Maggot Extract Interrupts Bacterial Biofilm Formation and Maturation in Combination with Antibiotics by Reducing the Expression of Virulence Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lucilia Sericata Larvae Extract
2.2. Bacteria Cultures
2.3. Isolation of Human Cutaneous Cells
2.4. Cell Co-Culture with Bacteria In Vitro
2.5. Skin Tissue Culture, Epidermal Wounding and Infection of Ex Vivo Dermal Model
2.6. Scanning Electron Microscopy (SEM)
2.7. RNA Preparation and cDNA Synthesis
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
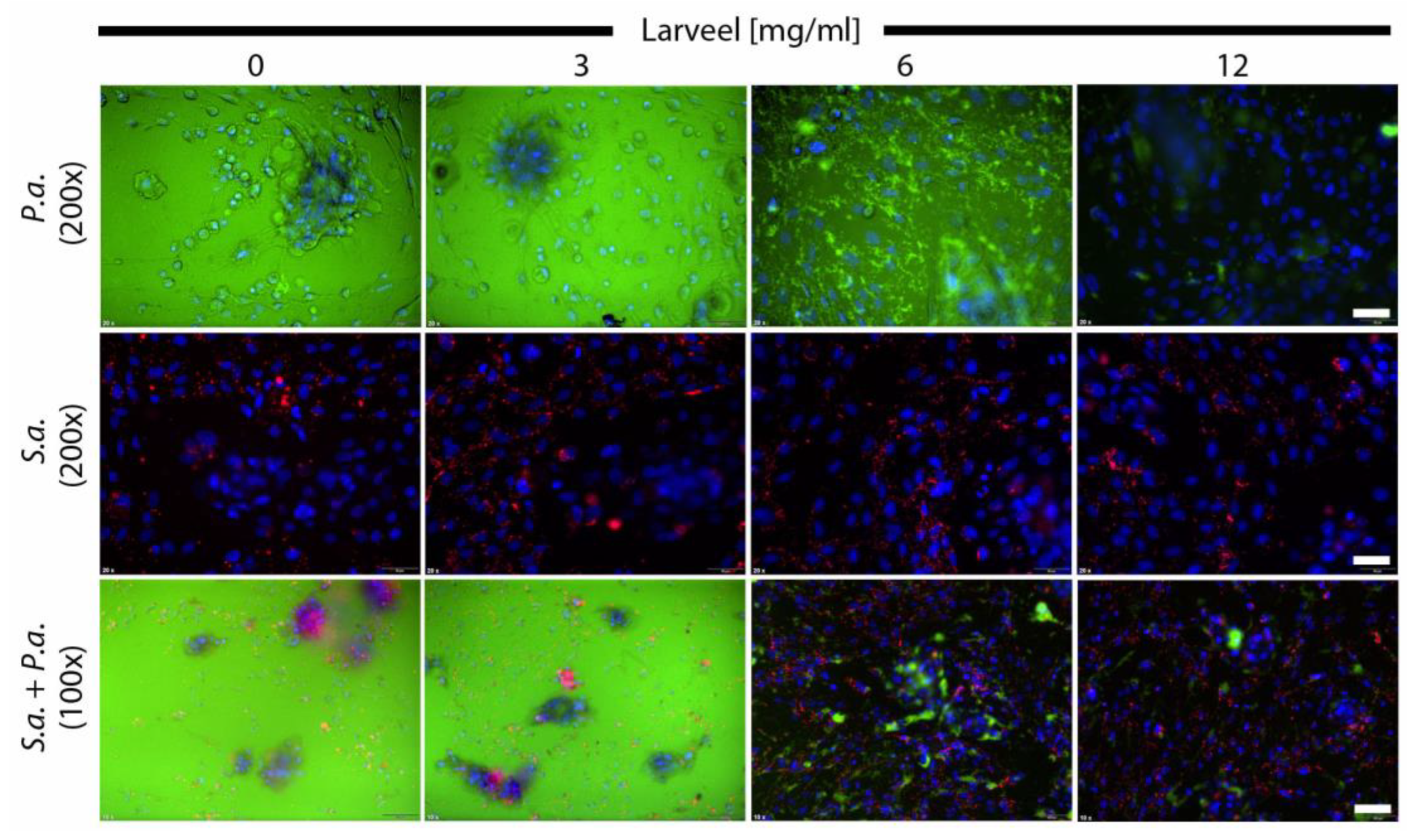
3.1. Cell Protective Effect of Larveel® towards Bacterial Infection in Combination with Antibiotics
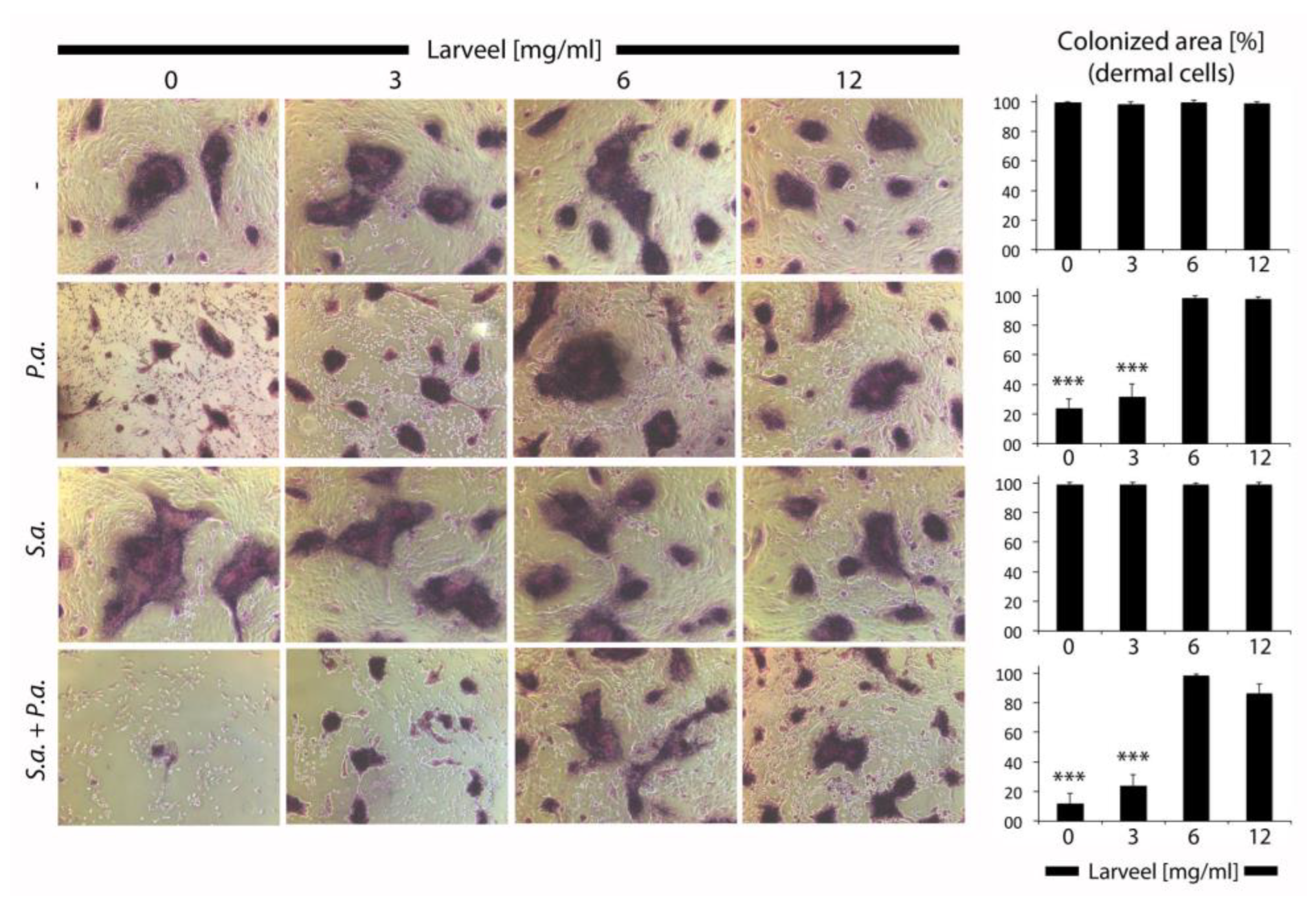
3.2. Generation of an Ex Vivo Human Dermal Wound Model
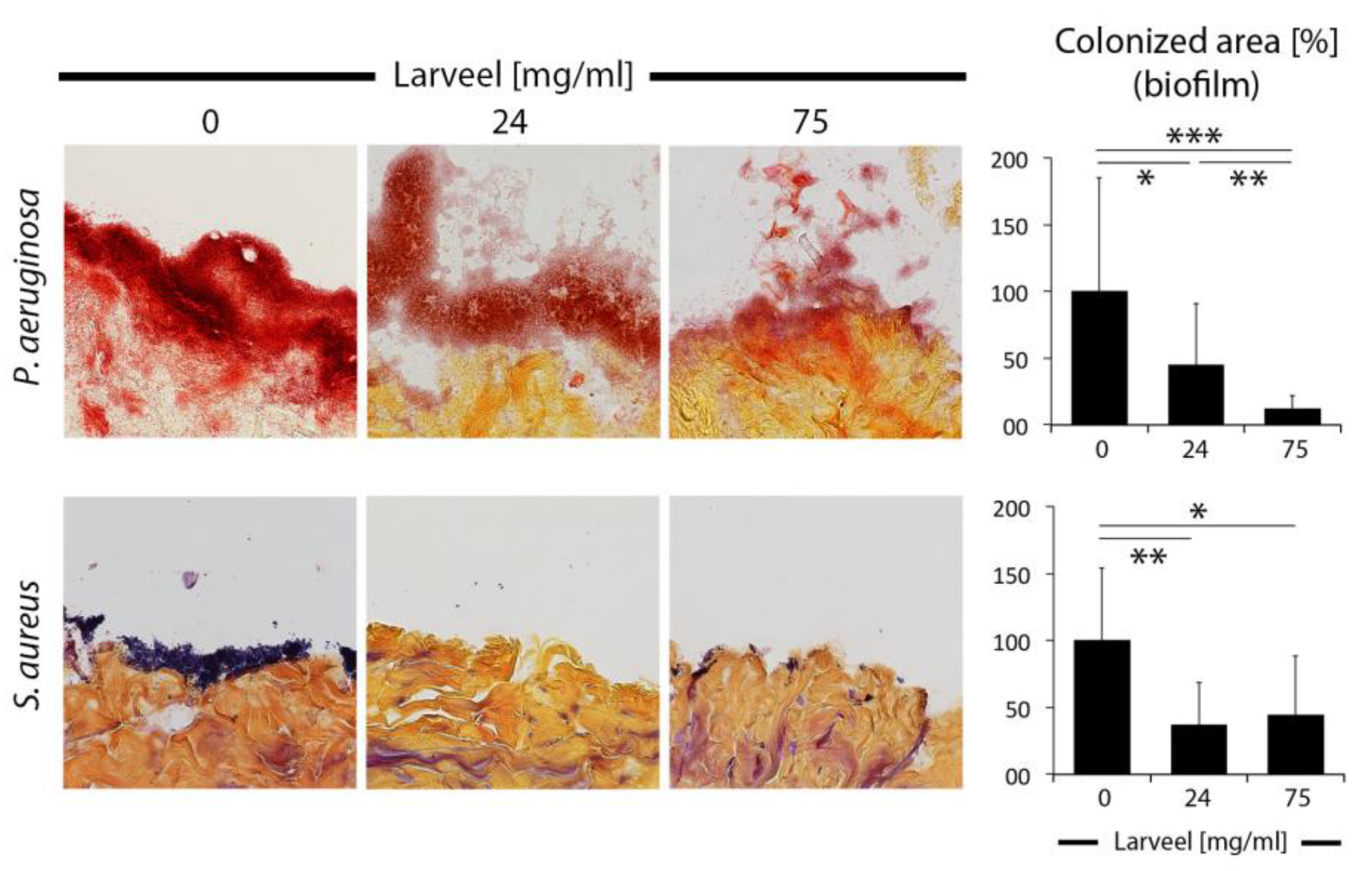
3.3. Impaired Biofilm Maturation and Stability by Larveel® Treatment in the Ex Vivo Human Dermal Wound Model
3.4. Crack Formation in P. aeruginosa Biofilm Surface after Larveel® Treatment in SEM
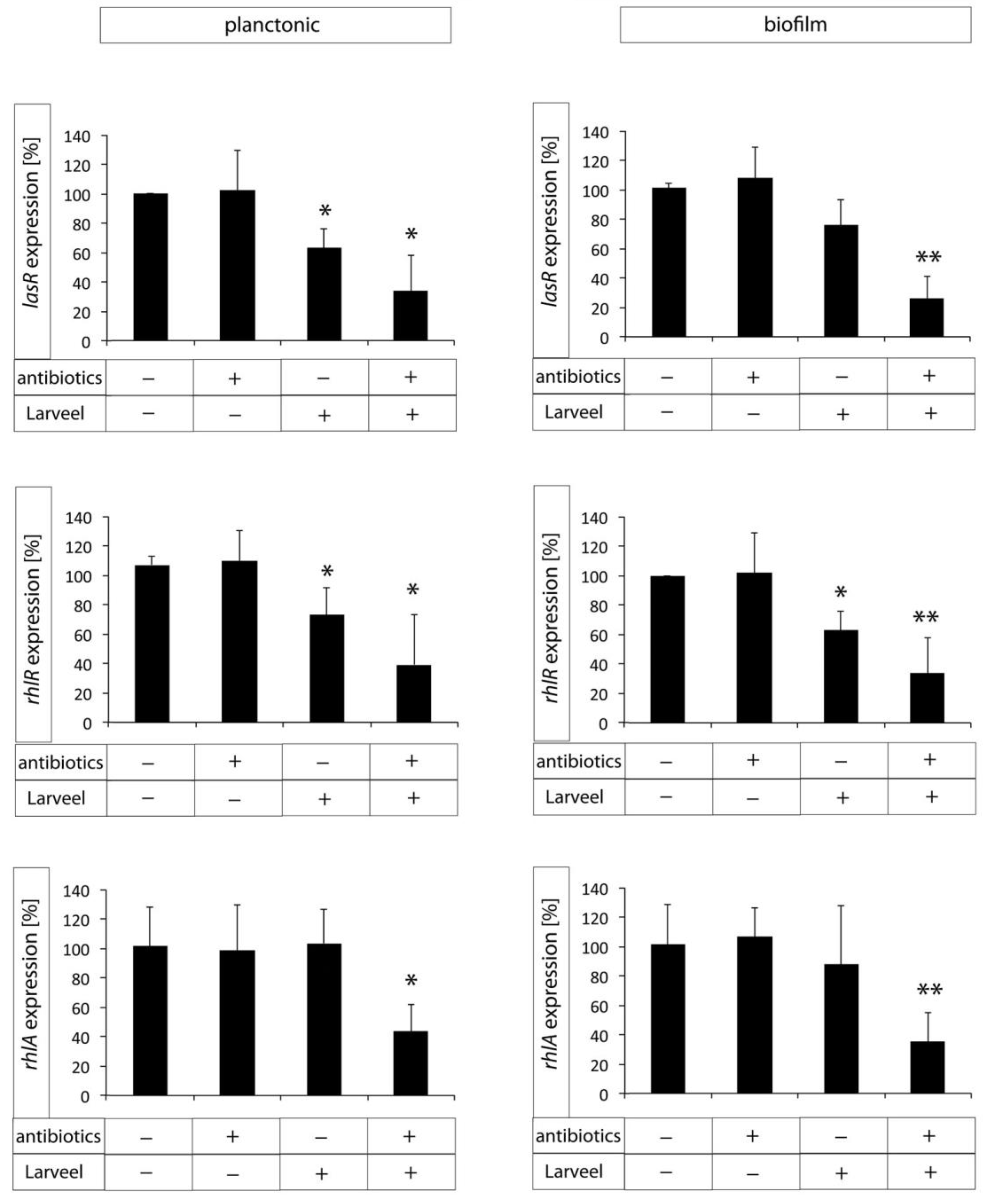
3.5. Decreased Expression of Biofilm Maturation and Virulence Genes after Larveel® Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall-Stoodley, L.; Stoodley, P.; Kathju, S.; Høiby, N.; Moser, C.; William Costerton, J.; Moter, A.; Bjarnsholt, T. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol. Med. Microbiol. 2012, 65, 127–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID* guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costerton, J.W. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Mah, T.-F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malic, S.; Hill, K.E.; Playle, R.; Thomas, D.W.; Williams, D.W. In vitro interaction of chronic wound bacteria in biofilms. J. Wound Care 2011, 20, 569–577. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rhoads, D.D. A study of biofilm-based wound management in subjects with critical limb ischaemia. J. Wound Care 2008, 17, 145–155. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef]
- Omar, A.; Wright, J.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial biofilms and chronic wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, D.G. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [Green Version]
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Møller, K.; Jørgensen, B.; Andersen, A.S.; Krogfelt, K.A.; Givskov, M.; Tolker-Nielsen, T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J. Clin. Microbiol. 2009, 47, 4084–4089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Lima, J.L.d.C.; Alves, L.R.; Jacomé, P.R.L.d.A.; Bezerra Neto, J.P.; Maciel, M.A.V.; Morais, M.M.C.d. Biofilm production by clinical isolates of Pseudomonas aeruginosa and structural changes in LasR protein of isolates non biofilm-producing. Braz. J. Infect. Dis. 2018, 22, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [Green Version]
- Baer, W.S. The classic: The treatment of chronic osteomyelitis with the maggot (larva of the blow fly). Clin. Orthop. Relat. Res. 2011, 469, 920–944. [Google Scholar] [CrossRef] [Green Version]
- Čeřovský, V.; Bém, R. Lucifensins, the insect defensins of biomedical importance: The story behind maggot therapy. Pharmaceuticals 2014, 7, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Becerikli, M.; Merwart, B.; Lam, M.C.; Suppelna, P.; Rittig, A.; Mirmohammedsadegh, A.; Stricker, I.; Theiss, C.; Singer, B.B.; Jacobsen, F.; et al. EPHB4 tyrosine-kinase receptor expression and biological significance in soft tissue sarcoma. Int. J. Cancer 2015, 136, 1781–1791. [Google Scholar] [CrossRef]
- Harati, K.; Daigeler, A.; Hirsch, T.; Jacobsen, F.; Behr, B.; Wallner, C.; Lehnhardt, M.; Becerikli, M. Tumor-associated fibroblasts promote the proliferation and decrease the doxorubicin sensitivity of liposarcoma cells. Int. J. Mol. Med. 2016, 37, 1535–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinstraesser, L.; Sorkin, M.; Niederbichler, A.D.; Becerikli, M.; Stupka, J.; Daigeler, A.; Kesting, M.R.; Stricker, I.; Jacobsen, F.; Schulte, M. A novel human skin chamber model to study wound infection ex vivo. Arch. Dermatol. Res. 2010, 302, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Becerikli, M.; Jaurich, H.; Wallner, C.; Wagner, J.M.; Dadras, M.; Jettkant, B.; Pöhl, F.; Seifert, M.; Jung, O.; Mitevski, B.; et al. P2000—A high-nitrogen austenitic steel for application in bone surgery. PLoS ONE 2019, 14, e0214384. [Google Scholar] [CrossRef]
- Becerikli, M.; Jaurich, H.; Schira, J.; Schulte, M.; Döbele, C.; Wallner, C.; Abraham, S.; Wagner, J.M.; Dadras, M.; Kneser, U.; et al. Age-dependent alterations in osteoblast and osteoclast activity in human cancellous bone. J. Cell. Mol. Med. 2017, 21, 2773–2781. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, M.R.; Fleuchot, B.; Lauciello, L.; Jafari, P.; Applegate, L.A.; Raffoul, W.; Que, Y.-A.; Perron, K. Effect of human burn wound exudate on pseudomonas aeruginosa virulence. mSphere 2016, 1, e00111-15. [Google Scholar] [CrossRef] [Green Version]
- Dauros Singorenko, P.; Rosario, R.; Windsor, J.A.; Phillips, A.R.; Blenkiron, C. The transcriptional responses of cultured wound cells to the excretions and secretions of medicinal L ucilia sericata larvae. Wound Repair Regen. 2017, 25, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Cazander, G.; van Veen, K.E.B.; Bernards, A.T.; Jukema, G.N. Do maggots have an influence on bacterial growth? A study on the susceptibility of strains of six different bacterial species to maggots of Lucilia sericata and their excretions/secretions. J. Tissue Viability 2009, 18, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, H.; Gestmann, F. Extracts from fly maggots and fly pupae as a “wound healer”. In Nature Helps...; Springer: Berlin/Heidelberg, Germany, 2011; pp. 325–348. [Google Scholar]
- Sretenovic, S.; Stojković, B.; Dogsa, I.; Kostanjšek, R.; Poberaj, I.; Stopar, D. An early mechanical coupling of planktonic bacteria in dilute suspensions. Nat. Commun. 2017, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; O’Neill, A.M.; Williams, M.R.; Cau, L.; Nakatsuji, T.; Horswill, A.R.; Gallo, R.L. Short chain fatty acids produced by Cutibacterium acnes inhibit biofilm formation by Staphylococcus epidermidis. Sci. Rep. 2020, 10, 21237. [Google Scholar] [CrossRef] [PubMed]
- Shimada, I. The stimulating effect of fatty acids and amino acid derivatives on the labellar sugar receptor of the fleshfly. J. Gen. Physiol. 1978, 71, 19–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrigan, J.J.; Srinivasan, N.G. The occurrence of certain D-amino acids in insects*. Biochemistry 1966, 5, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Warraich, A.A.; Mohammed, A.R.; Perrie, Y.; Hussain, M.; Gibson, H.; Rahman, A. Evaluation of anti-biofilm activity of acidic amino acids and synergy with ciprofloxacin on Staphylococcus aureus biofilms. Sci. Rep. 2020, 10, 9021. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti. Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Filkins, L.M.; Graber, J.A.; Olson, D.G.; Dolben, E.L.; Lynd, L.R.; Bhuju, S.; O’Toole, G.A. Coculture of Staphylococcus aureus with Pseudomonas aeruginosa drives S. aureus towards fermentative metabolism and reduced viability in a cystic fibrosis model. J. Bacteriol. 2015, 197, 2252–2264. [Google Scholar] [CrossRef] [Green Version]
- Dalton, T.; Dowd, S.E.; Wolcott, R.D.; Sun, Y.; Watters, C.; Griswold, J.A.; Rumbaugh, K.P. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS ONE 2011, 6, e27317. [Google Scholar] [CrossRef] [Green Version]
- Moura-Alves, P.; Puyskens, A.; Stinn, A.; Klemm, M.; Guhlich-Bornhof, U.; Dorhoi, A.; Furkert, J.; Kreuchwig, A.; Protze, J.; Lozza, L.; et al. Host monitoring of quorum sensing during Pseudomonas aeruginosa infection. Science 2019, 366, eaaw1629. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Kolter, R.; Greenberg, E.P. The superficial life of microbes. Nature 2006, 441, 300–302. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. QUORUM SENSING: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [Green Version]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Hentzer, M. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Christensen, Q.H.; Grove, T.L.; Booker, S.J.; Greenberg, E.P. A high-throughput screen for quorum-sensing inhibitors that target acyl-homoserine lactone synthases. Proc. Natl. Acad. Sci. USA 2013, 110, 13815–13820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starkey, M.; Lepine, F.; Maura, D.; Bandyopadhaya, A.; Lesic, B.; He, J.; Kitao, T.; Righi, V.; Milot, S.; Tzika, A.; et al. Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLoS Pathog. 2014, 10, e1004321. [Google Scholar] [CrossRef]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Skindersoe, M.E.; Alhede, M.; Phipps, R.; Yang, L.; Jensen, P.O.; Rasmussen, T.B.; Bjarnsholt, T.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 3648–3663. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.Y.-Y.; Chua, S.-L.; Chen, Y.; Rice, S.A.; Kjelleberg, S.; Nielsen, T.E.; Yang, L.; Givskov, M. Identification of five structurally unrelated quorum-sensing inhibitors of Pseudomonas aeruginosa from a natural-derivative database. Antimicrob. Agents Chemother. 2013, 57, 5629–5641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.A.K.S.; Rudden, M.; Smyth, T.J.; Dooley, J.S.G.; Marchant, R.; Banat, I.M. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotechnol. 2019, 103, 3521–3535. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerikli, M.; Wallner, C.; Dadras, M.; Wagner, J.M.; Dittfeld, S.; Jettkant, B.; Gestmann, F.; Mehlhorn, H.; Mehlhorn-Diehl, T.; Lehnhardt, M.; et al. Maggot Extract Interrupts Bacterial Biofilm Formation and Maturation in Combination with Antibiotics by Reducing the Expression of Virulence Genes. Life 2022, 12, 237. https://doi.org/10.3390/life12020237
Becerikli M, Wallner C, Dadras M, Wagner JM, Dittfeld S, Jettkant B, Gestmann F, Mehlhorn H, Mehlhorn-Diehl T, Lehnhardt M, et al. Maggot Extract Interrupts Bacterial Biofilm Formation and Maturation in Combination with Antibiotics by Reducing the Expression of Virulence Genes. Life. 2022; 12(2):237. https://doi.org/10.3390/life12020237
Chicago/Turabian StyleBecerikli, Mustafa, Christoph Wallner, Mehran Dadras, Johannes M. Wagner, Stephanie Dittfeld, Birger Jettkant, Falk Gestmann, Heinz Mehlhorn, Tim Mehlhorn-Diehl, Marcus Lehnhardt, and et al. 2022. "Maggot Extract Interrupts Bacterial Biofilm Formation and Maturation in Combination with Antibiotics by Reducing the Expression of Virulence Genes" Life 12, no. 2: 237. https://doi.org/10.3390/life12020237
APA StyleBecerikli, M., Wallner, C., Dadras, M., Wagner, J. M., Dittfeld, S., Jettkant, B., Gestmann, F., Mehlhorn, H., Mehlhorn-Diehl, T., Lehnhardt, M., & Behr, B. (2022). Maggot Extract Interrupts Bacterial Biofilm Formation and Maturation in Combination with Antibiotics by Reducing the Expression of Virulence Genes. Life, 12(2), 237. https://doi.org/10.3390/life12020237






