12–15. [Google Scholar] [CrossRef]
- Moghaddam, F.R.; Aminpanah, H. Green bean (Phaseolus vulgaris L.) growth and yield as affected by chemical phosphorus fertilizer and phosphate bio-fertilizer. Idesia 2015, 33, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Huda, A.I.; El-Behairy, U.A.; El-Desuki, M.; Bakry, M.O.; Abou-Hadid, A.F. Response of green bean to fertilization with potassium and magnesium. Res. J. Agric. Biol. Sci. 2010, 6, 834–839. [Google Scholar]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Rousk, J.; Brookes, P.C.; Baath, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Scharenbroch, B.C.; Johnston, D.P. A microcosm study of the common night crawler earthworm (Lumbricus terrestris) and physical, chemical and biological properties of a designed urban soil. Urban Ecosyst. 2011, 14, 119–134. [Google Scholar] [CrossRef]
- Koron, D.; Sonjak, S.; Regvar, M. Effects of non-chemical soil fumigant treatments on root colonisation with arbuscular mycorrhizal fungi and strawberry fruit production. Crop Prot. 2014, 55, 35–41. [Google Scholar] [CrossRef]
- Bonanomi, G.; Chiurazzi, M.; Caporaso, S.; Del Sorbo, G.; Moschetti, G.; Felice, S. Soil solarization with biodegradable materials and its impact on soil microbial communities. Soil Biol. Biochem. 2008, 40, 1989–1998. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ultra Jr., V. U.; Tanaka, S.; Sakurai, K.; Iwasaki, K. Effects of methyl bromide fumigation, chloropicrin fumigation and steam sterilization on soil nitrogen dynamics and microbial properties in a pot culture experiment. Soil Sci. Plant Nutr. 2008, 54, 886–894. [Google Scholar] [CrossRef]
- Yan, D.; Wang, Q.; Mao, L.; Ma, T.; Li, Y.; Ouyang, C.; Guo, M.; Cao, A. Interaction between nitrification, denitrification and nitrous oxide production in fumigated soils. Atmos. Environ. 2015, 103, 82–86. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Funct. Plant Biol. 2000, 27, 595–607. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Corneo, P.E.; Keitel, C.; Kertesz, M.A.; Dijkstra, F.A. Variation in specific root length among 23 wheat genotypes affects leaf δ13C and yield. Agric. Ecosyst. Environ. 2017, 246, 21–29. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Yang, H.; Chang, Z. Effect of biofumigation and chemical fumigation on soil microbial community structure and control of pepper Phytophthora blight. World J. Microbiol. Biotechnol. 2014, 30, 507–518. [Google Scholar] [CrossRef]
- Pasković, I.; Radić, T.; Perinčić, B.; Užila, Z.; Palčić, I.; Ban, D.; Romić, M.; Žnidarčić, D.; Ban, S.G. The effect of aqueous nettle extract on soil fertility and dwarf French bean vegetative growth. In Proceedings of the 52. Croatian and 12. International Symposium on Agriculture, Dubrovnik, Hrvatska, 12–17 February 2017; pp. 275–279. [Google Scholar]
- Dozet, G.; Ðukić, V.; Balešević-Tubić, S.; Ðurić, N.; Miladinov, Z.; Vasin, J.; Jakšić, S. Uticaj primene vodenih ekstrakata na prinos u organskoj proizvodnji soje. In Zbornik Radova 1, XXII Savetovanje o Biotehnologiji sa Međunarodnim Učešćem; Faculty of Agriculture, University of Kragujevac: Čačak, Serbia, 2017; pp. 81–86. [Google Scholar]
- Lian, B.; Wang, B.; Pan, M.; Liu, C.; Teng, H.H. Microbial release of potassium from K-bearing minerals by thermophilic fungus Aspergillus fumigatus. Geochim. Cosmochim. Acta 2008, 72, 87–98. [Google Scholar] [CrossRef]
- Araújo, A.S.; Leite, L.F.; Santos, V.B.; Carneiro, R.F. Soil microbial activity in conventional and organic agricultural systems. Sustainability 2009, 1, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Janušauskaite, D.; Kadžienė, G.; Auškalnienė, O. The effect of tillage system on soil microbiota in relation to soil structure. Pol. J. Environ. Stud. 2013, 22, 1387–1391. [Google Scholar]
- Bhatt, B.; Chandra, R.; Ram, S.; Pareek, N. Long-term effects of fertilization and manuring on productivity and soil biological properties under rice (Oryza sativa)—Wheat (Triticum aestivum) sequence in Mollisols. Arch. Agron. Soil Sci. 2016, 62, 1109–1122. [Google Scholar] [CrossRef]
- Arriagada, C.; Manquel, D.; Cornejo, P.; Soto, J.; Sampedro, I.; Ocampo, J. Effects of the co-inoculation with saprobe and mycorrhizal fungi on Vaccinium corymbosum growth and some soil enzymatic activities. J. Soil Sci. Plant Nutr. 2012, 12, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, K.K.; Dudeja, S.S. Ecology of legume root nodule bacteria. In Advances in Ecology and Environmental Sciences; 1st Chapter; Mishra, P.C., Behera, N., Senapati, B.K., Guru, B.C., Eds.; Ashish Publishing House: New Delhi, India, 1995; pp. 17–33. [Google Scholar]
- Adak, M.S.; Kibritci, M. Effect of nitrogen and phosphorus levels on nodulation and yield components in faba bean (Vicia faba L.). Legume Res. 2016, 39, 991–994. [Google Scholar] [CrossRef] [Green Version]
- Liese, R.; Schulze, J.; Cabeza, R.A. Nitrate application or P deficiency induce a decline in Medicago truncatula N2-fixation by similar changes in the nodule transcriptome. Sci. Rep. 2017, 7, 46264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Fernández, M.A.; Calvo-Magro, E.; Rodríguez-Sánchez, J.; Valentine, A. Differential growth costs and nitrogen fixation in Cytisus multiflorus (L′ Hér.) Sweet and Cytisus scoparius (L.) Link are mediated by sources of inorganic N. Plant Biol. 2017, 19, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Regus, J.U.; Wendlandt, C.E.; Bantay, R.M.; Gano-Cohen, K.A.; Gleason, N.J.; Hollowell, A.C.; O’Neill, M.R.; Sachs, J.L. Nitrogen deposition decreases the benefits of symbiosis in a native legume. Plant Soil 2017, 414, 159–170. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Hong, S.J.; Park, J.H.; Han, E.J.; Kim, J.H.; Kim, S.C. Effect of aerated compost tea on the growth promotion of lettuce, soybean, and sweet corn in organic cultivation. Plant Pathol. J. 2015, 31, 259. [Google Scholar] [CrossRef] [Green Version]
- Isoi, T.; Yoshida, S. Low nitrogen fixation of common bean (Phaseolus vulgaris L.). Soil Sci. Plant Nutr. 1991, 37, 559–563. [Google Scholar] [CrossRef]

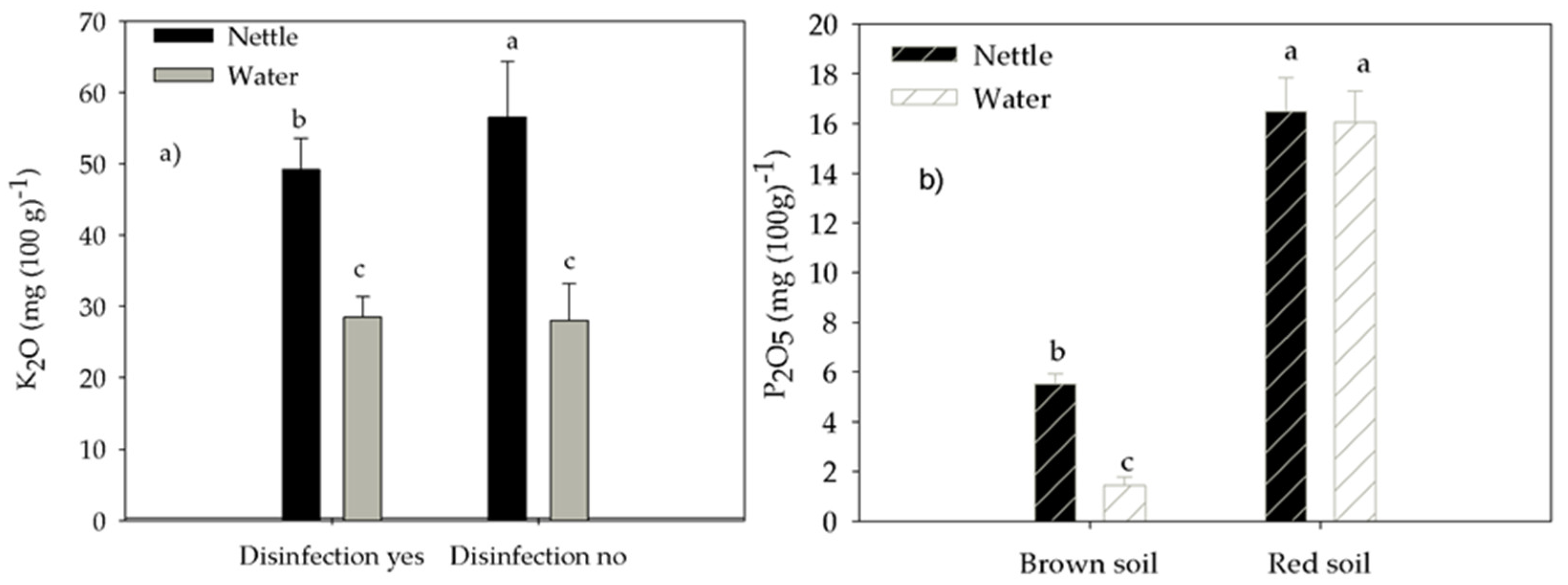

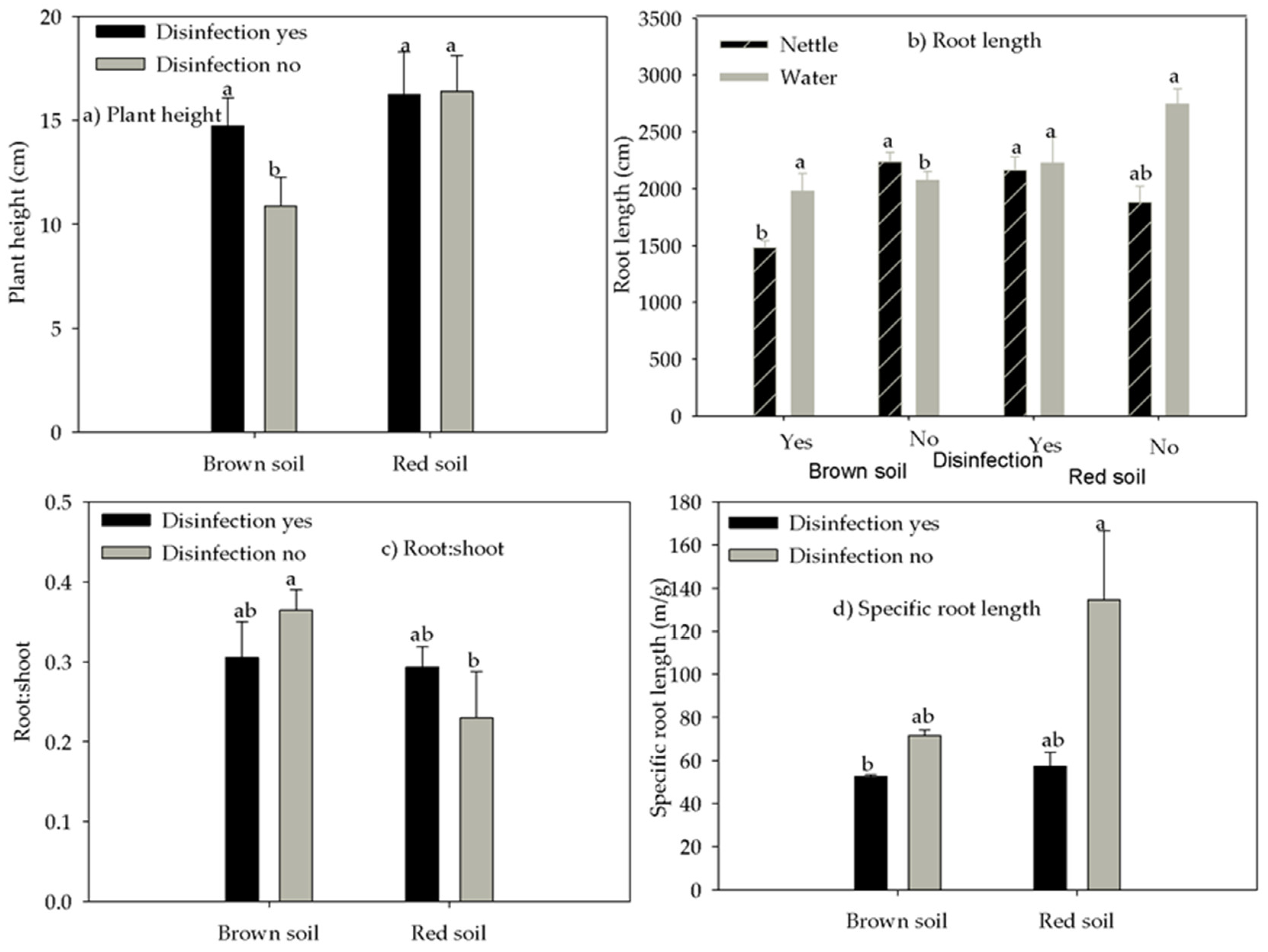
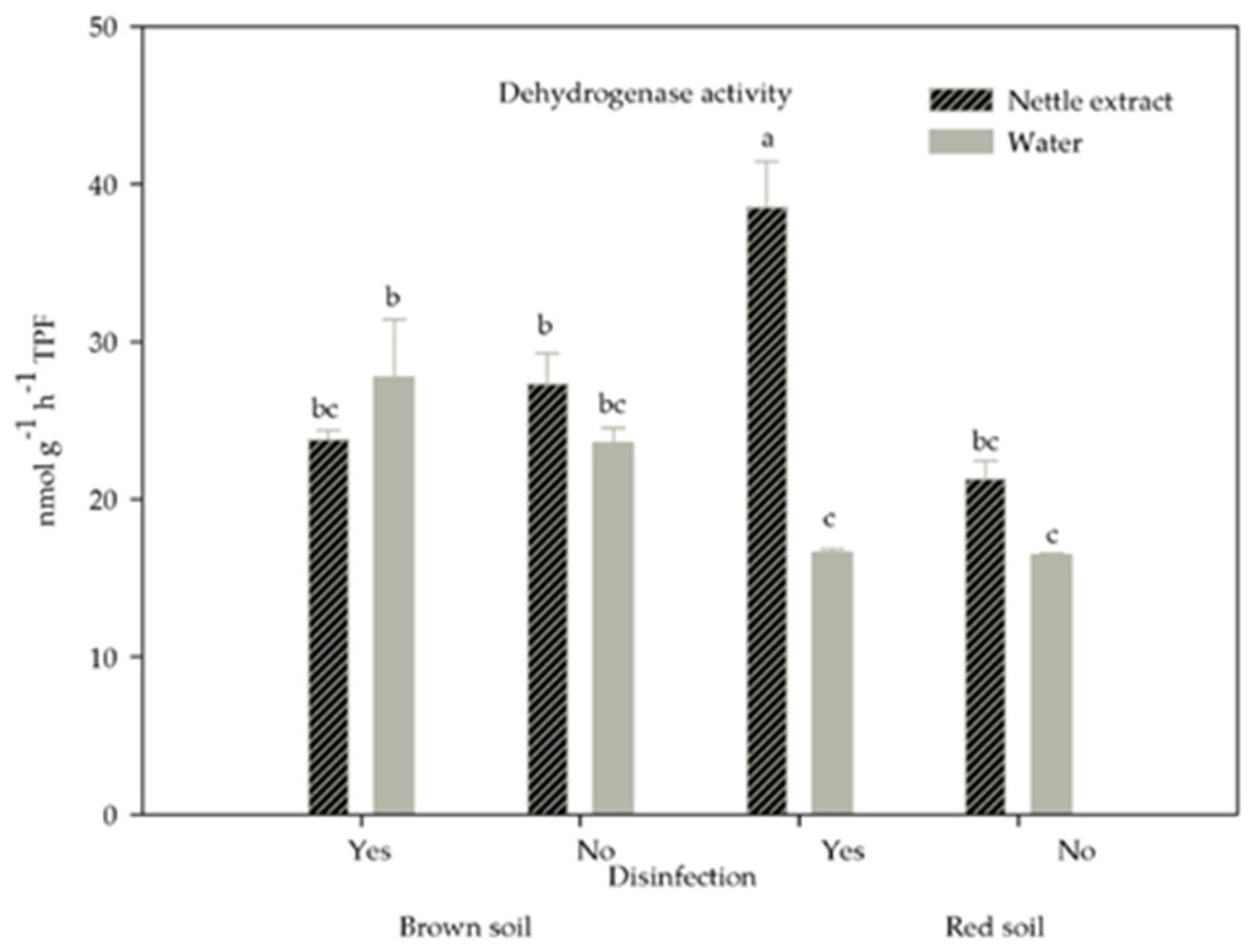

| Soil Type | pH (H2O) | pH (KCl) | N (%) | P (mg 100 g−1) | K (mg 100 g−1) | Humus (%) |
|---|---|---|---|---|---|---|
| Red soil | 7.99 ± 0.04 a | 7.24 ± 0.01 a | 0.17 ± 0.01 a | 31.05 ± 0.61 a | 32.25 ± 1.80 a | 2.75 ± 0.08 a |
| Brown soil | 7.85 ± 0.03 b | 7.20 ± 0.01 b | 0.12 ± 0.01 b | 5.46 ± 0.84 b | 13.55 ± 0.65 b | 2.02 ± 0.11 b |
| Parameter | Unit | Nettle Extract | Nettle Extract (Peterson and Jensen, 1985) | Hoagland’s Nutrient Solution |
|---|---|---|---|---|
| pH | 6.53 | 5.50–5.85 | 6.00 | |
| EC | mS/cm | 5.42 | 4.25–5.02 | 1.80 |
| NH4-N | mM | 8.90 | 13.60–18.40 | 2.00 |
| NO2, NO3-N | mM | 0.02 | 0.00–0.20 | 14.50 |
| P | mM | 0.37 | 1.30–4.00 | 2.00 |
| K | mM | 16.20 | 9.50–44.40 | 6.50 |
| Ca | mM | 15.20 | 10.90–18.30 | 4.00 |
| Mg | mM | 2.40 | 2.40–3.30 | 2.00 |
| S | mM | 1.60 | 2.30–2.40 | 2.00 |
| Fe | µM | 3.23 | 48.30–118.10 | 45.00 |
| B | µM | n.a. * | 71.20–99.00 | 4.60 |
| Mn | µM | 0.62 | 21.10–22.00 | 0.50 |
| Zn | µM | 3.80 | 11.50–26.70 | 0.20 |
| Cu | µM | 1.08 | 6.00–8.00 | 0.20 |
| Mo | µM | n.a. | 0.90–6.00 | 0.70 |
| Factor | Level | pH (H2O) | pH (KCl) | OM (mg g−1) | P2O5 (mg 100 g−1) | K2O (mg 100 g−1) |
|---|---|---|---|---|---|---|
| Soil (S) | Brown | 7.78 ± 0.04 | 7.20 ± 0.02 | 10.60 ± 0.57 | 3.50 ± 0.81 | 32.10 ± 4.05 |
| Red | 7.84 ± 0.03 | 7.17 ± 0.02 | 10.60 ± 0.22 | 16.30 ± 0.85 | 49.00 ± 5.73 | |
| Disinfection (D) | Yes | 7.83 ± 0.04 | 7.16 ± 0.02 | 10.40 ± 0.40 | 9.10 ± 2.00 | 38.90 ± 4.60 |
| No | 7.79 ± 0.03 | 7.21 ± 0.01 | 10.80 ± 0.45 | 10.70 ± 3.00 | 42.30 ± 6.90 | |
| Irrigation (I) | Nettle | 7.74 ± 0.02 | 7.20 ± 0.02 | 11.10 ± 0.20 | 11.00 ± 2.20 | 52.90 ± 4.35 |
| Water | 7.88 ± 0.03 | 7.17 ± 0.02 | 10.10 ± 0. 51 | 8.80 ± 2.80 | 28.30 ± 2.72 | |
| Significance | ||||||
| S | 0.065 | 0.027 | 1.000 | 0.001 | 0.001 | |
| D | 0.195 | 0.002 | 0.439 | 0.001 | 0.079 | |
| I | 0.001 | 0.027 | 0.057 | 0.001 | 0.001 | |
| SxD | 0.002 | 0.168 | 0.443 | 0.001 | 0.006 | |
| SxI | 0.520 | 0.622 | 0.347 | 0.001 | 0.060 | |
| DxI | 0.073 | 0.338 | 0.138 | 0.770 | 0.039 | |
| SxDxI | 0.164 | 0.001 | 0.137 | 0.707 | 0.442 |
| Factor | Level | Shoot Biomass (g) | Root Biomass (mg) | Root:Shoot |
|---|---|---|---|---|
| Soil (S) | Brown | 1.03 ± 0.12 | 330.00 ± 19.00 | 0.34 ± 0.03 |
| Red | 1.17 ± 0.08 | 290.00 ± 30.00 | 0.26 ± 0.03 | |
| Disinfection (D) | Yes | 1.22 ± 0.09 | 350.00 ± 17.00 | 0.30 ± 0.02 |
| No | 0.97 ± 0.10 | 270.00 ± 23.00 | 0.30 ± 0.11 | |
| Irrigation (I) | Nettle | 1.29 ± 0.09 | 310.00 ± 27.00 | 0.26 ± 0.03 |
| Water | 0.95 ± 0.07 | 310.00 ± 25.00 | 0.34 ± 0.028 | |
| Significance | ||||
| S | 0.140 | 0.440 | 0.032 | |
| D | 0.009 | 0.014 | 0.073 | |
| I | 0.004 | 1.000 | 0.032 | |
| SxD | 0.061 | 0.770 | 0.032 | |
| IxS | 0.300 | 1.000 | 0.350 | |
| IxD | 0.230 | 0.880 | 0.760 | |
| IxSxD | 0.590 | 0.490 | 0.093 |
| Factor | Level | Leaf Area (cm2) | Flower Bud Count | Root Nodule Count |
|---|---|---|---|---|
| Soil (S) | Brown | 210.00 ± 30.00 | 4.86 ± 0.51 | 90.60 ± 47.00 |
| Red | 248.00 ± 32.00 | 5.80 ± 0.46 | 84.00 ± 20.00 | |
| Disinfection (D) | Yes | 273.00 ± 29.00 | 5.94 ± 0.46 | 128.00 ± 42.00 |
| No | 181.00 ± 23.00 | 4.71 ± 0.46 | 47.00 ± 20.00 | |
| Irrigation (I) | Nettle | 292.00 ± 28.00 | 6.39 ± 0.37 | 32.60 ± 8.00 |
| Water | 176.00 ± 16.00 | 4.46 ± 0.35 | 142.00 ± 41.00 | |
| Significance | ||||
| S | 0.240 | 0.006 | 0.820 | |
| D | 0.004 | 0.004 | 0.001 | |
| I | 0.001 | 0.001 | 0.001 | |
| SxD | 0.710 | 0.160 | 0.002 | |
| IxS | 0.540 | 1.000 | 0.310 | |
| IxD | 0.710 | 0.260 | 0.007 | |
| IxSxD | 0.940 | 0.230 | 0.014 |
| Factor | Level | Pods Length (cm) | Pods Diameter (mm) | Total Yield (g) | Shoot Dry Weight(g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|
| Soil (S) | Brown | 8.68 ± 0.18 | 6.79 ± 0.15 b | 60.85 ± 13.46 b | 5.53 ± 0.30 | 2.03 ± 0.06 b |
| Red | 9.06 ± 0.16 | 7.41 ± 0.12 a | 90.70 ± 8.26 a | 6.21 ± 0.39 | 3.12 ± 0.17 a | |
| Disinfection (D) | Yes | 9.27 ± 0.12 a | 7.26 ± 0.12 | 104.06 ± 10.46 a | 6.46 ± 0.28 a | 2.64 ± 0.18 |
| No | 8.36 ± 0.21 b | 6.90 ± 0.17 | 47.49 ± 7.93 b | 5.29 ± 0.37 b | 2.51 ± 0.16 | |
| Irrigation (I) | Nettle | 9.29 ± 0.14 a | 7.38 ± 0.14 a | 90.51 ± 12.16 a | 6.79 ± 0.30 a | 3.77 ± 0.22 a |
| Water | 8.39 ± 0.18 b | 6.79 ± 0.13 b | 61.04 ± 10.11 b | 4.96 ± 0.29 b | 2.37 ± 0.10 b | |
| Significance | ||||||
| S | 0.07654 | 0.00013 | 0.013703 | 0.063119 | 0.000000 | |
| D | 0.000037 | 0.0651 | 0.000037 | 0.002095 | 0.412978 | |
| I | 0.00003 | 0.00177 | 0.014797 | 0.000007 | 0.019376 | |
| SxD | 0.23331 | 0.00011 | 0.174397 | 0.208528 | 0.876521 | |
| IxS | 0.76466 | 0.40371 | 0.830372 | 0.809223 | 0.003867 | |
| IxD | 0.02593 | 0.31899 | 0.629620 | 0.011883 | 0.852882 | |
| IxSxD | 0.1022 | 0.98859 | 0.364287 | 0.905730 | 0.718366 |
| Pods Diameter (mm) | Root Dry Weight (g) | |||
|---|---|---|---|---|
| Disinfection | Irrigation | |||
| Soil | Yes | No | Nettle | Water |
| Brown | 7.27 ± 0.16 a | 6.22 ± 0.22 b | 1.97 ± 0.10 c | 2.08 ± 0.08 c |
| Red | 7.26 ± 0.17 a | 7.62 ± 0.16 a | 3.57 ± 0.26 a | 2.66 ± 0.13 b |
| Pods length (cm) | Shoot dry weight (g) | |||
| Irrigation | Irrigation | |||
| Disinfection | Nettle | Water | Nettle | Water |
| Yes | 9.48 ± 0.19 a | 9.03 ± 0.15 a | 6.91 ± 0.43 a | 6.01 ± 0.34 a |
| No | 9.05 ± 0.22 a | 7.62 ± 0.31 b | 6.67 ± 0.43 a | 3.90 ± 0.19 b |
| Factor | Levels | Dehydrogenases (nmol g−1 h−1 TPF) | Acid Phosphatase (µmol g−1 h−1 pNP) | Alkaline Phosphatase (µmol g−1 h−1 pNP) | Soil Respiration (mg CO2 kg−1) |
|---|---|---|---|---|---|
| Soil (S) | Brown | 25.60 ± 1.10 | 1.15 ± 0.07 | 3.62 ± 3.20 | 889 ± 38 |
| Red | 23.20 ± 3.50 | 0.93 ± 0.06 | 2.29 ± 1.80 | 723 ± 80 | |
| Disinfection (D) | Yes | 26.60 ± 3.10 | 1.07 ± 0.05 | 3.04 ± 2.40 | 785 ± 65 |
| No | 22.10 ± 1.60 | 1.01 ± 0.10 | 2.87 ± 2.10 | 828 ± 74 | |
| Irrigation (I) | Nettle | 27.70 ± 2.60 | 1.11 ± 0.10 | 3.34 ± 2.70 | 945 ± 35 |
| Water | 21.10 ± 2.00 | 0.97 ± 0.05 | 2.57 ± 1.90 | 667 ± 55 | |
| Significance | |||||
| S | 0.078 | 0.037 | 0.0011 | 0.00415 | |
| D | 0.010 | 0.71 | 0.31 | 0.334 | |
| I | 0.0023 | 0.20 | 0.0011 | 0.00016 | |
| SxD | 0.0065 | 0.20 | 0.17 | 0.479 | |
| SxI | 0.0011 | 0.71 | 0.54 | 0.0284 | |
| DxI | 0.12 | 0.44 | 0.71 | 0.249 | |
| SxDxI | 0.0011 | 0.65 | 0.17 | 0.834 |
| EC | DHA | AcP | AlP | %C | Ph H2O | pH KCl | P2O5 | K2O | CO2 | Plant Height | Stem Diameter | Leaf Area | Flower no. | Shoot Dry Weight | Root Nodule Count | Spec Root Length | Root:shoot | Root Dry Weight | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | 1 | ||||||||||||||||||
| DHA | 0.32 | 1 | |||||||||||||||||
| AcP | 0.45 | 0.48 | 1 | ||||||||||||||||
| AlP | 0.75 | 0.55 | 0.75 | 1 | |||||||||||||||
| OM | 0.52 | 0.05 | 0.01 | 0.15 | 1 | ||||||||||||||
| pH H2O | −0.85 | −0.38 | −0.44 | −0.58 | −0.6 | 1 | |||||||||||||
| pH KCl | 0.56 | −0.28 | 0.03 | 0.26 | 0.44 | −0.42 | 1 | ||||||||||||
| P2O5 | −0.32 | −0.26 | −0.51 | −0.74 | 0.07 | 0.21 | −0.13 | 1 | |||||||||||
| K2O | 0.35 | 0.19 | −0.13 | −0.09 | 0.25 | −0.3 | 0.22 | 0.68 | 1 | ||||||||||
| CO2 | 0.76 | 0.54 | 0.47 | 0.76 | 0.16 | −0.56 | 0.42 | −0.3 | 0.43 | 1 | |||||||||
| Plant height | 0.22 | 0.34 | −0.14 | −0.08 | 0.21 | −0.26 | −0.06 | 0.58 | 0.84 | 0.31 | 1 | ||||||||
| Stem diameter | 0.28 | 0.03 | 0.09 | 0.07 | −0.03 | −0.19 | 0.17 | −0.02 | 0.18 | 0.06 | 0.26 | 1 | |||||||
| Leaf area | 0.21 | 0.54 | 0.02 | 0.11 | 0.24 | −0.31 | −0.16 | 0.24 | 0.57 | 0.31 | 0.83 | 0.02 | 1 | ||||||
| Flower no. | 0.18 | 0.48 | 0.02 | 0.07 | 0.12 | −0.26 | −0.27 | 0.41 | 0.66 | 0.32 | 0.92 | 0.07 | 0.86 | 1 | |||||
| Shoot dw | 0.13 | 0.28 | −0.11 | 0.01 | 0.21 | −0.16 | −0.1 | 0.37 | 0.54 | 0.11 | 0.84 | 0.18 | 0.84 | 0.84 | 1 | ||||
| Root nodule count | −0.57 | −0.21 | −0.25 | −0.23 | −0.58 | 0.79 | −0.51 | −0.09 | −0.39 | −0.37 | −0.17 | −0.09 | −0.17 | −0.07 | −0.02 | 1 | |||
| Spec root length | −0.13 | −0.37 | −0.53 | −0.47 | 0.22 | 0.1 | 0.23 | 0.51 | 0.27 | −0.26 | 0.2 | 0.29 | −0.14 | −0.09 | 0.16 | −0.11 | 1 | ||
| Root:shoot | −0.05 | −0.04 | 0.27 | 0.2 | −0.29 | 0.12 | −0.08 | −0.58 | −0.57 | 0.02 | −0.68 | −0.18 | −0.5 | −0.53 | −0.78 | 0.26 | −0.66 | 1 | |
| Root dw | 0.03 | 0.27 | 0.27 | 0.28 | −0.3 | 0.08 | −0.4 | −0.32 | −0.16 | 0.12 | 0.05 | −0.1 | 0.28 | 0.32 | 0.1 | 0.46 | −0.83 | 0.52 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maričić, B.; Brkljača, M.; Ban, D.; Palčić, I.; Franin, K.; Marcelić, Š.; Goreta Ban, S. Non-Aerated Common Nettle (Urtica dioica L.) Extract Enhances Green Beans (Phaseolus vulgaris L.) Growth and Soil Enzyme Activity. Life 2022, 12, 2145. https://doi.org/10.3390/life12122145
Maričić B, Brkljača M, Ban D, Palčić I, Franin K, Marcelić Š, Goreta Ban S. Non-Aerated Common Nettle (Urtica dioica L.) Extract Enhances Green Beans (Phaseolus vulgaris L.) Growth and Soil Enzyme Activity. Life. 2022; 12(12):2145. https://doi.org/10.3390/life12122145
Chicago/Turabian StyleMaričić, Branka, Mia Brkljača, Dean Ban, Igor Palčić, Kristijan Franin, Šime Marcelić, and Smiljana Goreta Ban. 2022. "Non-Aerated Common Nettle (Urtica dioica L.) Extract Enhances Green Beans (Phaseolus vulgaris L.) Growth and Soil Enzyme Activity" Life 12, no. 12: 2145. https://doi.org/10.3390/life12122145





