Smart Device-Driven Corticolimbic Plasticity in Cognitive-Emotional Restructuring of Space-Related Neuropsychiatric Disease and Injury
Abstract
:1. Introduction
- Memories of extreme emotional experiences may produce psychiatric problems whether they vividly enter or remain inaccessible to consciousness in Earth and non-terran environments, causing health, wellbeing, and performance decrements in dangerous contexts.
- Newer psychotherapeutic programs for the selective reconsolidation of traumatic patient memories aim to modulate vagal tone to treat mood, affect, and anxiety disorders, and may be useful in treating space-induced neuropsychiatric illness and injury.
- Though neuroscience research findings support these types of intervention, the program in its present form is beset by methodological imprecision.
- Optimizing therapeutic efficacy by combining these programs with the personalized advantages offered from the use of minimally invasive smart neuroprosthetic technologies, such as brain stimulation methods, improves clinical options, but needs further study in humans and animal models.
- Such technologies drive vagal activity, corticolimbic plasticity, and the cognitive–emotional restructuring of patients suffering from psychiatric symptoms of varying severities and pathophysiologies.
- Current and future state-of-art must adopt seamless integrated and interoperable systems with open- and closed-loop capabilities that enable remote human and (semi)autonomous robotic and virtual digibot clinicians for better monitoring and treatment of inflight astronaut patients.
- Medical governing bodies must establish best policies and practice guidelines for the ethical design and use of these technological systems on Earth and beyond.
2. The Problem of Optimal Cognitive-Emotional Restructuring
3. Smart Solutions for the Problem of Optimal Cognitive-Emotional Restructuring
4. Cognitive-Emotional Restructuring via Vagus Nerve Stimulation
4.1. Anatomy and Afferent Pathways of the Vagal System
4.2. Neurochemistry of the Vagal System and Its Ascending Targets
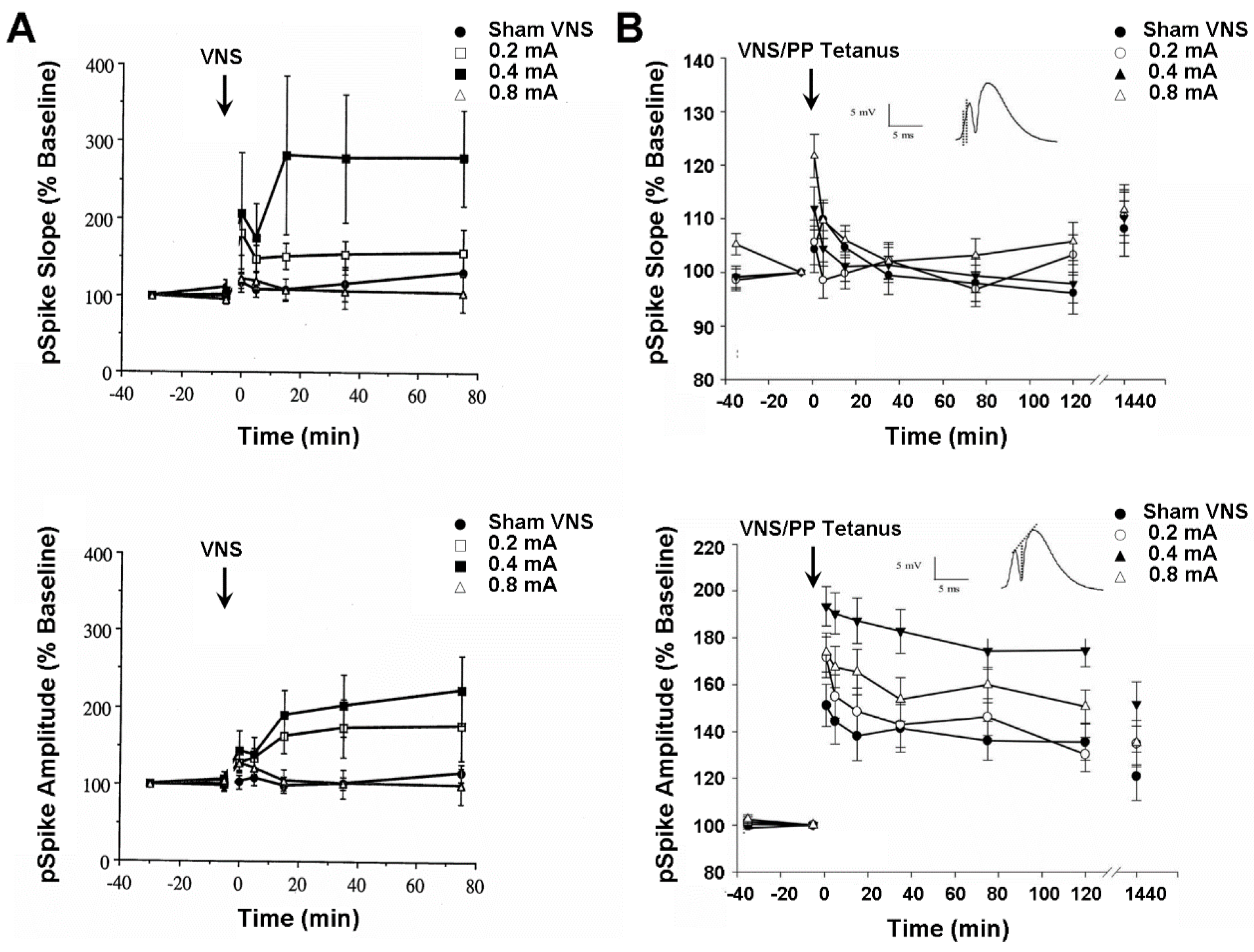
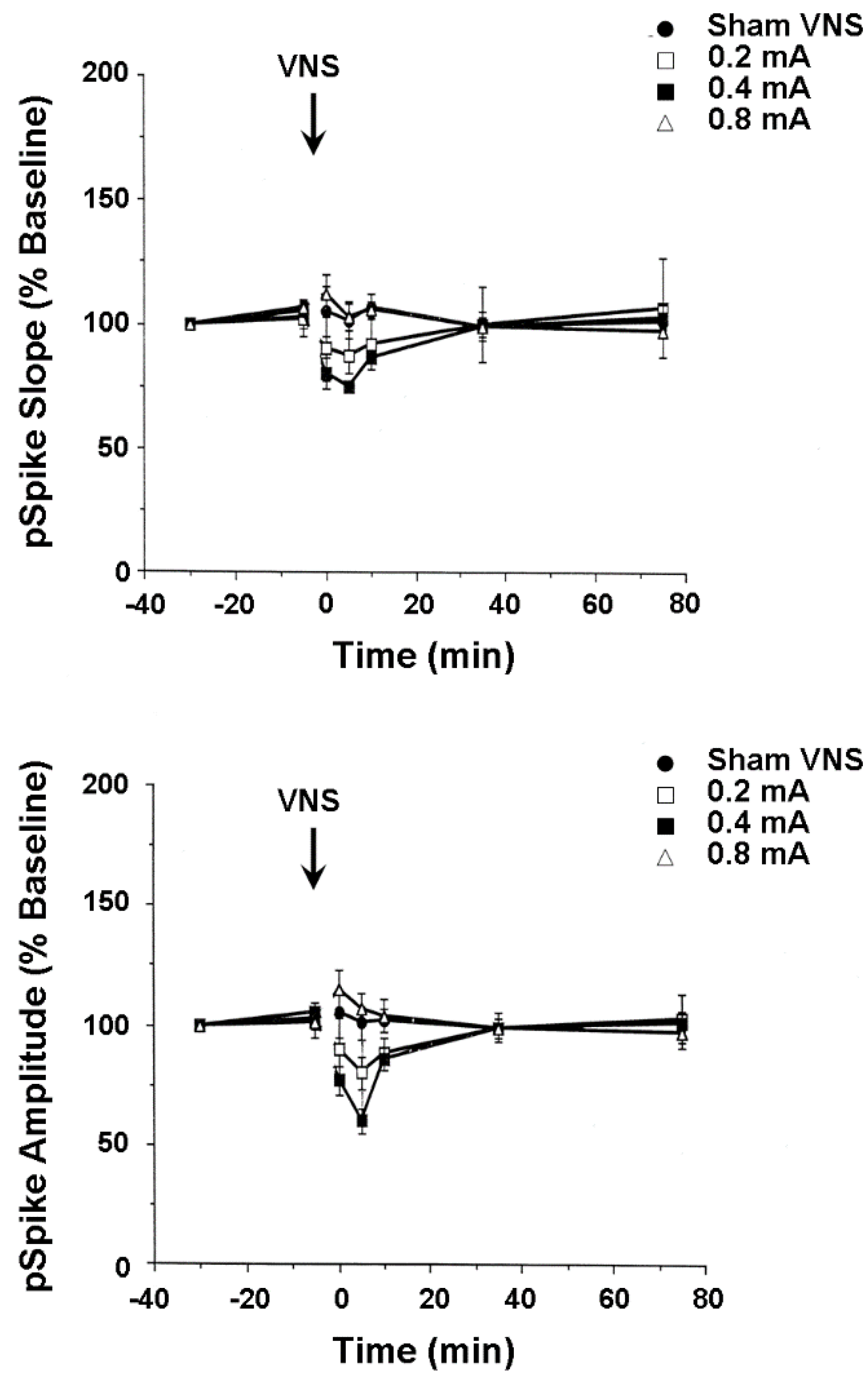
4.3. Plasticity of Hippocampus and Medial Frontal Cortex Evoked Population Responses
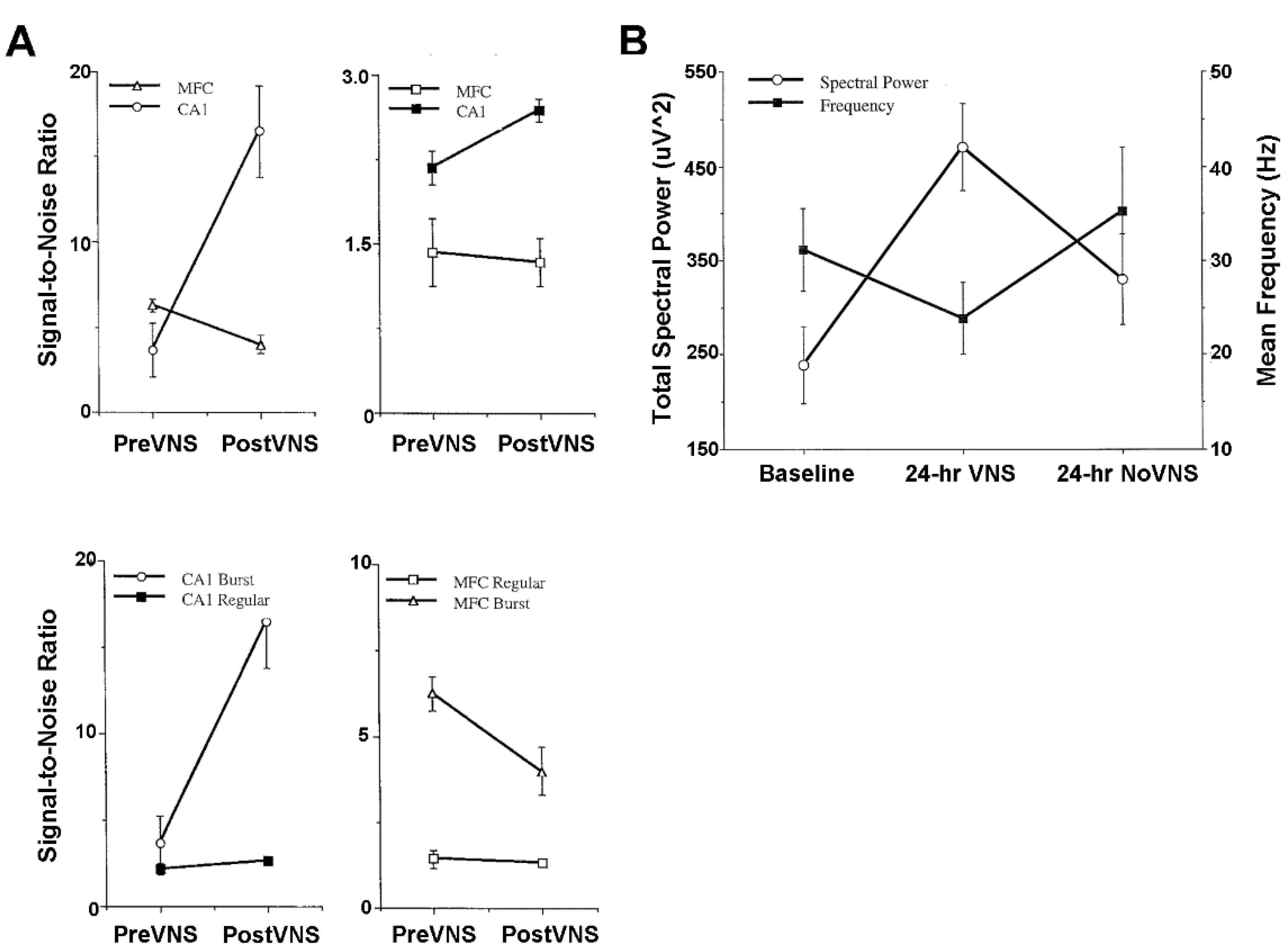
4.4. Activity of Hippocampus and Medial Frontal Cortex Single Neurons and Local Field Potentials
5. Alternative Cognitive-Emotional Restructuring via Transcranial Magnetic Stimulation
6. Nervous System-Computer Interfaces, Medical Robots and Digibots, and the Future of Seamless Integrated Device-Driven Theragnostic Cognitive-Emotional Restructuring
7. Concluding Remarks about Medical Ethics
8. Contributions to the Field Statement
Funding
Conflicts of Interest
References
- Clark, K.B. Topical: Smart Theragnostic Cognitive-Emotional Restructuring for Space-Related Neuropsychiatric Disease and Injury. White Paper Submitted to the Committee on the Decadal Survey for Biological and Physical Sciences Research in Space 2023-2032; National Research Council: Washington, DC, USA, in press.
- Jandial, R.; Hoshide, R.; Waters, J.D.; Limoli, C.L. Space-brain: The negative effects of space exposure on the central nervous system. Surg. Neurol. Int. 2018, 9, 9. [Google Scholar] [CrossRef]
- Kanas, N. Psychological, psychiatric, and interpersonal aspects of long-term space missions. J. Spacecr. Rockets 2018, 27, 457–463. [Google Scholar] [CrossRef]
- Kanas, N. Psychosocial value of space simulation for extended spaceflight. Adv. Space Biol. Med. 1997, 6, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Mann, V.; Sundaresan, A.; Chaganti, M. Cellular changes in the nervous system to gravitational variation. Neurol. India 2019, 67, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Marshall-Goebel, K.; Damanu, R.; Bershad, E.M. Brain physiological response and adaptation during spaceflight. Neurosurgery 2019, 85, E815–E821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nday, C.M.; Frantzidis, C.; Jackson, G.; Bamidis, P.; Kourtidou-Papadeli, C. Neurophysiological changes in simulated microgravity: An animal model. Neurol. India 2019, 67, S221–S226. [Google Scholar] [CrossRef]
- Ohuwafemi, F.A.; Abdelbaki, R.; Lai, J.C.-Y.; Mora-Almanza, J.G.; Afolavan, E.M. A review of astronaut mental health in manned missions: Potential interventions for cognitive and mental challenges. Life Sci. Space Res. 2021, 28, 26–31. [Google Scholar] [CrossRef]
- Romanella, S.M.; Sprugnoli, G.; Ruffini, G.; Sevedmadam, K.; Rossi, S.; Santarnecchi, E. Noninvasive brain stimulation & space exploration: Opportunities and challenges. Neurosci. Biobehav. Rev. 2020, 119, 294–319. [Google Scholar] [CrossRef]
- Roy-O’Reilly, M.; Mulavara, A.; Williams, T. A review of alterations to the brain during spaceflight and the potential relevance to crew in long-duration space exploration. NPJ Microgravity 2021, 7, 5. [Google Scholar] [CrossRef]
- Lane, R.D.; Ryan, L.; Nadel, L.; Greenberg, L. Memory reconsolidation, emotional arousal and the process of change in psychotherapy: New insights from brain science. Behav. Brain Sci. 2014, 38, e1. [Google Scholar] [CrossRef] [Green Version]
- Helmick, K. Cognitive rehabilitation for military personnel with mild traumatic brain injury and chronic post-concussional disorder: Results of April 2009 consensus conference. NeuroRehabilitation 2010, 26, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Samuelkamaleshkumar, S.; Viswanathan, A.; Macaden, A. Cognitive rehabilitation for adults with traumatic brain injury to improve occupational outcomes. Cochrane Database Syst. Rev. 2017, 6, CD007935. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B. Studies Investigating the Role Played by Vagus Nerve Stimulation in the Modulation of Memory Formation. Ph.D. Thesis, Southern Illinois University, Carbondale, IL, USA, 1999. [Google Scholar]
- Clark, K.B.; Naritoku, D.K.; Smith, D.C.; Browning, R.A.; Jensen, R.A. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat. Neurosci. 1999, 2, 94–98. [Google Scholar] [CrossRef]
- McGaugh, J.L. Memory and Emotion: The Making of Lasting Memories; Columbia University Press: New York, NY, USA, 2003; ISBN-13: 978-0231120234. [Google Scholar]
- McIntyre, C.K.; McGaugh, J.L.; Williams, C.L. Interacting brain systems modulate memory consolidation. Neurosci. Biobehav. Rev. 2012, 36, 1750–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadel, L.; Samsonovich, A.; Ryan, L.; Moscovitch, M. Multiple trace theory of human memory: Computational, neuroimaging, and neuropsychological results. Hippocampus 2000, 10, 352–368. [Google Scholar] [CrossRef]
- Nadel, L.; Campbell, J.; Ryan, L. Autobiographical memory retrieval and hippocampal activation as a function of repetition and the passage of time. Neural. Plast. 2007, 2007, 90472. [Google Scholar] [CrossRef]
- Nader, K.; Schafe, G.E.; Le Doux, J.E. Reconsolidation: The labile nature of consolidation theory. Nat. Rev. Neurosci. 2000, 1, 216–219. [Google Scholar] [CrossRef]
- Phelps, E.A. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 2004, 14, 198–202. [Google Scholar] [CrossRef]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, memory and the amygdale. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- Williams, C.L.; Jensen, R.A. Effects of vagotomy on Leu-enkephalin-induced changes in memory storage processes. Physiol. Behav. 1993, 54, 659–663. [Google Scholar] [CrossRef]
- Wilson, M.A.; McNaughton, B.L. Reactivation of hippocampal ensemble memories during sleep. Science 1994, 265, 676–679. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.B. The humanness of artificial nonnormative personalities. Behav. Brain Sci. 2017, 40, e259. [Google Scholar] [CrossRef]
- Clark, K.B. Digital life, a theory of minds, and mapping human and machine cultural universals. Behav. Brain Sci. 2020, 43, e98. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.A.; Rennaker, R.L.; Kilgard, M.P. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog. Brain Res. 2013, 207, 275–299. [Google Scholar] [CrossRef] [Green Version]
- Howland, R.H.; Shutt, L.S.; Berman, S.R.; Spots, C.R.; Denko, T. The emerging use of technology for the treatment of depression and other neuropsychiatric disorders. Ann. Clin. Psychiatry 2011, 23, 48–62. [Google Scholar] [PubMed]
- Naritoku, D.N.; Jensen, R.A.; Browning, R.A.; Clark, K.B.; Smith, D.C.; Terry, R.S., Jr. Methods of Modulating Aspects of Brain Neural Plasticity by Vagus Nerve Stimulation. U.S. Patent 6339725, 15 January 2002. Available online: https://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=6,339,725.PN.&OS=PN/6,339,725&RS=PN/6,339,725 (accessed on 10 December 2021).
- Naritoku, D.K.; Jensen, R.A.; Browning, R.A.; Clark, K.B.; Smith, D.C.; Terry, R.S., Jr. Methods of Improving Learning or Memory by Vagus Nerve Stimulation. U.S. Patent 6556868, 29 April 2003. Available online: https://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=6,556,868.PN.&OS=PN/6,556,868&RS=PN/6,556,868 (accessed on 10 December 2021).
- Peña, D.F.; Engineer, N.D.; McIntyre, C.K. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol. Psychiatry 2013, 73, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Agyare, E.K.; Curran, G.L.; Ramakrishnan, M.; Yu, C.C.; Poduslo, J.F.; Kandimalla, K.K. Development of a smart nano-vehicle to target cerebrovascular amyloid deposits and brain parenchymal plaques observed in Alzheimer’s disease and cerebral amyloid angiopathy. Pharm. Res. 2008, 25, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G. MicroRNAs as a target for novel antipsychotic: A systematic review of an emerging field. Int. J. Neuropsychopharmacol. 2010, 13, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Modi, G.; Pillay, V.; Choonara, Y.E. Advances in the treatment of neurodegenerative disorders employing nanotechnology. Ann. N. Y. Acad. Sci. 2010, 1184, 154–172. [Google Scholar] [CrossRef] [PubMed]
- De Oliveria Barros, A.; Yang, J. A review of magnetically actuated milli/micro-scale robots locomotion and features. Crit. Rev. Biomed. Eng. 2019, 47, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Nummelin, S.; Shen, B.; Piskunen, P.; Liu, Q.; Kostiainen, M.A.; Linko, V. Robotic DNA nanostructures. ACS Synth. Biol. 2020, 9, 1923–1940. [Google Scholar] [CrossRef]
- Li, M.; Xi, N.; Wang, Y.; Liu, L. Progress in Nanorobotics for Advancing Biomedicine. IEEE Trans. Biomed. Eng. 2021, 68, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Pedram, A.; Pishkenari, H.N. Smart micro/nano-robotic systems for gene delivery. Curr. Gene Ther. 2017, 17, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yu, Y.; Chen, Z.; Bian, F.; Ye, F.; Sun, L.; Zhao, Y. Biohybrid robotics with living cell actuation. Chem. Soc. Rev. 2020, 49, 4043–4069. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, S.E.; Purcell, R.; Garner, B.; Parslow, R. Combined pharmacotherapy and psychological therapies for post-traumatic stress disorder (PTSD). Cochrane Database Sys. Rev. 2010, 7, CD007316. [Google Scholar] [CrossRef] [Green Version]
- Von Wolff, A.; Hölzel, L.P.; Westphal, A.; Härter, M.; Kriston, L. Combination pharmacotherapy and psychotherapy in the treatment of chronic depression: A systematic review and meta-analysis. BMC Psychiatry 2012, 12, 61. [Google Scholar] [CrossRef] [Green Version]
- Fraschinim, M.; Puligheddu, M.; Demuru, M.; Polizzi, L.; Maleci, A.; Tamburini, G.; Congia, S.; Bortolato, M.; Marrosu, F. VNS induced desynchronization in gamma bands correlates with positive clinical outcomes in temporal lobe pharmacoresistant epilepsy. Neurosci. Lett. 2013, 536, 14–18. [Google Scholar] [CrossRef]
- Lyubashina, O.; Panteleev, S. Effects of cervical vagus nerve stimulation on amygdala-evoked responses of the medial prefrontal cortex neurons in rat. Neurosci. Res. 2009, 65, 122–125. [Google Scholar] [CrossRef]
- Nahas, Z.; Teneback, C.; Chae, J.H.; Mu, Q.; Molnar, C.; Kozel, F.A.; Walker, J.; Anderson, B.; Koola, J.; Kose, S.; et al. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology 2007, 32, 1649–1660. [Google Scholar] [CrossRef]
- Roosevelt, R.W.; Smith, D.C.; Clough, R.W.; Jensen, R.A.; Browning, R.A. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006, 1119, 124–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, K.B.; Krahl, S.E.; Smith, D.C.; Jensen, R.A. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol. Learn. Mem. 1995, 63, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B.; Smith, D.C.; Hassert, D.L.; Browning, R.A.; Naritoku, D.K.; Jensen, R.A. Posttraining electrical stimulation of vagal afferents with concomitant efferent inactivation enhances memory storage processes in the rat. Neurobiol. Learn. Mem. 1998, 70, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Critchley, H.D.; Lewis, P.A.; Orth, M.; Josephs, O.; Deichmann, R.; Trimble, M.R.; Dolan, R.J. Vagus nerve stimulation for treatment-resistant depression: Behavioural and neural effects on encoding negative material. Psychosom. Med. 2007, 69, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.B.; Smith, D.C.; Jensen, R.A. Vagus nerve stimulation induces both long-term potentiation and depression in the rat hippocampus. Soc. Neurosci. Abst. 1997, 23, 787. [Google Scholar]
- Zuo, Y.; Smith, D.C.; Jensen, R.A. Vagus nerve stimulation potentiates hippocampal LTP in freely-moving rats. Physiol. Behav. 2007, 90, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.B.; Smith, D.C.; Browning, R.A. Long-term continuous, intermittent vagus nerve stimulation produces nerve adaptation that may suppress seizures. Soc. Neurosci. Abstr. 1998, 24, 718. [Google Scholar]
- Usami, K.; Kano, R.; Kawai, K.; Noda, T.; Shiramatsu, T.I.; Saito, N.; Takahashi, H. Modulation of cortical synchrony by vagus nerve stimulation in adult rats. Annu. Int. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 2013, 5348–5351. [Google Scholar] [CrossRef]
- Krahl, S.E.; Clark, K.B.; Smith, D.C.; Browning, R.A. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 1998, 39, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Hassert, D.L.; Miyashita, T.; Williams, C.L. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdale. Behav. Neurosci. 2004, 118, 79–88. [Google Scholar] [CrossRef]
- Krahl, S.E.; Clark, K.B. Vagus nerve stimulation for epilepsy: A review of central mechanisms. Surg. Neurol. Int. 2012, 3, S255–S259. [Google Scholar] [CrossRef]
- Benchenane, K.; Peyrache, A.; Khamassi, M.; Tierney, P.L.; Gioanni, Y.; Battaglia, F.P.; Wiener, S.I. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron 2010, 66, 921–936. [Google Scholar] [CrossRef]
- Fisher, R.S. Therapeutic devices for epilepsy. Ann. Neurol. 2012, 71, 157–168. [Google Scholar] [CrossRef] [Green Version]
- George, M.S.; Nahas, Z.; Borckardt, J.J.; Anderson, B.; Foust, M.J.; Burns, C.; Kose, S.; Short, E.B. Brain stimulation for the treatment of psychiatric disorders. Curr. Opin. Psychiatry 2007, 20, 250–254. [Google Scholar] [CrossRef]
- Miranda, R.A.; Casebeer, W.D.; Hein, A.M.; Judy, J.W.; Krotkov, E.P.; Laabs, T.L.; Manzo, J.E.; Pankratz, K.G.; Pratt, G.A.; Sanchez, J.C.; et al. DARPA-funded efforts in the development of novel brain–computer interface technologies. J. Neurosci. Methods 2015, 244, 52–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barraco, I.R.A. (Ed.) . Nucleus of the Solitary Tract; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Kalia, M.; Mesulam, M.-M. Brain stem projections of the sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J. Comp. Neurol. 1980, 193, 467–508. [Google Scholar] [CrossRef]
- Kalia, M.; Mesulam, M.-M. Brain stem projections of the sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J. Comp. Neurol. 1980, 193, 467–508. [Google Scholar] [CrossRef]
- Ricardo, J.A.; Koh, E.T. Anatomical evidence for direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978, 153, 1–26. [Google Scholar] [CrossRef]
- Rutecki, P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia 1990, 31, S1–S6. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Shipley, M.T.; Chouvert, G.; Ennis, M.; Van Bockstaele, E.; Pieribone, V.; Shiekhattar, R.; Akaoka, H.; Drolet, G.; Astier, B.; et al. Afferent regulation of locus coeruleus neurons: Anatomy, physiology, and pharmacology. In Progress in Brain Research: Neurobiology of the Locus Coeruleus; Barnes, C.D., Pompeiano, O., Eds.; Elsevier: New York, NY, USA, 1991; Volume 88, pp. 47–75. [Google Scholar]
- Ter Horst, G.J.; Streefland, C. Ascending projections of the solitary tract nucleus. In Nucleus of the Solitary Tract; Barraco, I.R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 93–103. [Google Scholar]
- Cornwall, J.; Cooper, J.D.; Phillipson, O.T. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Br. Res. Bull. 1990, 25, 271–284. [Google Scholar] [CrossRef]
- Herbert, H.; Saper, C.B. Organization of medullary adrenergic and noradrenergic projections to the periaqueductal gray matter in the rat. J. Comp. Neurol. 1992, 315, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, G.J.; De Boer, P.; Luiten, P.G.M.; Van Willigen, J.D. Ascending projections from the solitary tract nucleus to the hypothalamus: A phaseolus vulgaris lectin tracing study in the rat. Neuroscience 1989, 31, 785–797. [Google Scholar] [CrossRef]
- Cechetto, D.F. Central representations of visceral function. Fed. Proceed. 1986, 46, 17–23. [Google Scholar]
- Ferino, F.; Thierry, A.M.; Glowinski, J. Anatomical and electrophysiological evidence for a direct projection from Ammon’s horn to the medial prefrontal cortex in the rat. Exp. Brain Res. 1987, 65, 421–426. [Google Scholar] [CrossRef]
- Dorr, A.E.; Debonnel, G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J. Pharmacol. Exp. Ther. 2006, 318, 890–898. [Google Scholar] [CrossRef]
- Groves, D.A.; Bowman, E.M.; Brown, V.J. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci. Lett. 2005, 379, 174–179. [Google Scholar] [CrossRef]
- Naritoku, D.K.; Terry, W.J.; Helfert, R.H. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res. 1995, 22, 53–62. [Google Scholar] [CrossRef]
- Takigawa, M.; Mogenson, G.J. A study of inputs to antidromically identified neurons of the locus coeruleus. Brain Res. 1977, 135, 217–230. [Google Scholar] [CrossRef]
- Garcia, R. Stress, metaplasticity, and antidepressants. Curr. Mol. Med. 2002, 2, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Bakish, D.; Beaulieu, S. The neurobiology of treatment response to antidepressants and mood stabilizing medications. J. Psychiatry Neurosci. 2002, 27, 260–265. [Google Scholar] [PubMed]
- Masada, T.; Itano, T.; Fujisawa, M.; Miyamoto, O.; Tokuda, M.; Matsui, H.; Nagao, S.; Hatase, O. Protective effect of vagus nerve stimulation on forebrain ischaemia in gerbil hippocampus. Neuroreport 1996, 7, 446–448. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Koyama, N.; Yoshida, Y.; Yokota, T. Activation of ascending antinociceptive system by vagal afferent input as revealed in the nucleus ventralis posteromedialis. Brain Res. 1999, 833, 108–111. [Google Scholar] [CrossRef]
- Browning, R.A.; Clark, K.B.; Naritoku, D.K.; Smith, D.C.; Jensen, R.A. Loss of anticonvulsant effect of vagus nerve stimulation in the pentylenetetrazol seizure model following treatment with 6-hydroxydopamine or 5,7-dihydroxytryptamine. Soc. Neurosci. Abst. 1997, 23, 2424. [Google Scholar]
- Ben-Menachem, E.; Hamberger, A.; Hedner, T.; Hammond, E.J.; Uthman, B.M.; Slater, J.; Treig, T.; Stefan, H.; Ramsay, R.E.; Wernicke, J.F.; et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995, 20, 221–227. [Google Scholar] [CrossRef]
- Rush, A.J.; George, M.S.; Sackeim, H.A.; Marangell, L.B.; Husain, M.M.; Giller, C.; Nahas, Z.; Haines, S.; Simpson, R.K.; Goodman, R. Vagus nerve stimulation (VNS) for treatment-resistant depressions: A multicenter study, Biol. Psychiatry 2000, 47, 273–275. [Google Scholar] [CrossRef]
- Brasil-Neto, J.P. Learning, memory, and transcranial direct current stimulation. Front. Psychiatry 2012, 3, 80. [Google Scholar] [CrossRef] [Green Version]
- Breton, J.; Robertson, E.M. Flipping the switch: Mechanisms that regulate memory consolidation. Trends Cogn. Sci. 2014, 18, 629–634. [Google Scholar] [CrossRef]
- Clark, V.P.; Parasuraman, R. Neuroenhancement: Enhancing brain and mind in health and in disease. NeuroImage 2014, 85, 889–894. [Google Scholar] [CrossRef] [Green Version]
- George, M.S.; Massimini, M. Using brain stimulation to create thoughts, retrieve and alter memories, and measure consciousness—A discussion of research. Brain Stim. 2013, 6, 835–836. [Google Scholar] [CrossRef] [PubMed]
- Robertson, E.M. New insights in human memory interference and consolidation, Curr. Biol. 2012, 22, R66–R71. [Google Scholar] [CrossRef] [Green Version]
- Spiers, H.J.; Bendor, D. Enhance, delete, incept: Manipulating hippocampus-dependent memories. Brain Res. Bull. 2014, 105, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Baek, K.; Chae, J.H.; Jeong, J. The effect of repetitive transcranial magnetic stimulation on fear extinction in rats. Neuroscience 2012, 200, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Ferrari, C. rTMS stimulation on left DLPFC increases the correct recognition of memories for emotional target and distractor words. Cogn. Affect. Behav. Neurosci. 2012, 12, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Balconi, M.; Ferrari, C. Repeated transcranial magnetic stimulation on dorsolateral prefrontal cortex improves performance in emotional memory retrieval as a function of level of anxiety and stimulus valence. Psychiatry Clin. Neurosci. 2013, 67, 210–218. [Google Scholar] [CrossRef]
- Balconi, M.; Ferrari, C. Left DLPFC rTMS stimulation reduced the anxiety bias effect or how to restore the positive memory processing in high-anxiety subjects. Psychiatry Res. 2013, 209, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.; Grimm, S.; Astalosch, A.; Guo, J.S.; Briesemeister, B.B.; Lisanby, S.H.; Luber, B.; Baiboui, M. Lateralized effects of prefrontal repetitive transcranial magnetic stimulation on emotional working memory. Exp. Brain Res. 2013, 227, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.; Richtermeier, A.; Feeser, M.; Guo, J.S.; Briesemeister, B.B.; Grimm, S.; Baiboui, M. State-dependent effects of prefrontal repetitive transcranial magnetic stimulation on emotional working memory. Brain Stim. 2013, 6, 905–912. [Google Scholar] [CrossRef]
- Balconi, M.; Ferrari, C. Emotional memory retrieval. rTMS stimulation on the left DLPFC increases the positive memories. Brain Imaging Behav. 2012, 6, 454–461. [Google Scholar] [CrossRef]
- Balconi, M.; Ferrari, C. rTMS stimulation on left DLPFC affects emotional cue retrieval as a function of anxiety level and gender. Depress. Anxiety 2012, 29, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Campanella, F.; Fabbro, F.; Ungesi, C. Cognitive and anatomical underpinnings of the conceptual knowledge for common objects and familiar people: A repetitive transcranial magnetic stimulation study. PLoS ONE 2013, 8, e64596. [Google Scholar] [CrossRef] [Green Version]
- Censor, N.; Davan, E.; Cohen, L.G. Cortico-subcortico neuronal circuitry associated with reconsolidation of human procedural memories. Cortex 2014, 58, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, C.; De Graaf, T.A.; Goebel, R.; Sack, A.T. The temporal dynamics of early visual cortex involvement in behavioral priming. PLoS ONE 2012, 7, e48808. [Google Scholar] [CrossRef] [Green Version]
- Kongthong, N.; Minami, T.; Nakauchi, S. Semantic processing in subliminal face stimuli: An EEG and tDCS study. Neurosci. Lett. 2013, 544, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Meehan, S.K.; Zabukovec, J.R.; Dao, E.; Cheung, K.L.; Linsdell, M.A.; Boyd, L.A. One hertz repetitive transcranial magnetic stimulation over dorsal premotor cortex enhances offline motor memory consolidation for sequence-specific implicit learning. Eur. J. Neurosci. 2013, 38, 3071–3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantone, M.; Di Pino, G.; Capone, F.; Piombo, M.; Chiarell, D.; Cheeran, B.; Pennisi, G.; Di Lazzaro, V. The contribution of trancranial magnetic stimulation in the diagnosis and in the management of dementia. Clin. Neurophysiol. 2014, 125, 1509–1532. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.O.; Lepage, M. Neural correlates of recognition memory of social information in people with schizophrenia. J. Psychiatry Neurosci. 2014, 39, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koski, L.; Kolivakis, T.; Yu, C.; Chen, J.K.; Delaney, S.; Ptito, A. Noninvasive brain stimulation for persistent postconcussive symptoms in mild traumatic brain injury. J. Neurotrauma 2014, 32, 38–44. [Google Scholar] [CrossRef]
- Lett, T.A.; Voineskos, A.N.; Kennedy, J.L.; Levine, B.; Daskalakis, Z. Treating working memory deficits in schizophrenia: A review of the neurobiology. Biol. Psychiatry 2014, 75, 361–370. [Google Scholar] [CrossRef]
- Leuchter, A.F.; Cook, I.A.; Jin, Y.; Philips, B. The relationship between brain oscillatory activity and therapeutic effectiveness of transcranial magnetic stimulation in the treatment of major depressive disorder. Front. Hum. Neurosci. 2013, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Marin, M.F.; Camprodon, J.A.; Dougherty, D.D.; Milad, M.R. Device-based brain stimulation to augment fear extinction: Implications for PTSD treatment and beyond. Depress. Anxiety 2014, 31, 269–278. [Google Scholar] [CrossRef]
- Nadeau, S.E.; Bowers, D.; Jones, T.L.; Wu, S.S.; Triggs, W.J.; Heilman, K.M. Cognitive effects of treatment of depression with repetitive transcranial magnetic stimulation. Cogn. Behav. Neurol. 2014, 27, 77–87. [Google Scholar] [CrossRef]
- Wang, J.X.; Rogers, L.M.; Gross, E.Z.; Ryals, A.J.; Dokucu, M.E.; Brandstatt, K.L.; Hermiler, M.S.; Voss, J.L. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 2014, 345, 1054–1057. [Google Scholar] [CrossRef] [Green Version]
- Bilek, E.; Schäfer, A.; Ochs, E.; Esslinger, C.; Zangl, M.; Plichta, M.M.; Braun, U.; Kirsch, P.; Schulze, T.G.; Rietschel, M.; et al. Application of high-frequency repetitive transcranial magnetic stimulation to the DLPFC alters human prefrontal-hippocampal functional interaction. J. Neurosci. 2013, 33, 7050–7056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Censor, N.; Horovitz, S.G.; Cohen, L.G. Interference with existing memories alters offline intrinsic functional brain connectivity. Neuron 2014, 81, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanslmayer, S.; Matuschek, J.; Fellner, M.C. Entrainment of prefrontal beta oscillations induce an endogenous echo and impairs memory formation. Curr. Biol. 2014, 24, 9049. [Google Scholar] [CrossRef] [Green Version]
- Van de Ven, V.; Sack, A.T. Transcranial magnetic stimulation of visual cortex in memory: Cortical state, intereference and reactivation of visual content in memory. Behav. Brain Res. 2013, 236, 67–77. [Google Scholar] [CrossRef]
- Zanto, T.P.; Chadick, J.Z.; Satris, G.; Gazzaley, A. Rapid functional reorganization in human cortex following neural perturbation. J. Neurosci. 2013, 33, 16268–16274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batsikadze, G.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Effect of serotonin on paired associative stimulation-induced plasticity in the human motor cortex. Neuropsychopharmology 2013, 38, 2260–2267. [Google Scholar] [CrossRef] [Green Version]
- Chajeb, L.; Antal, A.; Ambrus, G.G.; Paulus, W. Brain-derived neurotrophic factor: Its impact upon neuroplasticity inducing transcranial brain stimulation protocols. Neurogenetics 2014, 15, 1–11. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Z.; Kang, L.; Geng, D.; Wang, Y.; Wang, M.; Cui, H. Repetitive transcranial magnetic stimulation (rTMS) influences spatial cognition and modulates hippocampal structural synaptic plasticity in aging mice. Exp. Gerontol. 2014, 58, 256–268. [Google Scholar] [CrossRef]
- Rogasch, N.C.; Daskalakis, Z.; Fitzgerald, P.B. Cortical inhibition of distinct mechanisms in the dorsalateral prefrontal cortex is related to working memory performance: A TMS-EEG study. Cortex 2014, 64C, 68–77. [Google Scholar] [CrossRef]
- Tan, T.; Xie, J.; Liu, T.; Chen, X.; Zheng, X.; Tong, Z.; Tian, X. Low-frequency (1 Hz) repetitive transcranial magnetic stimulation (rTMS) reverses Aβ(1-41)-mediated memory deficits in rats. Exp. Gerontol. 2013, 48, 786–794. [Google Scholar] [CrossRef]
- Yang, H.; Shi, O.; Jin, Y.; Henrich-Noack, P.; Qiao, H.; Cai, C.; Tao, H.; Tian, X. Functional protection of learning and memory abilities in rats with vascular dementia. Restor. Neurol. Neurosci. 2014, 32, 689–700. [Google Scholar] [CrossRef]
- Lindsey., A.; Ellison, R.A.H.; Aaronson, A.; Kletzel, S.; Stika, M.; Guernon, A.T. rTMS/iTBS and Cognitive Rehabilitation: A Theoretical Framework and Review Examining Paired Treatment to Remediate Deficits Associated with TBI and PTSD. Neurosci. Biobehav. Rev. (in press).
- Fiske, A.; Henningsen, P.; Buyx, A. Your robot therapist will see you now: Ethical implications of embodied artificial intelligence in psychiatry, psychology, and psychotherapy. J. Med. Internet Res. 2019, 21, e13216. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Nevejans, N.; Allen, C.; Blyth, A.; Leonard, S.; Pagallo, U.; Holzinger, K.; Holzinger, A.; Sajid, M.I.; Ashrafian, H. Legal, regulatory, and ethical frameworks for development of standards in artificial intelligence (AI) and autonomous robotic surgery. Int. J. Med. Robot. 2019, 15, e1968. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.R.; Medina, M.G.; Dwyer, A.M. Telemedicine and telerobotics: From science fiction to reality. Updates Surg. 2018, 70, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.; Guang-Zhong, Y. Hand-held medical robots. Ann. Biomed. Eng. 2014, 42, 1594–1605. [Google Scholar] [CrossRef]
- Umay, I.; Fidan, B.; Barshan, B. Localization and tracking of implantable biomedical sensors. Sensors 2017, 17, 583. [Google Scholar] [CrossRef]
- Vilela, M.; Hochberg, L.R. Applications of brain-computer interfaces to the control of robotic and prosthetic arms. Handb. Clin. Neurol. 2020, 168, 87–99. [Google Scholar] [CrossRef]
- Bedi, G.; Carrillo, F.; Cecchi, G.A.; Slezak, D.F.; Sigman, M.; Mota, N.B.; Ribeiro, S.; Javitt, D.C.; Copelli, M.; Corcoran, C.M. Automated analysis of free speech predicts psychosis onset in high-risk youths. Schizophrenia 2015, 1, 15030. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B. Evolution of affective and linguistic disambiguation under social eavesdropping pressures. Behav. Brain Sci. 2014, 37, 551–552. [Google Scholar] [CrossRef]
- Clark, K.B. Dialect Structural Priming in Endangered Language Evolution, Devolution and Protection. Proc. Roy. Soc. B. Biol. Sci. eLetter 2017. Available online: https://royalsocietypublishing.org/doi/suppl/10.1098/rspb.2014.1574 (accessed on 10 December 2021).
- Clark, K.B. Natural chunk-and-pass language processing: Just another joint source-channel coding model? Commun. Integr. Biol. 2018, 11, e1445899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowski, K.; Rynkiewicz, A.; Lassalle, A.; Baron-Cohen, S.; Schuller, B.; Cummins, N.; Baird, A.; Podgόrska-Bednarz, J.; Pieniażek, A.; Łucka, I. Emotional expression in psychiatric conditions: New technology for clinicians. Psychiatry Clin. Neurosci. 2019, 73, 50–62. [Google Scholar] [CrossRef]
- Lake, B.M.; Ullman, T.D.; Tenebaum, J.D.; Gershman, S.J. Building machines that learn and think like people. Behav. Brain Sci. 2017, 40, e253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, K.B. Topical: Intelligent Meta-Learning Inferential Social Robotic and Virtual Space Medicine Clinicians. White Paper Submitted to the Committee on the Decadal Survey for Biological and Physical Sciences Research in Space 2023–2032; National Research Council: Washington, DC, USA, in press.
- Mooney, S.J.; Pejaver, V. Big data in public health: Terminology, machine learning, and privacy. Ann. Rev. Public Health 2018, 39, 95–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, K.B. Smart Device-Driven Corticolimbic Plasticity in Cognitive-Emotional Restructuring of Space-Related Neuropsychiatric Disease and Injury. Life 2022, 12, 236. https://doi.org/10.3390/life12020236
Clark KB. Smart Device-Driven Corticolimbic Plasticity in Cognitive-Emotional Restructuring of Space-Related Neuropsychiatric Disease and Injury. Life. 2022; 12(2):236. https://doi.org/10.3390/life12020236
Chicago/Turabian StyleClark, Kevin B. 2022. "Smart Device-Driven Corticolimbic Plasticity in Cognitive-Emotional Restructuring of Space-Related Neuropsychiatric Disease and Injury" Life 12, no. 2: 236. https://doi.org/10.3390/life12020236
APA StyleClark, K. B. (2022). Smart Device-Driven Corticolimbic Plasticity in Cognitive-Emotional Restructuring of Space-Related Neuropsychiatric Disease and Injury. Life, 12(2), 236. https://doi.org/10.3390/life12020236




