Pathomechanisms and Treatment Implications for Stroke in COVID-19: A Review of the Literature
Abstract
:1. Introduction
2. Basic Epidemiology
Comparison between COVID-19-Associated and Non-COVID-19 Strokes
3. Biological Mechanisms
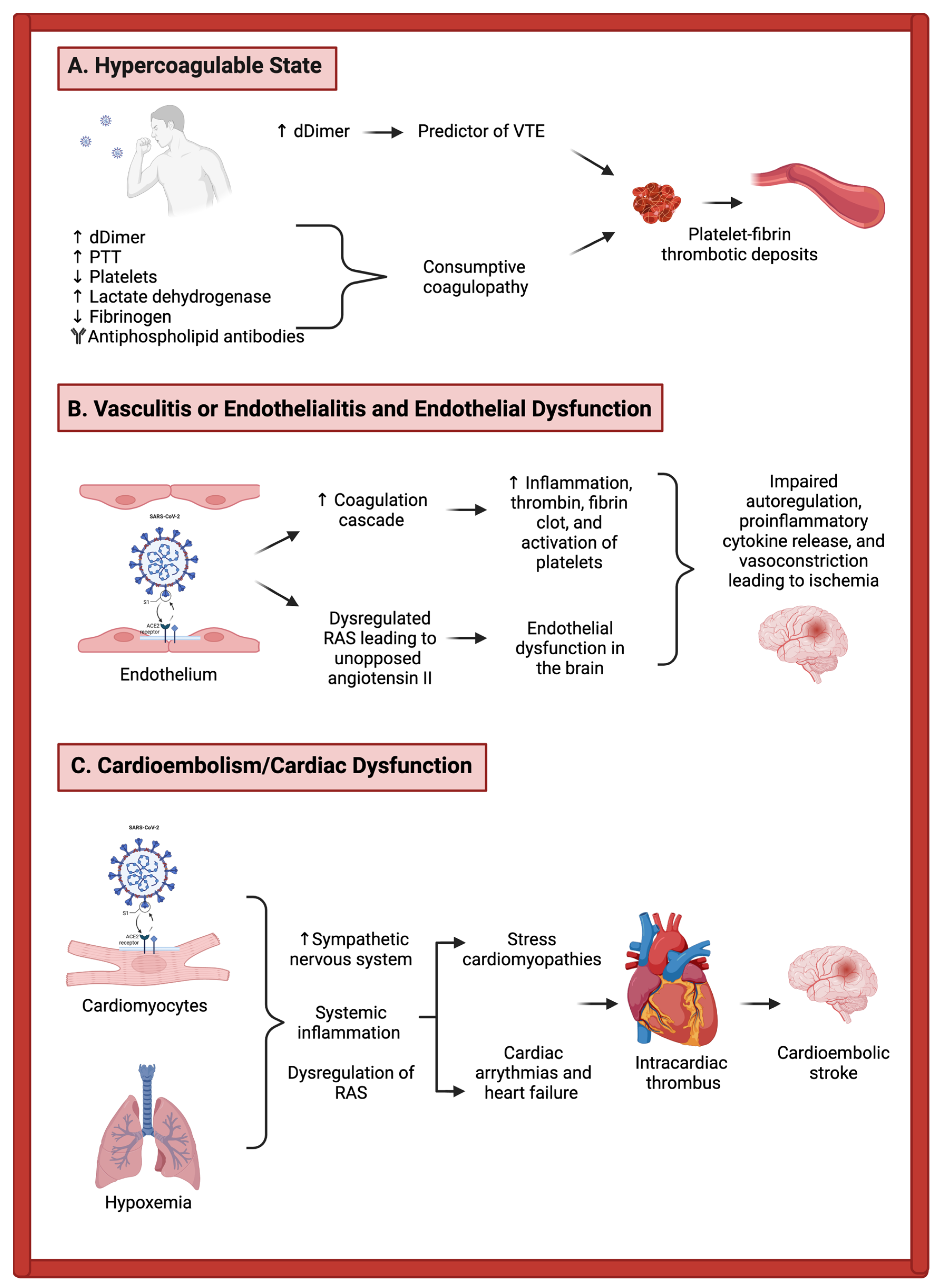
3.1. Mechanisms of Acute Ischemic Stroke in COVID-19 Patients
3.1.1. Vasculitis, Endothelialitis, and Endothelial Dysfunction
3.1.2. Hypercoagulability
3.1.3. Cardiac Dysfunction
3.1.4. Overlap between Mechanistic Categories
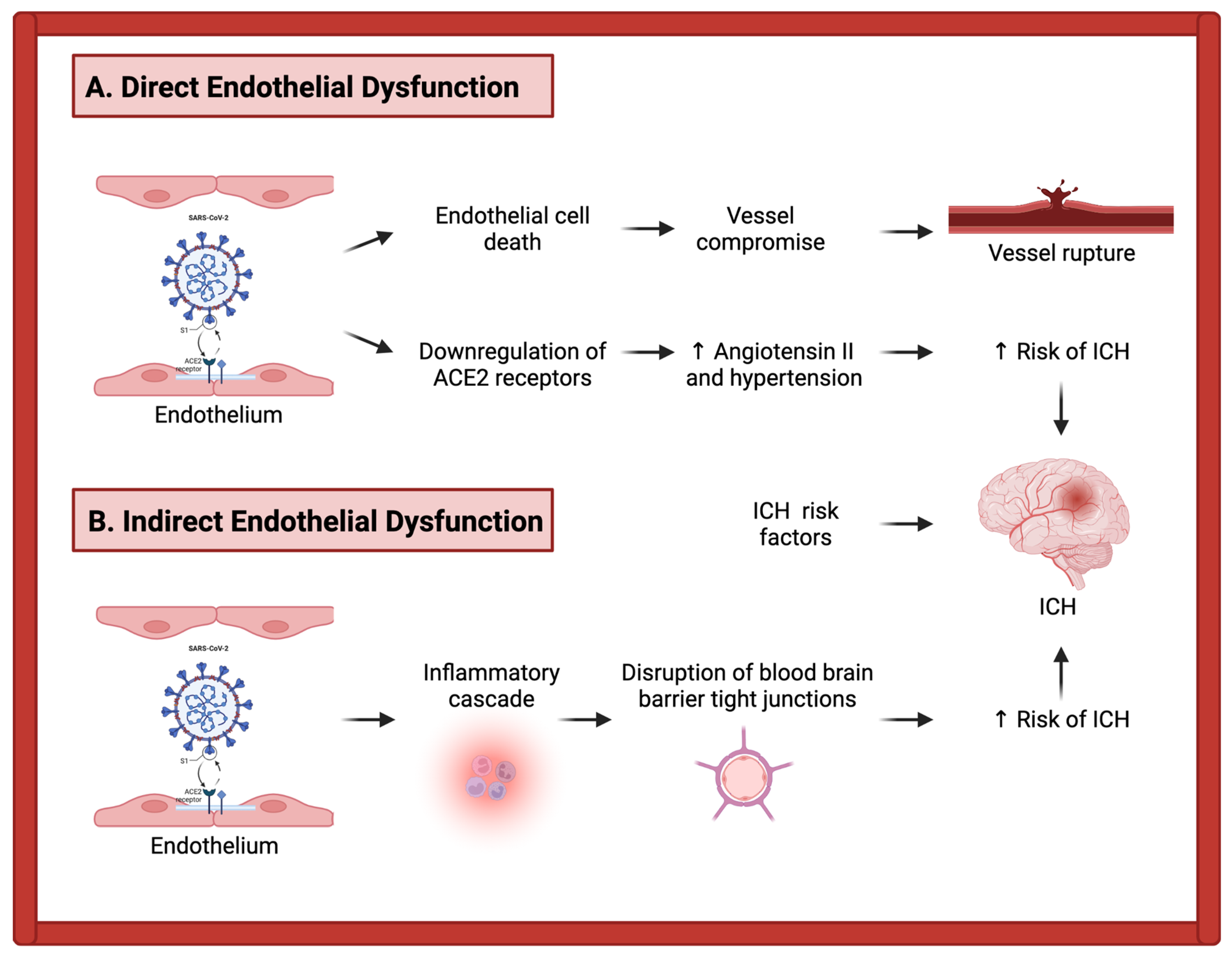
3.2. Mechanisms of Intracerebral Hemorrhage in COVID-19 Patients
4. Case Illustration
5. Delays in Care
6. Potential Treatment Options
6.1. IV Thrombolysis
6.2. Mechanical Thrombectomy
6.3. Therapeutic Anticoagulation
6.4. ACE-Inhibitors and Angiotensin Receptor Blockers
6.5. Experimental Therapies in Ongoing Trials
7. Ancillary Treatment Approaches and Other Considerations
7.1. Tele-Stroke
7.2. Rehabilitation and Prevention of Secondary Stroke
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worobey, M. Dissecting the early COVID-19 cases in Wuhan. Science 2021, 374, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Liotta, E.M.; Batra, A.; Clark, J.R.; Shlobin, N.A.; Hoffman, S.C.; Orban, Z.S.; Koralnik, I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in COVID-19 patients. Ann. Clin. Transl. Neurol. 2020, 7, 2221–2230. [Google Scholar] [CrossRef] [PubMed]
- Nannoni, S.; de Groot, R.; Bell, S.; Markus, H.S. Stroke in COVID-19: A systematic review and meta-analysis. Int. J. Stroke 2021, 16, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; Leacy, R.A.D.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of COVID-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef] [PubMed]
- Sagris, D.; Papanikolaou, A.; Kvernland, A.; Korompoki, E.; Frontera, J.A.; Troxel, A.B.; Gavriatopoulou, M.; Milionis, H.; Lip, G.Y.H.; Michel, P.; et al. COVID-19 and ischemic stroke. Eur. J. Neurol. 2021, 28, 3826–3836. [Google Scholar] [CrossRef] [PubMed]
- Dhamoon, M.S.; Thaler, A.; Gururangan, K.; Kohli, A.; Sisniega, D.; Wheelwright, D.; Mensching, C.; Fifi, J.T.; Fara, M.G.; Jette, N.; et al. Acute Cerebrovascular Events with COVID-19 Infection. Stroke 2021, 52, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; Fifi, J.T.; Ladner, T.R.; Lara-Reyna, J.; Yaeger, K.A.; Yim, B.; Dangayach, N.; Oxley, T.J.; Shigematsu, T.; Kummer, B.R.; et al. Emergent Large Vessel Occlusion Stroke during New York City’s COVID-19 Outbreak: Clinical Characteristics and Paraclinical Findings. Stroke 2020, 51, 2656–2663. [Google Scholar] [CrossRef] [PubMed]
- Kolominsky-Rabas, P.L.; Weber, M.; Gefeller, O.; Neundoerfer, B.; Heuschmann, P.U. Epidemiology of ischemic stroke subtypes according to TOAST criteria: Incidence, recurrence, and long-term survival in ischemic stroke subtypes: A population-based study. Stroke 2001, 32, 2735–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothstein, A.; Oldridge, O.; Schwennesen, H.; Do, D.; Cucchiara, B.L. Acute Cerebrovascular Events in Hospitalized COVID-19 Patients. Stroke 2020, 51, e219–e222. [Google Scholar] [CrossRef] [PubMed]
- Merkler, A.E.; Parikh, N.S.; Mir, S.; Gupta, A.; Kamel, H.; Lin, E.; Lantos, J.; Schenck, E.J.; Goyal, P.; Bruce, S.S.; et al. Risk of Ischemic Stroke in Patients with Coronavirus Disease 2019 (COVID-19) vs. Patients with Influenza. JAMA Neurol. 2020, 77, 1366–1372. [Google Scholar] [CrossRef]
- Avula, A.; Nalleballe, K.; Narula, N.; Sapozhnikov, S.; Dandu, V.; Toom, S.; Glaser, A.; Elsayegh, D. COVID-19 presenting as stroke. Brain Behav. Immun. 2020, 87, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Fara, M.G.; Stein, L.K.; Skliut, M.; Morgello, S.; Fifi, J.T.; Dhamoon, M.S. Macrothrombosis and stroke in patients with mild COVID-19 infection. J. Thromb. Haemost. 2020, 18, 2031–2033. [Google Scholar] [CrossRef] [PubMed]
- Elkind, M.S.V.; Boehme, A.K.; Smith, C.J.; Meisel, A.; Buckwalter, M.S. Infection as a Stroke Risk Factor and Determinant of Outcome After Stroke. Stroke 2020, 51, 3156–3168. [Google Scholar] [CrossRef]
- Ehaideb, S.N.; Abdullah, M.L.; Abuyassin, B.; Bouchama, A. Evidence of a wide gap between COVID-19 in humans and animal models: A systematic review. Crit. Care 2020, 24, 594. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.C.; Eldahshan, W.; Rutkowski, E. COVID-19-Related Stroke. Transl. Stroke Res. 2020, 11, 322–325. [Google Scholar] [CrossRef]
- Albrecht, L.; Bishop, E.; Jay, B.; Lafoux, B.; Minoves, M.; Passaes, C. COVID-19 Research: Lessons from Non-Human Primate Models. Vaccines 2021, 9, 886. [Google Scholar] [CrossRef]
- Kumari, P.; Rothan, H.A.; Natekar, J.P.; Stone, S.; Pathak, H.; Strate, P.G.; Arora, K.; Brinton, M.A.; Kumar, M. Neuroinvasion and Encephalitis Following Intranasal Inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses 2021, 13, 132. [Google Scholar] [CrossRef]
- Khateb, M.; Bosak, N.; Muqary, M. Coronaviruses and Central Nervous System Manifestations. Front. Neurol. 2020, 11, 715. [Google Scholar] [CrossRef]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-Y.; Lee, H.-K.; Park, J.H.; Cho, S.-J.; Kwon, M.; Jo, C.; Koh, Y.H. Altered COVID-19 receptor ACE2 expression in a higher risk group for cerebrovascular disease and ischemic stroke. Biochem. Biophys. Res. Commun. 2020, 528, 413–419. [Google Scholar] [CrossRef]
- Spence, J.D.; de Freitas, G.R.; Pettigrew, L.C.; Ay, H.; Liebeskind, D.S.; Kase, C.S.; Brutto, O.H.D.; Hankey, G.J.; Venketasubramanian, N. Mechanisms of Stroke in COVID-19. Cerebrovasc. Dis. 2020, 49, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Venketasubramanian, N.; Anderson, C.; Ay, H.; Aybek, S.; Brinjikji, W.; de Freitas, G.R.; Brutto, O.H.D.; Fassbender, K.; Fujimura, M.; Goldstein, L.B.; et al. Stroke Care during the COVID-19 Pandemic: International Expert Panel Review. Cerebrovasc. Dis. 2021, 50, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Palaiodimou, L.; Zand, R.; Lioutas, V.A.; Krogias, C.; Katsanos, A.H.; Shoamanesh, A.; Sharma, V.K.; Shahjouei, S.; Baracchini, C.; et al. COVID-19 and cerebrovascular diseases: A comprehensive overview. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420978004. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Janardhan, V.; Janardhan, V.; Kalousek, V. COVID-19 as a Blood Clotting Disorder Masquerading as a Respiratory Illness: A Cerebrovascular Perspective and Therapeutic Implications for Stroke Thrombectomy. J. Neuroimaging 2020, 30, 555–561. [Google Scholar] [CrossRef]
- Schulman, S.; Hu, Y.; Konstantinides, S. Venous thromboembolism in COVID-19. J. Thromb. Haemost. 2020, 120, 1642–1653. [Google Scholar] [CrossRef]
- Leisman, D.E.; Deutschman, C.S.; Legrand, M. Facing COVID-19 in the ICU: Vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020, 46, 1105–1108. [Google Scholar] [CrossRef]
- Sardu, C.; Gambardella, J.; Morelli, M.B.; Wang, X.; Marfella, R.; Santulli, G. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. J. Clin. Med. Res. 2020, 9, 1417. [Google Scholar] [CrossRef]
- Kirschenbaum, D.; Imbach, L.L.; Rushing, E.J.; Frauenknecht, K.B.M.; Gascho, D.; Ineichen, B.V.; Keller, E.; Kohler, S.; Lichtblau, M.; Reimann, R.R.; et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol. Appl. Neurobiol. 2021, 47, 454–459. [Google Scholar] [CrossRef]
- Lee, S.G.; Fralick, M.; Sholzberg, M. Coagulopathy associated with COVID-19. CMAJ 2020, 192, E583. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Ishida, K.; Torres, J.; Mac Grory, B.; Raz, E.; Humbert, K.; Henniger, N.; Trivedi, T.; Lillemoe, K.; Alam, S.; et al. SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke 2020, 51, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H.; Warkentin, T.E.; Thachil, J.; van der Poll, T.; Levi, M.; Scientific and Standardization Committee on DIC, and the Scientific and Standardization Committee on Perioperative and Critical Care of the International Society on Thrombosis and Haemostasis. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemost. 2019, 17, 1989–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghayari Sheikh Neshin, S.; Shahjouei, S.; Koza, E.; Friedenberg, I.; Khodadadi, F.; Sabra, M.; Kobeissy, F.; Ansari, S.; Tsivgoulis, G.; Li, J.; et al. Stroke in SARS-CoV-2 Infection: A Pictorial Overview of the Pathoetiology. Front. Cardiovasc. Med. 2021, 8, 649922. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Connors, J.M.; Warkentin, T.E.; Thachil, J.; Levi, M. The unique characteristics of COVID-19 coagulopathy. Crit. Care 2020, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 1–13. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; der Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Akhmerov, A.; Marbán, E. COVID-19 and the heart. Circ. Res. 2020, 126, 1443–1455. [Google Scholar] [CrossRef] [Green Version]
- Adão, R.; Guzik, T.J. Inside the Heart of COVID-19. Cardiovascular Research; Oxford University Press (OUP): Oxford, UK, 2020; pp. e59–e61. [Google Scholar]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [Green Version]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19–related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawdey, M.S.; Loskutoff, D.J. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J. Clin. Investig. 1991, 88, 1346–1353. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhang, J.; Wang, C.; Chen, X.; Zhao, X.; Jing, H.; Liu, H.; Li, Z.; Wang, L.; Shi, J. COVID-19 and ischemic stroke: Mechanisms of hypercoagulability (Review). Int. J. Mol. Med. 2021, 47, 4854. [Google Scholar] [CrossRef] [PubMed]
- Navi, B.B.; Iadecola, C. Ischemic stroke in cancer patients: A review of an underappreciated pathology. Ann. Neurol. 2018, 83, 873–883. [Google Scholar] [CrossRef]
- Margos, N.P.; Meintanopoulos, A.S.; Filioglou, D.; Ellul, J. Intracerebral hemorrhage in COVID-19: A narrative review. J. Clin. Neurosci. 2021, 89, 271–278. [Google Scholar] [CrossRef]
- Cheruiyot, I.; Sehmi, P.; Ominde, B.; Bundi, P.; Mislani, M.; Ngure, B.; Olabu, B.; Ogeng’o, J.A. Intracranial hemorrhage in coronavirus disease 2019 (COVID-19) patients. Neurol. Sci. 2021, 42, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Melmed, K.R.; Cao, M.; Dogra, S.; Zhang, R.; Yaghi, S.; Lewis, A.; Jain, R.; Bilaloglu, S.; Chen, J.; Czeisler, B.M.; et al. Risk factors for intracerebral hemorrhage in patients with COVID-19. J. Thromb. Thrombolysis 2021, 51, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Benger, M.; Williams, O.; Siddiqui, J.; Sztriha, L. Intracerebral haemorrhage and COVID-19: Clinical characteristics from a case series. Brain Behav. Immun. 2020, 88, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, X.; Fan, H.; Luo, Y.; Song, Y.; Xu, Y.; Chen, Y. Potential mechanisms of hemorrhagic stroke in elderly COVID-19 patients. Aging 2020, 12, 10022–10034. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, S.; Liu, J.; Li, L.; Li, Y.; Wu, X.; Li, Z.; Deng, P.; Zhang, J.; Zhong, N.; et al. Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005, 41, 1089–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Samkari, H.; Karp Leaf, R.S.; Dzik, W.H.; Carlson, J.C.T.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Gupta, S.; et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef]
- Deshotels, M.R.; Xia, H.; Sriramula, S.; Lazartigues, E.; Filipeanu, C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 2014, 64, 1368–1375. [Google Scholar] [CrossRef] [Green Version]
- Altschul, D.J.; Unda, S.R.; de La Garza Ramos, R.; Zampolin, R.; Benton, J.; Holland, R.; Fortunel, A.; Haranhalli, N. Hemorrhagic presentations of COVID-19: Risk factors for mortality. Clin. Neurol. Neurosurg. 2020, 198, 106112. [Google Scholar] [CrossRef] [PubMed]
- Daly, S.R.; Nguyen, A.V.; Zhang, Y.; Feng, D.; Huang, J.H. The Relationship Between COVID-19 Infection and Intracranial Hemorrhage: A systematic review. Brain Hemorrhages 2021, 2, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Batra, A.; Clark, J.R.; LaHaye, K.; Shlobin, N.A.; Hoffman, S.C.; Orban, Z.S.; Colton, K.; Dematte, J.E.; Sorond, F.A.; Koralnik, I.J.; et al. Transcranial Doppler Ultrasound Evidence of Active Cerebral Embolization in COVID-19. J. Stroke Cerebrovasc. Dis. 2021, 30, 105542. [Google Scholar] [CrossRef]
- Bansal, S.; Roy, M.; Chatterjee, T.; Roy, A.K. Deaths due to delayed presentation to the hospital from fear of contracting COVID-19 during lockdown period: A tertiary care center experience. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 299–301. [Google Scholar] [CrossRef]
- Findling, M.G.; Blendon, R.J.; Benson, J.M. Delayed Care with Harmful Health Consequences—Reported Experiences from National Surveys During Coronavirus Disease 2019. JAMA Health Forum 2020, 1, e201463. [Google Scholar]
- Eswaran, V.; Wang, R.C.; Vashi, A.A.; Kanzaria, H.K.; Fahimi, J.; Raven, M.C. Patient reported delays in obtaining emergency care during COVID19. Am. J. Emerg. Med. 2021; in press. [Google Scholar] [CrossRef]
- Altschul, D.J.; Haranhalli, N.; Esenwa, C.; Unda, S.R.; Garza Ramos, R.d.L.; Dardick, J.; Fernandez-Torres, J.; Toma, A.; Labovitz, D.; Cheng, N.; et al. The Impact of COVID-19 on Emergent Large-Vessel Occlusion: Delayed Presentation Confirmed by ASPECTS. AJNR Am. J. Neuroradiol. 2020, 41, 2271–2273. [Google Scholar]
- Schirmer, C.M.; Ringer, A.J.; Arthur, A.S.; Binning, M.J.; Fox, W.C.; James, R.F.; Levitt, M.R.; Tawk, R.G.; Veznedaroglu, E.; Walker, M.; et al. Delayed presentation of acute ischemic strokes during the COVID-19 crisis. J. Neurointerv. Surg. 2020, 12, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, H.; Kung, D.; Fisher, M.; Shen, Y.; Liu, R. Impact of the COVID-19 Epidemic on Stroke Care and Potential Solutions. Stroke 2020, 51, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Kerleroux, B.; Fabacher, T.; Bricout, N.; Moïse, M.; Testud, B.; Vingadassalom, S.; Ifergan, H.; Janot, K.; Consoli, A.; Hassen, W.B.; et al. Mechanical Thrombectomy for Acute Ischemic Stroke Amid the COVID-19 Outbreak: Decreased Activity, and Increased Care Delays. Stroke 2020, 51, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Khosravani, H.; Rajendram, P.; Notario, L.; Chapman, M.G.; Menon, B.K. Protected Code Stroke: Hyperacute Stroke Management During the Coronavirus Disease 2019 (COVID-19) Pandemic. Stroke 2020, 51, 1891–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warach, S.J.; Saver, J.L. Stroke Thrombolysis with Tenecteplase to Reduce Emergency Department Spread of Coronavirus Disease 2019 and Shortages of Alteplase. JAMA Neurol. 2020, 77, 1203–1204. [Google Scholar] [CrossRef]
- Escalard, S.; Maïer, B.; Redjem, H.; Delvoye, F.; Hébert, S.; Smajda, S.; Ciccio, G.; Desilles, J.; Mazighi, M.; Blanc, R.; et al. Treatment of Acute Ischemic Stroke due to Large Vessel Occlusion with COVID-19: Experience From Paris. Stroke 2020, 51, 2540–2543. [Google Scholar] [CrossRef]
- Wang, A.; Mandigo, G.K.; Yim, P.D.; Meyers, P.M.; Lavine, S.D. Stroke and mechanical thrombectomy in patients with COVID-19: Technical observations and patient characteristics. J. Neurointerv. Surg. 2020, 12, 648–653. [Google Scholar] [CrossRef]
- Papanagiotou, P.; Parrilla, G.; Pettigrew, L.C. Thrombectomy for Treatment of Acute Stroke in the COVID-19 Pandemic. Cerebrovasc. Dis. 2021, 50, 20–25. [Google Scholar] [CrossRef]
- Yavagal, D.R.; Saini, V.; Inoa, V.; Gardener, H.E.; Martins, S.O.; Fakey, M.; Ortega, S.; Mansour, O.; Leung, T.; Al-Mufti, F.; et al. MT2020 Global Executive Committee. International Survey of Mechanical Thrombectomy Stroke Systems of Care during COVID-19 Pandemic. J. Stroke Cerebrovasc. Dis. 2021, 30, 105806. [Google Scholar] [CrossRef]
- Sarfo, F.S.; Mensah, N.O.; Opoku, F.A.; Adusei-Mensah, N.; Ampofo, M.; Ovbiagele, B. COVID-19 and stroke: Experience in a Ghanaian healthcare system. J. Neurol. Sci. 2020, 416, 117044. [Google Scholar] [CrossRef]
- Bach, I.; Surathi, P.; Montealegre, N.; Abu-Hadid, O.; Rubenstein, S.; Redko, S.; Gupta, S.; Hillen, M.; Patel, P.; Khandelwal, P.; et al. Stroke in COVID-19: A single-centre initial experience in a hotspot of the pandemic. Stroke Vasc. Neurol. 2020, 5, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Smith, C.J.; Roffe, C.; Simister, R.; Narayanamoorthi, S.; Marigold, R.; Willmot, M.; Dixit, A.; Hassan, A.; Quinn, T.J.; et al. Characteristics and outcomes of COVID-19 associated stroke: A UK multicentre case-control study. J. Neurol. Neurosurg. Psychiatry. 2021, 92, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Chocron, R.; Galand, V.; Cellier, J.; Gendron, N.; Pommier, T.; Bory, O.; Khider, L.; Trimaille, A.; Goudot, G.; Weizman, O.; et al. Anticoagulation Before Hospitalization Is a Potential Protective Factor for COVID-19: Insight From a French Multicenter Cohort Study. J. Am. Heart Assoc. 2021, 10, e018624. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; De Sancho, M.T.; Diuguid, D.; Griffin, D.O.; et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: July 2021 update on post-discharge thromboprophylaxis. Blood Adv. 2021, 5, 872–888. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health (US): Bethesda, MD, USA, 2021.
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Kase, C.S.; Shoamanesh, A.; Abdalkader, M.; Pikula, A.; Sathya, A.; Catanese, L.; Ellis, A.T.; Nguyen, T.N. Stroke and Thromboprophylaxis in the Era of COVID-19. J. Stroke Cerebrovasc. Dis. 2021, 30, 105392. [Google Scholar] [CrossRef]
- Beyrouti, R.; Adams, M.E.; Benjamin, L.; Cohen, H.; Farmer, S.F.; Goh, Y.Y.; Humphries, F.; Jager, H.R.; Losseff, N.A.; Perry, R.J.; et al. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry 2020, 91, 889–891. [Google Scholar] [CrossRef]
- Rico-Mesa, J.S.; White, A.; Anderson, A.S. Outcomes in Patients with COVID-19 Infection Taking ACEI/ARB. Curr. Cardiol. Rep. 2020, 22, 31. [Google Scholar] [CrossRef] [Green Version]
- Kurdi, A.; Abutheraa, N.; Akil, L.; Godman, B. A systematic review and meta-analysis of the use of renin-angiotensin system drugs and COVID-19 clinical outcomes: What is the evidence so far? Pharmacol. Res. Perspect. 2020, 8, e00666. [Google Scholar] [CrossRef]
- Lopes, R.D.; Macedo, A.V.S.; de Barros ESilva, P.G.M.; Moll-Bernardes, R.J.; Dos Santos, T.M.; Mazza, L.; Feldman, A.; Arruda, G.D.S.; de Albuquerque, D.C.; Camiletti, A.S.; et al. Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted with COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Harky, A.; Chor, C.Y.T.; Nixon, H.; Jeilani, M. The controversy of using angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in COVID-19 patients. J. Renin Angiotensin Aldosterone Syst. 2021, 22, 1470320320987118. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Ndiwane, N.; Orner, M.B.; Palacios, N.; Mittler, B.; Berlowitz, D.; Kazis, L.E.; Xia, W. The association of COVID-19 occurrence and severity with the use of angiotensin converting enzyme inhibitors or angiotensin-II receptor blockers in patients with hypertension. PLoS ONE 2021, 16, e0248652. [Google Scholar]
- Study to Assess the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Inhaled APN01 Developed as Treatment for COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT05065645 (accessed on 22 November 2021).
- Evaluation of The Efficacy of Triazavirin Versus Oseltamivir in Egyptian Patients Infected with COVID-19—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04973462 (accessed on 22 November 2021).
- Efficacy and Safety of Direct Anti HCV Drugs in the Treatment of SARS-CoV-2 (COVID-19)—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04535869 (accessed on 22 November 2021).
- Hubert, G.J.; Corea, F.; Schlachetzki, F. The role of telemedicine in acute stroke treatment in times of pandemic. Curr. Opin. Neurol. 2021, 34, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Telestroke at Comprehensive Stroke Center during the COVID-19 Pandemic—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04761874?term=stroke&cond=COVID-19&draw=2&rank=4 (accessed on 27 November 2021).
- Huang, J.F.; Greenway, M.R.; Nasr, D.M.; Chukwudelunzu, F.E., Sr.; Demaerschalk, B.M.; O’Carroll, C.B.; Nord, C.A.; Pahl, E.A.; Barrett, K.M.; Williams, L.N. Telestroke in the Time of COVID-19: The Mayo Clinic Experience. Mayo Clin. Proc. 2020, 95, 1704–1708. [Google Scholar] [CrossRef]
- Mayo, N.E. Stroke Rehabilitation at Home: Lessons Learned and Ways Forward. Stroke 2016, 47, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-C.; Chao, J.-K.; Wang, M.-L.; Yang, Y.-P.; Chien, C.-S.; Lai, W.-Y.; Yang, Y.-C.; Chang, Y.-H.; Chou, C.-L.; Kao, C.-L. Care for Patients with Stroke During the COVID-19 Pandemic: Physical Therapy and Rehabilitation Suggestions for Preventing Secondary Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105182. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Abd-Allah, F.; Al-Senani, F.; Aytac, E.; Borhani-Haghighi, A.; Ciccone, A.; Gomez, C.R.; Gurkas, E.; Hsu, C.Y.; Jani, V.; et al. Management of acute ischemic stroke in patients with COVID-19 infection: Insights from an international panel. Am. J. Emerg. Med. 2020, 38, 1548.e5–1548.e7. [Google Scholar] [CrossRef]

| COVID-19 Associated Stroke | Non-COVID-19 Associated Stroke | |
|---|---|---|
| Age at stroke onset mean ± SD | 65.9 ± 14.3 for all strokes 59 ± 13 for LVOs | 66.7 ± 15.5 for all strokes 73 ± 18 for LVOs |
| Pathomechanisms | Predominant ischemic stroke mechanisms include: hypercoagluable state, endothelial dysfunction/endothelialitis, and cardiac dysfunction | Prior classication systems for ischemic stroke mechanisms have been published, e.g. TOAST, which includes: large artery atherosclerosis, cardioembolism, small vessel occlusion, stroke of other determined etiology or undetermined etiology |
| Acute treatments | ||
| IV thrombolysis | Alteplase or tenecteplase, logistical benefits of tenecteplase due to faster infusion times & decreased exposures | Either alteplase or tenecteplase, whichever is the approved standard of care at the treating facility |
| Endovascular thrombectomy | High proportion of LVOs, and EVT is standard of care, but particular challenges arise in COVID-19 patients, including: multi-vessel territory infarcts, re-occlusions, clot fragmentation, and high clot burden | Standard of care for LVOs presenting within the treatment window |
| Outcomes | ||
| LOS, mean ± SD | 17.4 ± 14.8 days | 8.0 ± 6.4 days |
| Requiring ICU care | 58.7% | 44.7% |
| In-hospital death | 33% | 12.9% |
| Potential Treatment/Care | Challenges | |
|---|---|---|
| Prehospitalization/diagnosis | Timely presentation and diagnosis with proper stroke workup, available resources, and staff trained to efficiently handle infection control |
|
| Hospitalization | IV Thrombolysis |
|
| Mechanical Thrombectomy |
| |
| Therapeutic Anticoagulation |
| |
| Poststroke | Telestroke |
|
| Rehabilitation and Prevention of Secondary Stroke |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamm, B.; Huang, D.; Royan, R.; Lee, J.; Marquez, J.; Desai, M. Pathomechanisms and Treatment Implications for Stroke in COVID-19: A Review of the Literature. Life 2022, 12, 207. https://doi.org/10.3390/life12020207
Stamm B, Huang D, Royan R, Lee J, Marquez J, Desai M. Pathomechanisms and Treatment Implications for Stroke in COVID-19: A Review of the Literature. Life. 2022; 12(2):207. https://doi.org/10.3390/life12020207
Chicago/Turabian StyleStamm, Brian, Deborah Huang, Regina Royan, Jessica Lee, Joshua Marquez, and Masoom Desai. 2022. "Pathomechanisms and Treatment Implications for Stroke in COVID-19: A Review of the Literature" Life 12, no. 2: 207. https://doi.org/10.3390/life12020207
APA StyleStamm, B., Huang, D., Royan, R., Lee, J., Marquez, J., & Desai, M. (2022). Pathomechanisms and Treatment Implications for Stroke in COVID-19: A Review of the Literature. Life, 12(2), 207. https://doi.org/10.3390/life12020207








