The Potential Role of Nutrition in Lung Cancer Establishment and Progression
Abstract
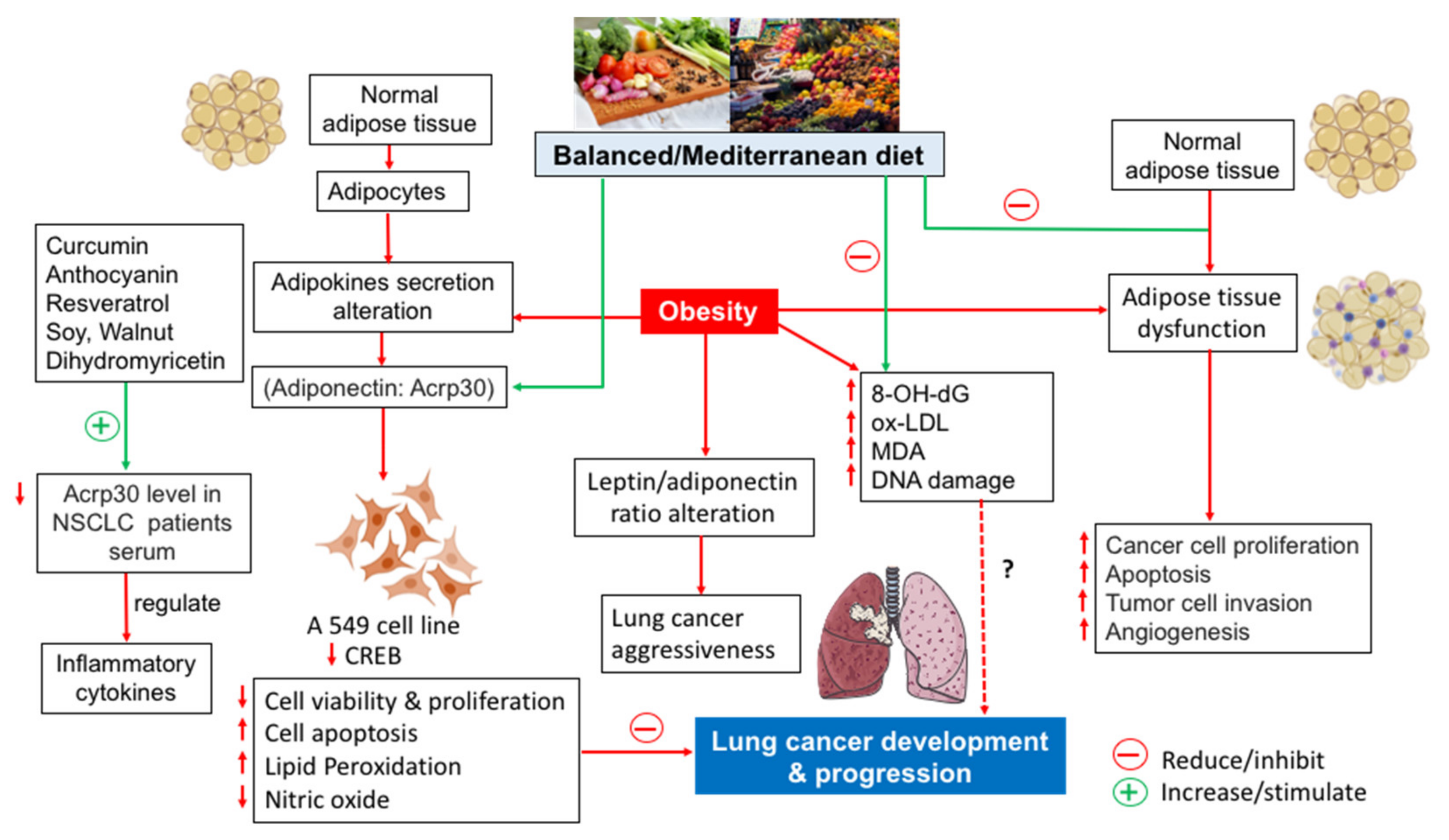
:1. Introduction
2. Oxidative Stress and Lung Cancer
3. The Importance of Adipose Tissue in Cancer Development and/or Progression
4. Adiponectin and Lung Cancer
5. Anti-Inflammatory and Antioxidant Foods to Prevent Cancer
6. Foods and Lung Cancer
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, A.; Messina, G.; Capaccio, D.; Santini, M. Recurrent spontaneous pneumomediastinum: A rare but possible event! J. Thorac. Dis. 2012, 4, 431–433. [Google Scholar] [PubMed]
- Clark, S.B.; Alsubait, S. Non Small Cell Lung Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021; p. 9. [Google Scholar]
- Kligerman, S.; White, C. Epidemiology of lung cancer in women: Risk factors, survival, and screening. AJR Am. J. Roentgenol. 2011, 196, 287–295. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cress, D.; Munoz-Antonia, T. Racial and Ethnic Differences in the Epidemiology and Genomics of Lung Cancer. Cancer Control. 2016, 23, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Van Meerbeeck, J.P.; Fennell, D.A.; De Ruysscher, D.K. Small-cell lung cancer. Lancet 2011, 378, 1741–1755. [Google Scholar] [CrossRef]
- Santini, M.; Fiorelli, A.; Messina, G.; Mazzella, A.; Accardo, M. The Feasibility of LigaSure to Create Intestinal Anastomosis: Results of Ex Vivo Study. Surg. Innov. 2015, 22, 266–273. [Google Scholar] [CrossRef]
- Ebell, M.H.; Bentivegna, M.; Hulme, C. Cancer-Specific Mortality, All-Cause Mortality, and Overdiagnosis in Lung Cancer Screening Trials: A Meta-Analysis. Ann. Fam. Med. 2020, 18, 545–552. [Google Scholar] [CrossRef]
- Cozzolino, I.; Ronchi, A.; Messina, G.; Montella, M.; Morgillo, F.; Vicidomini, G.; Tirino, V.; Grimaldi, A.; Zito Marino, F.; Santini, M.; et al. Adequacy of Cytologic Samples by Ultrasound-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology of Peripheral Pulmonary Nodules for Morphologic Diagnosis and Molecular Evaluations: Comparison with Computed Tomography–Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology. Arch. Pathol. Lab. Med. 2020, 144, 361–369. [Google Scholar]
- Fiorelli, A.; Accardo, M.; Carelli, E.; Del Prete, A.; Messina, G.; Reginelli, A.; Berritto, D.; Papale, F.; Armenia, E.; Chiodini, P.; et al. Harmonic tecnology versus neodymium-doped yttrium aluminium garnet laser and electrocautery for lung metastasectomy: An experimental study. Interactive Cardiovasc. Thorac Surg. 2016, 23, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santini, M.; Fiorelli, A.; Messina, G.; Laperuta, P.; Mazzella, A.; Accardo, M. Use of the LigaSure device and the Stapler for closure of the small bowel: A comparative ex vivo study. Surg. Today 2013, 43, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Arribas, L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 2018, 29 (Suppl. 2), ii1e9. [Google Scholar] [CrossRef] [PubMed]
- Baldessari, C.; Guaitoli, G.; Valoriani, F.; Bonacini, R.; Marcheselli, R.; Reverberi, L.; Pecchi, A.; Menozzi, R.; Torricelli, P.; Bertolini, F.; et al. Impact of body composition, nutritional and inflammatory status on outcome of non-small cell lung cancer patients treated with immunotherapy. Clin. Nutr. ESPEN 2021, 43, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Mengheri, E.; Nobili, F.; Crocchioni, G.; Lewis, J.A. Protein starvation impairs the ability of activated lymphocytes to produce interferon-gamma. J. Interferon. Res. 1992, 12, 17e21. [Google Scholar] [CrossRef]
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012, 18, 363e74. [Google Scholar] [CrossRef]
- Yang, H.; Youm, Y.H.; Vandanmagsar, B.; Ravussin, A.; Gimble, J.M.; Greenway, F.; Stephens, J.M.; Mynatt, R.L.; Dixit, V.D. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: Implications for systemic inflammation and insulin resistance. J. Immunol. 2010, 185, 1836e45. [Google Scholar] [CrossRef] [Green Version]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and clinical significance. JPEN-J Parenter Enter Nutr. 2019, 43, 181e93. [Google Scholar] [CrossRef] [Green Version]
- Masri, F. Role of nitric oxide and its metabolites as potential markers in lungcancer. Ann. Thorac. Med. 2010, 5, 123–127. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine, 4th ed.; Oxford University: Oxford, UK, 2007. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Afonso, V.; Champy, R.; Mitrovic, D.; Collin, P.; Lomri, A. Reactive oxygen species andsuperoxide dismutases: Role in joint diseases. J. Bone Spine 2007, 74, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Filaire, E.; Dupuis, C.; Galvaing, G.; Aubreton, S.; Laurent, H.; Richard, R.; Filaire, M. Lung cancer: What are the links with oxidative stress, physical activity and nutrition. Lung Cancer 2013, 82, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Crohns, M. Antioxidants, Cytokines and Markers of Oxidative Stress in Lungcancer: Associations with Adverse Events, Response and Survival, 1st ed.; Lambert Academic Publishing: Saarbrücken, Germany, 2010. [Google Scholar]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress: Relationship with exercise and training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Jeong, E.K.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Regulation of Tumor Progression by Programmed Necrosis. Oxid. Med. Cell Longev. 2018, 2018, 3537471. [Google Scholar] [CrossRef] [Green Version]
- Ottavio, F.G.; Handy, D.E.; Loscalzo, J. Redox regulation in the extracellular environment. Circ. J. 2008, 72, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Cerutti, P.A. Prooxidant states and tumor promotion. Science 1985, 227, 375–380. [Google Scholar] [CrossRef]
- Dreher, D.; Junod, A.F. Role of oxygen free radicals in cancer development. Eur. J. Cancer 1996, 32A, 30–38. [Google Scholar] [CrossRef]
- Toyokuni, S. Molecular mechanisms of oxidative stress-induced carcinogenesis: From epidemiology to oxygenomics. Int. Union Biochem. Mol. Biol. Life 2008, 60, 441–447. [Google Scholar] [CrossRef]
- Hahn, W.C.; Weinberg, R.A. Modeling the molecular circuitry of cancer. Nat. Rev. Cancer 2002, 2, 331–341. [Google Scholar] [CrossRef]
- Azad, N.; Rojanasakul, Y.; Vallyathan, V. Inflammation and lungcancer: Roles of reactive oxygen/nitrogen species. J. Toxicol. Environ. Health B Crit. Rev. 2008, 11, 1–15. [Google Scholar] [CrossRef]
- Weinberg, R.A. The Biology of Cancer; Garland Science (Taylor & Francis Group): New York, NY, USA, 2006. [Google Scholar]
- Ziech, D.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Reactive oxygen speciers (ROS)-induced genetic and epigenetic alterations in human carcinogenesis. Mutat. Res. 2011, 711, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Gardi, C.; Valacchi, G. Cigarette smoke and ozone effect on murine inflammatory responses. Ann. N. Y. Acad. Sci. 2012, 1259, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Sangani, R.G.; Ghio, A.J. Lung injury after cigarette smoking is particle related. Int. J. Chron. Obstruct. Pulmon. Dis. 2011, 6, 191–198. [Google Scholar] [PubMed] [Green Version]
- Pryor, W.A. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ. Health Perspect. 1997, 105, 875. [Google Scholar] [PubMed]
- Hannan, M.A.; Recio, L.; Deluca, P.P.; Enoch, H. Co-mutagenic effects of 2-aminoanthracene and cigarette smoke condensate on smoker’s urine in the Ames Salmonella assay system. Cancer Lett. 1981, 13, 203–212. [Google Scholar] [CrossRef]
- Møller, P.; Folkmann, J.K.; Forchhammer, L.; Bräuner, E.V.; Danielsen, P.H.; Risom, L.; Loft, S. Air pollution, oxidative damage to DNA, and carcinogenesis. Cancer Lett. 2008, 266, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Taneja, V.; Vassallo, R. Cigarette smoking and inflammation: Cellular and molecular mechanisms. J. Dent. Res. 2012, 91, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Mons, U.; Muscat, J.E.; Modesto, J.; Richie, J.P., Jr.; Brenner, H. Effect of smoking reduction and cessation on the plasma levels of the oxidative stress biomarker glutathione--Post-hoc analysis of data from a smoking cessation trial. Free Radic. Biol. Med. 2016, 91, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Field, R.W.; Withers, B.L. Occupational and environmental causes of lung cancer. Clin. Chest Med. 2012, 33, 681–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, B.; Sarker, A.H.; Havel, C.; Saha, S.; Hazra, T.K.; Schick, S.; Jacob, P., 3rd; Rehan, V.K.; Chenna, A.; Sharan, D.; et al. Thirdhand smoke causes DNA damage in human cells. Mutagenesis 2013, 28, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldkorn, T.; Filosto, S.; Chung, S. Lung injury and lung cancer caused by cigarette smoke-induced oxidative stress: Molecular mechanisms and therapeutic opportunities involving the ceramide-generating machinery and epidermal growth factor receptor. Antioxid. Redox Signal. 2014, 15, 2149–2174. [Google Scholar] [CrossRef] [Green Version]
- Ntikoudi, E.; Kiagia, M.; Boura, P.; Syrigos, K.N. Hormones of adipose tissue and their biologic role in lung cancer. Cancer Treat Rev. 2014, 1, 22–30. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 91–200. [Google Scholar]
- Ackerman, S.E.; Blackburn, O.A.; Marchildon, F.; Cohen, P. Insights into the link between obesity and cancer. Curr. Obes. Rep. 2017, 6, 195–203. [Google Scholar] [CrossRef]
- Polito, R.; Nigro, E.; Messina, A.; Monaco, M.L.; Monda, V.; Scudiero, O.; Cibelli, G.; Valenzano, A.; Picciocchi, E.; Zammit, C.; et al. Adiponectin and Orexin-A as a Potential Immunity Link Between Adipose Tissue and Central Nervous System. Front. Physiol. 2018, 9, 982. [Google Scholar] [CrossRef]
- Bifulco, M.; Ciaglia, E. Updates on “adiponcosis”: More new incoming evidence strengthening the obesity-cancer link. Eur. J. Intern. Med. 2017, 41, e19–e20. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Zazzo, E.; Polito, R.; Bartollino, S.; Nigro, E.; Porcile, C.; Bianco, A.; Daniele, A.; Moncharmont, B. Adiponectin as Link Factor between Adipose Tissue and Cancer. Int. J. Mol. Sci. 2019, 20, 839. [Google Scholar] [CrossRef] [Green Version]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Frühbeck, G. Adipose tissue immunity and cancer. Front. Physiol. 2013, 4, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lengyel, E.; Makowski, L.; DiGiovanni, J.; Kolonin, M.G. Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends Cancer 2018, 4, 374–384. [Google Scholar] [CrossRef]
- Eheman, C.; Henley, S.J.; Ballard-Barbash, R.; Jacobs, E.J.; Schymura, M.J.; Noone, A.M.; Pan, L.; Anderson, R.N.; Fulton, J.E.; Kohler, B.A.; et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer 2012, 118, 2338–2366. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Cozzo, A.J.; Fuller, A.M.; Makowski, L. Contribution of Adipose Tissue to Development of Cancer. Compr. Physiol. 2017, 8, 237–282. [Google Scholar]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y.; Li, X.; Zhang, Y.; Gulbins, E.; Zhang, Y. Enhancement of endothelial permeability by free fatty acid through lysosomal cathepsin B-mediated Nlrp3 inflammasome activation. Oncotarget 2016, 7, 73229–73241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Incio, J.; Ligibel, J.A.; McManus, D.T.; Suboj, P.; Jung, K.; Kawaguchi, K.; Pinter, M.; Babykutty, S.; Chin, S.M.; Vardam, T.D.; et al. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci. Transl. Med. 2018, 10, eaag0945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, A.K.; Grant, F.D.; Slavin, S.A. Lower-extremity lymphedema and elevated body-mass index. N. Engl. J. Med. 2012, 366, 2136–2137. [Google Scholar] [CrossRef] [PubMed]
- Nishita, M.; Enomoto, M.; Yamagata, K.; Minami, Y. Cell/tissue-tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol. 2010, 20, 346–354. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Zhang, Y.; Leroith, D.; Bernlohr, D.A.; Chen, X. The role of lipocalin 2 in the regulation of inflammation in adi-pocytes and macrophages. Mol. Endocrinol. 2008, 22, 1416–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Izaguirre, M.; Hernández-Lizoain, J.L.; Baixauli, J.; Martí, P.; Valentí, V.; Moncada, R.C.; et al. Increased obesity-associated circulating levels of the extracellular matrixproteins osteopontin, chitinase-3 Like-1 and tenascin C are associated with colon cancer. PLoS ONE 2016, 11, e0162189. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Silva, C.; Rotellar, F.; Hernández-Lizoain, J.L.; Baixauli, J.; Valentí, V.; Pardo, F.; et al. Up-regulationof the novel proinflammatory adipokines lipocalin-2, chitinase-3like-1 and osteopontin as well as angiogenic-related factors invisceral adipose tissue of patients with colon cancer. J. Nutr. Bio-Chem. 2011, 22, 634–641. [Google Scholar] [CrossRef]
- Nielsen, A.R.; Erikstrup, C.; Johansen, J.S.; Fischer, C.P.; Plomgaard, P.; Krogh-Madsen, R.; Taudorf, S.; Lindegaard, B.; Pedersen, B.K. Plasma YKL-40: A BMI-independent marker of type 2 diabetes. Diabetes 2008, 57, 3078–3082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, H.O. An Explanation for the Adiponectin Paradox. Pharmaceuticals 2021, 14, 1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.P.; Wang, X.H.; Li, Z.M.; Sun, J.R. Changes in serum inflammatory factors, adiponectin, intestinal flora and immunity in patients with non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10566–10572. [Google Scholar]
- Illiano, M.; Nigro, E.; Sapio, L.; Caiafa, I.; Spina, A.; Scudiero, O.; Bianco, A.; Esposito, S.; Mazzeo, F.; Pedone, P.V.; et al. Adiponectin down-regulates CREB and inhibits proliferation of A549 lung cancer cells. Pulm. Pharmacol. Ther. 2017, 45, 114–120. [Google Scholar] [CrossRef]
- Nigro, E.; Stiuso, P.; Matera, M.G.; Monaco, M.L.; Caraglia, M.; Maniscalco, M.; Perrotta, F.; Mazzarella, G.; Daniele, A.; Bianco, A. The anti-proliferative effects of adiponectin on human lung adenocarcinoma A549 cells and oxidative stress involvement. Pulm. Pharmacol. Ther. 2019, 55, 25–30. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, L. Expression of adiponectin in non-small cell lung cancer and its relationship with MMP-9 and angiogenesis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2015, 40, 579–584. [Google Scholar]
- Nigro, E.; Perrotta, F.; Monaco, M.L.; Polito, R.; Pafundi, P.C.; Matera, M.G.; Daniele, A.; Bianco, A. Implications of the Adiponectin System in Non-Small Cell Lung Cancer Patients: A Case-Control Study. Biomolecules 2020, 10, 926. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, C.; Ji, M.; Fan, J.; Xie, J.; Huang, Y.; Jiang, X.; Xu, J.; Yin, R.; Du, L.; et al. Diet and Risk of Incident Lung Cancer: A Large Prospective Cohort Study in UK Biobank. Am. J. Clin. Nutr. 2021, 114, 2043–2051. [Google Scholar] [CrossRef]
- Widiatmaja, D.M.; Lutvyani, A.; Sari, D.R.; Kurniasari, H.; Meiliana, I.D.; Fasitasari, M.; Yamaoka, Y.; Rejeki, P.S. The effect of long-term ketogenic diet on serum adiponectin and insulin-like growth factor-1 levels in mice. J. Basic Clin. Physiol. Pharmacol. 2021. [CrossRef]
- Atazadegan, M.A.; Bagherniya, M.; Fakheran, O.; Sathyapalan, T.; Sahebkar, A. The Effect of Herbal Medicine and Natural Bioactive Compounds on Plasma Adiponectin: A Clinical Review. Adv. Exp. Med. Biol. 2021, 1328, 37–57. [Google Scholar]
- Galbete, C.; Schwingshackl, L.; Schwedhelm, C.; Boeing, H.; Schulze, M.B. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: An umbrella review of meta-analyses. Eur. J. Epidemiol. 2018, 33, 909–931. [Google Scholar] [CrossRef] [Green Version]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Niaz, M.A.; Ghosh, S.; Beegum, R.; Bishnoi, I.; Agarwal, P.; Agarwal, A. Dietary intake and plasma levels of antioxidant vitamins in health and disease. A hospital based case control study. J. Nutr. Environ. Med. 1995, 5, 235–242. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Grekas, A.; Christou, A.; Chatzigeorgiou, M.; Skoumas, I.; Tousoulis, D.; et al. Adherence to Mediterranean diet and 10-year incidence (2002–2012) of diabetes: Correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab. Res. Rev. 2016, 32, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Estruch, R. Mediterranean diet, antioxidants and cancer: The need for randomized trials. Eur. J. Cancer Prev. 2004, 13, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Trichopoulou, A. Observational Epidemiology, Lifestyle, and Health: The Paradigm of the Mediterranean Diet. Am. J. Health Promot. 2020, 34, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Bibiloni, M.D.; Martorell, M.; Buil-Cosiales, P.; Marti, A.; Pons, A.; Tur, J.A.; Martinez-Gonzalez, M.Á.; PREDIMED Study Investigators. Mediterranean diets supplemented with virgin olive oil and nuts enhance plasmatic antioxidant capabilities and decrease xanthine oxidase activity in people with metabolic syndrome: The PREDIMED study. Mol. Nutr. Food Res. 2016, 60, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean Diet Enriched in High Quality Extra Virgin Olive Oil on Oxidative Stress, Inflammation and Gut Microbiota in Obese and Normal Weight Adult Subjects. Front. Pharmacol. 2019, 10, 1366. [Google Scholar] [CrossRef] [Green Version]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Skoumas, J.; Stefanadis, C. Status and management of blood lipids in Greek adults and their relation to socio-demographic, lifestyle and dietary factors: The ATTICA Study. Blood lipids distribution in Greece. Atherosclerosis 2004, 173, 353–361. [Google Scholar] [CrossRef]
- Azzini, E.; Polito, A.; Fumagalli, A.; Intorre, F.; Venneria, E.; Durazzo, A.; Zaccaria, M.; Ciarapica, D.; Foddai, M.S.; Mauro, B.; et al. Mediterranean Diet Effect: An Italian picture. Nutr. J. 2011, 10, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribezzo, F.; Shiloh, Y.; Schumacher, B. Systemic DNA damage responses in aging and diseases. Semin. Cancer Biol. 2016, 37–38, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, B.C.; Dizdaroglu, M. Implications of DNA damage and DNA repair on human diseases. Mutagenesis 2020, 35, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Hernández-Galiot, A. Intake of Nutrient and Non-Nutrient Dietary Antioxidants. Contribution of Macromolecular Antioxidant Polyphenols in an Elderly Mediterranean Population. Nutrients 2019, 11, 2165. [Google Scholar] [CrossRef] [Green Version]
- Blaak, E.E. Current metabolic perspective on malnutrition in obesity: Towards more subgroup-based nutritional approaches? Proc. Nutr. Soc. 2020, 79, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Engin, A. The Pathogenesis of Obesity-Associated Adipose Tissue Inflammation. In Obesity and Lipotoxicity; Springer: New York, NY, USA, 2017; pp. 221–245. [Google Scholar]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; de la Higuera, M.; Frühbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef] [Green Version]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Romagnolo, D.F.; Selmin, O.I. Flavonoids and cancer prevention: A review of the evidence. J. Nutr. Gerontol. Geriatr. 2012, 31, 206–238. [Google Scholar] [CrossRef]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and vegetables and cancer risk: A review of southern European studies. Br. J. Nutr. 2015, 113, 102–110. [Google Scholar] [CrossRef]
- Russo, A.; Acquaviva, R.; Campisi, A.; Sorrenti, V.; Giacomo, C.D.; Virgata, G.; Barcellona, M.L.; Vanella, A. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol. Toxicol. 2000, 16, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Arif, H.; Rehmani, N.; Farhan, M.; Ahmad, A.; Hadi, S.M. Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study. Int. J. Mol. Sci. 2015, 16, 26754–26769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nani, A.; Belarbi, M.; Ksouri-Megdiche, W.; Abdoul-Azize, S.; Benammar, C.; Ghiringhelli, F.; Hichami, A.; Khan, N.A. Effects of polyphenols and lipids from Pennisetum glaucum grains on T-cell activation: Modulation of Ca2+ and ERK1/ERK2 signaling. BMC Complement. Altern. Med. 2015, 15, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiselli, A.; Nardini, M.; Baldi, A.; Scaccini, C. Antioxidant activity of different phenolic fractions separated from an Italian red wine. J. Agric. Food Chem. 1998, 46, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Sinatra, D.; Blanco, I.; Mulè, S.; La Verde, M.; Marranzano, M. Association between dietary phenolic acids and hypertension in a mediterranean cohort. Nutrients 2017, 9, 1069. [Google Scholar] [CrossRef] [Green Version]
- Kopp, C.; Singh, S.P.; Regenhard, P.; Müller, U.; Sauerwein, H.; Mielenz, M. Trans-cinnamic acid increases adiponectin and the phosphorylation of AMP-activated protein kinase through G-protein-coupled receptor signaling in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2014, 15, 2906–2915. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Luna-Vital, D.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.; Loarca-Piña, G.; de Mejia, E.G. Maize extract rich in ferulic acid and anthocyanins prevents high-fat-induced obesity in mice by modulating SIRT1, AMPK and IL-6 associated metabolic and inflammatory pathways. J. Nutr. Biochem. 2020, 79, 108343. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visioli, F.; Bellomo, G.; Montedoro, G.; Galli, C. Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis 1995, 117, 25–32. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.-Y.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Li, Z.; Henning, S.M.; Zhang, Y.; Zerlin, A.; Li, L.; Gao, K.; Lee, R.-P.; Karp, H.; Thames, G.; Bowerman, S.; et al. Antioxidant-rich spice added to hamburger meat during cooking results in reduced meat, plasma, and urine malondialdehyde concentrations. Am. J. Clin. Nutr. 2010, 91, 1180–1184. [Google Scholar] [CrossRef] [Green Version]
- Marnett, L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008, 22, 41–46. [Google Scholar] [CrossRef]
- Griffiths, K.; Adlercretz, H.; Boyle, P.; Denis, L.; Nicholson, R.I.; Morton, M.S. Nutrition and Cancer; ISIS Medical Media Ltd.: Oxford, UK, 1996. [Google Scholar]
- Wolska, K.; Gorska, A.; Antosik, K.; Lugowska, K. Immunomodulatory effects of propolis and its components on basic immune cell functions. Indian J. Pharm. Sci. 2019, 81, 575–588. [Google Scholar] [CrossRef] [Green Version]
- Liao, B.C.; Hsieh, C.W.; Liu, Y.C.; Tzeng, T.T.; Sun, Y.W.; Wung, B.S. Cinnamaldehyde inhibits the tumor necrosis factor-alpha-induced expression of cell adhesion molecules in endothelial cells by suppressing NF-kappaB activation: Effects upon IkappaB and Nrf2. Toxicol. Appl. Pharmacol. 2008, 229, 161–171. [Google Scholar] [CrossRef]
- Bang, J.S.; Oh, D.H.; Choi, H.M.; Sur, B.J.; Lim, S.J.; Kim, J.Y.; Yang, H.I.; Yoo, M.C.; Hahm, D.H.; Kim, K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49. [Google Scholar] [CrossRef] [Green Version]
- Wang-Sheng, C.; Jie, A.; Jian-Jun, L.; Lan, H.; Zeng-Bao, X.; Chang-Qing, L. Piperine attenuates lipopolysaccharide (LPS)-induced inflammatory responses in BV2 microglia. Int. Immunopharmacol. 2017, 42, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Antioxidant and Anti-Inflammatory Components of Foods; ILSI International Life Sciences Institute: Washington, DC, USA, 2015. [Google Scholar]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. CMAJ 2000, 163, 739–744. [Google Scholar]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcuminandcurcumin-likemolecules: Fromspice to drugs. Curr. Med. Chem. 2014, 21, 204–222. [Google Scholar] [CrossRef]
- Panaro, M.A.; Corrado, A.; Benameur, T.; Paolo, C.F.; Cici, D.; Porro, C. The Emerging Role of Curcumin in the Modulation of TLR-4 Signaling Pathway: Focus on Neuroprotective and Anti-Rheumatic Properties. Int. J. Mol. Sci. 2020, 21, 2299. [Google Scholar] [CrossRef] [Green Version]
- Porro, C.; Cianciulli, A.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Curcumin Regulates Anti-Inflammatory Responses by JAK/STAT/SOCS Signaling Pathway in BV-2 Microglial Cells. Biology 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [PubMed]
- Benameur, T.; Soleti, R.; Panaro, M.A.; La Torre, M.E.; Monda, V.; Messina, G.; Porro, C. Curcumin as Prospective Anti-Aging Natural Compound: Focus on Brain. Molecules 2021, 26, 4794. [Google Scholar] [CrossRef]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whyand, T.; Hurst, J.R.; Beckles, M.; Caplin, M.E. Pollution and respiratory disease: Can diet or supplements help? A review. Respir. Res. 2018, 19, 79. [Google Scholar] [CrossRef] [Green Version]
- De Nuccio, F.; Cianciulli, A.; Porro, C.; Kashyrina, M.; Ruggiero, M.; Calvello, R.; Miraglia, A.; Nicolardi, G.; Lofrumento, D.D.; Panaro, M.A. Inflammatory Response Modulation by Vitamin C in an MPTP Mouse Model of Parkinson’s Disease. Biology 2021, 10, 1155. [Google Scholar] [CrossRef]
- Vázquez-Fresno, R.; Rosana, A.R.R.; Sajed, T.; Onookome-Okome, T.; Wishart, N.A.; Wishart, D.S. Herbs and spices- biomarkers of intake based on human intervention studies—A systematic review. Genes Nutr. 2019, 14, 18. [Google Scholar] [CrossRef]
- USDA National Nutrient Database. Foods Highest in Vitamin C and Iron in Spices and Herbs. 2008. Available online: https://nutritiondata.self.com/foods-002101119000000000000-1w.html (accessed on 27 May 2020).
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The Effectiveness of Vitamin E Treatment in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef] [Green Version]
- La Torre, M.E.; Villano, I.; Monda, M.; Messina, A.; Cibelli, G.; Valenzano, A.; Pisanelli, D.; Panaro, M.A.; Tartaglia, N.; Ambrosi, A.; et al. Role of Vitamin E and the Orexin System in Neuroprotection. Brain Sci. 2021, 11, 1098. [Google Scholar] [CrossRef]
- Shahidi, F.; de Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H. Selenium and inflammation: Underlying anti-inflammatory mechanisms. Horm. Metab. Res. 2009, 41, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.C.; Spallholz, J.E.; Yun, H.K.; Kim, S.W. Selenium-enriched garlic and cabbage as a dietary selenium source for broilers. J. Med. Food 2008, 11, 687–692. [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Hamishehkar, H.; Shadvar, K.; Ostadi, Z.; Sanaie, S.; Saghaleini, S.H.; Nader, N.D. The effect of intravenous selenium on oxidative stress in critically Ill patients with acute respiratory distress syndrome. Immunol. Investig. 2019, 48, 147–159. [Google Scholar] [CrossRef]
- Stone, C.A.; Kawai, K.; Kupka, R.; Fawzi, W.W. Role of Selenium in HIV infection. Nutr. Rev. 2010, 68, 671–681. [Google Scholar] [CrossRef]
- Dworkin, B.M. Selenium deficiency in HIV infection and the acquired immunodeficiency syndrome (AIDS). Chem. Biol. Interact. 1994, 91, 181–186. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Giovannucci, E.L.; Willett, W.C. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N. Engl. J. Med. 1995, 332, 977–982. [Google Scholar] [CrossRef]
- Kromhout, D.; Bosschieter, E.B.; de Lezenne Coulander, C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N. Engl. J. Med. 1985, 312, 1205–1209. [Google Scholar] [CrossRef]
- Daviglus, M.L.; Stamler, J.; Orencia, A.J.; Dyer, A.R.; Liu, K.; Greenland, P.; Walsh, M.K.; Morris, D.; Shekelle, R.B. Fish consumption and the 30-year risk of fatal myocardial infarction. N. Engl. J. Med. 1997, 336, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the neuroprotective role of astaxanthin: New perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labonté, M.-È.; Couture, P.; Richard, C.; Desroches, S.; Lamarche, B. Impact of dairy products on biomarkers of inflammation: A systematic review of randomized controlled nutritional intervention studies in overweight and obese adults. Am. J. Clin. Nutr. 2013, 97, 706–717. [Google Scholar] [CrossRef] [Green Version]
- Bordoni, A.; Danesi, F.; Dardevet, D.; Dupont, D.; Fernandez, A.S.; Gille, D.; Nunes Dos Santos, C.; Pinto, P.; Re, R.; Rémond, D.; et al. Dairy products and inflammation: A review of the clinical evidence. Crit. Rev. Food Sci. Nutr. 2017, 57, 2497–2525. [Google Scholar] [CrossRef]
- Zelaya, H.; Alvarez, S.; Kitazawa, H.; Villena, J. Respiratory antiviral immunity and immunobiotics: Beneficial effects on inflammation-coagulation interaction during influenza virus infection. Front. Immunol. 2016, 7, 633. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J. Biol. Chem. 2013, 288, 19450–19458. [Google Scholar] [CrossRef] [Green Version]
- Wimalawansa, S.J. Vitamin D deficiency: Effects on oxidative stress, epigenetics, gene regulation, and aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [Green Version]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Huang, X.; Ahn, D.U. Antioxidant, angiotensin-converting enzyme inhibitory activity and other functional properties of egg white proteins and their derived peptides—A review. Poult. Sci. 2018, 97, 1462–1468. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Jo, C.; Suh, J.W.; Ahn, D.U. Enzymatic hydrolysis of ovomucin and the functional structural characteristics of peptides in the hydrolysates. Food Chem. 2016, 192, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, S.; Asamura, H.; Suzuki, K.; Tsuchiya, R. Recent results of postoperative mortality for surgical resections in lung cancer. Ann. Thorac. Surg. 2004, 78, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Lung Cancer (Non-Small Cell); American Cancer Society: Atlanta, GA, USA, 2010; Available online: http://www.cancer.org/Cancer/LungCancer-Non-SmallCell/DetailedGuide/non-small-cell-lungcancer-what-is-non-small-cell-lung-cancer (accessed on 27 May 2020).
- American Cancer Society. Lung Cancer (Small Cell); American Cancer Society: Atlanta, GA, USA, 2010; Available online: http://www.cancer.org/Cancer/LungCancer-SmallCell/DetailedGuide/index (accessed on 27 May 2020).
- Australian Cancer Network. Clinical Practice Guidelines for the Prevention, Diagnosis and Management of Lung Cancer: National Health and Medical Research Council. 2004. Available online: www.nhmrc.gov.au/guidelines (accessed on 27 May 2020).
- Chang, J.Y.; Bradley, J.D.; Govindan, R.; Komaki, R. Lung. In Principles and Practices of Radiation Oncology, 5th ed.; Halperin, E.C., Perez, G.A., Brady, L.W., Eds.; Lippincott Williams & Wilkins K: Philadelphia, PA, USA, 2008; pp. 1076–1108. [Google Scholar]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O., Jr.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 1980, 69, 491–497. [Google Scholar]
- Tewari, N.; Martin-Ucar, A.E.; Black, E.; Beggs, L.; Beggs, F.D.; Duffy, J.P.; Morgan, W.E. Nutritional status affects long term survival after lobectomy for lung cancer. Lung Cancer 2007, 57, 389–394. [Google Scholar] [CrossRef]
- Bozzetti, F.; Arends, J.; Lundholm, K.; Micklewright, A.; Zurcher, G.; Muscaritoli, M. ESPEN guidelines on parenteral nutrition: Non-surgical oncology. Clin. Nutr. 2009, 28, 445–454. [Google Scholar] [CrossRef]
- Isenring, E.; Bauer, J.; Capra, S. The scored Patient-Generated Subjective Global Assessment (PG-SGA) and its association with quality of life in ambulatory patients receiving radiotherapy. Eur. J. Clin. Nutr. 2003, 57, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Nourissat, A.; Vasson, M.P.; Merrouche, Y.; Bouteloup, C.; Goutte, M.; Mille, D.; Jacquin, J.P.; Collard, O.; Michaud, P.; Chauvin, F. Relationship between nutritional status and quality of life in patients with cancer. Eur. J. Cancer 2008, 44, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Ovesen, L.; Hannibal, J.; Mortensen, E.L. The interrelationship of weight loss, dietary intake, and quality of life in ambulatory patients with cancer of the lung, breast, and ovary. Nutr. Cancer 1993, 19, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Jager-Wittenaar, H.; Dijkstra, P.U.; Vissink, A.; van der Laan, B.F.A.M.; van Oort, R.P.; Roodenburg, J.L. Malnutrition and quality of life in patients treated for oral or oropharyngeal cancer. Head Neck 2011, 33, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Norman, K.; Stobaus, N.; Smoliner, C.; Zocher, D.; Scheufele, R.; Valentini, L.; Lochs, H.; Pirlich, M. Determinants of hand grip strength, knee extension strength and functional status in cancer patients. Clin. Nutr. 2010, 29, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Kiss, N.; Hodgson, B.; Crowe, T.C.; Walsh, A.D. Associations between nutritional status, weight loss, radiotherapy treatment toxicity and treatment outcomes in gastrointestinal cancer patients. Clin. Nutr. 2011, 30, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, H.J.N.; Norman, A.R.; Oates, J.; Cunningham, D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur. J. Cancer 1998, 34, 503–509. [Google Scholar] [CrossRef]
- Ross, P.J.; Ashley, S.; Norton, A.; Priest, K.; Waters, J.S.; Eisen, T.; Smith, I.E.; O’Brien, M.E. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br. J. Cancer 2004, 90, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Cranganu, A.; Camporeale, J. Nutrition aspects of lung cancer. Nutr. Clin. Pract. 2009, 24, 688–700. [Google Scholar] [CrossRef]
- Clamon, G.; Gardner, L.; Pee, D.; Stumbo, P.; Feld, R.; Evans, W.; Weiner, R.; Moran, E.; Blum, R.; Hoffman, F.A.; et al. The effect of intravenous hyperalimentation on the dietary intake of patients with small cell lung cancer. A randomized trial. Cancer 1985, 55, 1572–1578. [Google Scholar] [CrossRef]
- Evans, W.K.; Makuch, R.; Clamon, G.H.; Feld, R.; Weiner, R.S.; Moran, E.; Blum, R.; Shepherd, F.A.; Jeejeebhoy, K.N.; DeWys, W.D. Limited impact of total parenteral nutrition on nutritional status during treatment for small cell lung cancer. Cancer Res. 1985, 45, 3347–3353. [Google Scholar]
- Valdivieso, M.; Frankmann, C.; Murphy, W.K.; Benjamin, R.S.; Barkley, H.T.; McMurtrey, M.J.; Jeffries, D.G.; Welch, S.R.; Bodey, G.P. Long-term effects of intravenous hyperalimentation administered during intensive chemotherapy for small cell bronchogenic carcinoma. Cancer 1987, 59, 362–369. [Google Scholar] [CrossRef]
- Kiss, N.; Isenring, E.; Gough, K.; Wheeler, G.; Wirth, A.; Campbell, B.A.; Krishnasamy, M. Early and intensive dietary counselling in lung cancer patients receiving (chemo)radiotherapy—A pilot randomized controlled trial. Supportive Care Cancer 2014, 22 (Suppl. 1), S116. [Google Scholar]
- Uy, K.L.; Darling, G.; Xu, W.; Yi, Q.L.; De Perrot, M.; Pierre, A.F.; Waddell, T.K.; Johnston, M.R.; Bezjak, A.; Shepherd, F.A.; et al. Improvedresults of induction chemoradiation before surgical intervention for selectedpatients with stage IIIA-N2 non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2007, 134, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Berthon, B.S.; Wood, L.G. Nutrition and respiratory health--feature review. Nutrients 2015, 7, 1618–1643. [Google Scholar] [CrossRef] [PubMed]
- Peto, R.; Doll, R.; Buckley, J.D.; Sporn, M.B. Can dietary beta-carotene materially reduce human cancer rates? Nature 1981, 290, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- UNESCO. Representative List of the Intangible Cultural Heritage of Humanity. 2010. Available online: www.unesco.org/culture/ich/index.php?lg=en&pg=00011&RL=00394 (accessed on 20 October 2017).
- Van den Brandt, P.A.; Schulpen, M. Mediterranean diet adherence and risk of postmenopausal breast cancer: Results of a cohort study and meta-analysis. Int. J. Cancer 2017, 140, 2220–2231. [Google Scholar] [CrossRef]
- Gorlova, O.Y.; Weng, S.F.; Hernandez, L.; Spitz, M.R.; Forman, M.R. Dietary patterns affect lung cancer risk in never smokers. Nutr. Cancer 2011, 63, 842–849. [Google Scholar] [CrossRef]
- Smith-Warner, S.A.; Spiegelman, D.; Yaun, S.S.; Albanes, D.; Beeson, W.L.; van den Brandt, P.A.; Feskanich, D.; Folsom, A.R.; Fraser, G.E.; Freudenheim, J.L.; et al. Fruits, vegetables and lung cancer: A pooled analysis of cohortstudies. Int. J. Cancer 2003, 107, 1001–1011. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sobue, T.; Otani, T.; Tsugane, S. Vegetables, fruit consumption and risk oflung cancer among middle-aged Japanese men and women: JPHC study. Cancer Causes Control 2004, 15, 349–357. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- Holick, C.N.; Michaud, D.S.; Stolzenberg-Solomon, R.; Mayne, S.T.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. Am. J. Epidemiol. 2002, 156, 536–547. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Abdala-Valencia, H.; Hartert, T. Two faces of vitamin E in the lung. Am. J. Respir. Crit. Care Med. 2013, 188, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014, 6, 221ra215. [Google Scholar] [CrossRef] [PubMed]
- Keck, A.S.; Finley, J.W. Cruciferous vegetables: Cancer protective mechanisms of glucosinolate hydrolysis products and selenium. Integr. Cancer Ther. 2004, 3, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.; Hsu, C.C.; Moullan, N.; Szeszenia-Dabrowska, N.; Lissowska, J.; Zaridze, D.; Rudnai, P.; Fabianova, E.; Mates, D.; Bencko, V. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: A mendelian randomisation approach. Lancet 2005, 366, 1558–1560. [Google Scholar] [CrossRef]
- Moyad, M.A. Results and lessons from clinical trials using dietary supplements for cancer: Direct and indirect investigations. Semin. Urol. Oncol. 2001, 19, 232–246. [Google Scholar] [PubMed]
- Gonzalez, M.J.; Miranda-Massari, J.R.; Mora, E.M.; Guzmán, A.; Riordan, N.H.; Riordan, H.D.; Casciari, J.J.; Jackson, J.A.; Román-Franco, A. Orthomolecular oncology review: Ascorbic acid and cancer 25 years later. Integr. Cancer Ther. 2005, 4, 32–44. [Google Scholar] [CrossRef]
- Hochstein, P.; Atallah, A.S. The nature of oxidants and antioxidant systems in the inhibition of mutation and cancer. Mutat. Res. 1988, 202, 363–375. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chang, M.Y.; Park, C.H.; Kim, H.Y.; Kim, J.H.; Son, H.; Lee, Y.S.; Lee, S.H. Ascorbate-induced differentiation of embryonic cortical precursors into neurons and astrocytes. J. Neurosci. Res. 2003, 73, 156–165. [Google Scholar] [CrossRef]
- Kang, J.H.; Shi, Y.M.; Zheng, R.L. Effects of ascorbic acid on human hepatoma cell proliferation and redifferentiation. Zhongguo Yao Li Xue Bao 1999, 20, 1019–1024. [Google Scholar]
- Riordan, H.D.; Hunninghake, R.B.; Riordan, N.H.; Jackson, J.J.; Meng, X.; Taylor, P.; Casciari, J.J.; González, M.J.; Miranda-Massari, J.R.; Mora, E.M.; et al. Intravenous ascorbic acid: Protocol for its application and use. Health Sci. J. 2003, 22, 287–290. [Google Scholar]
- Luo, G.; Xie, Z.Z.; Liu, F.Y.; Zhang, G.B. Effects of vitamin C on myocardial mitochondrial function and ATP content in hypoxic rats. Zhongguo Yao Li Xue Bao 1998, 19, 351–355. [Google Scholar] [PubMed]
- Camarena, V.; Wang, G. The epigenetic role of vitamin C in health and disease. Cell. Mol. Life Sci. 2016, 73, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, J.; Karlsson, O.; Wang, G.; Li, J.; Guo, Y.; Lin, X.; Zemplenyi, M.; Sanchez-Guerra, M.; Trevisi, L.; Urch, B.; et al. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc. Natl. Acad. Sci. USA 2017, 114, 3503–3508. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Perkins, J.T.; Hennig, B. EGCG prevents PCB 126-induced endothelial cell inflammation via epigenetic modifications of NF-_B target genes in human endothelial cells. J. Nutr. Biochem. 2016, 28, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, E.; Kanwal, R.; Candamo, M.; Gupta, S. Dietary phytochemicals as epigenetic modifiers in cancer: Promise and challenges. Semin. Cancer Biol. 2016, 40–41, 82–99. [Google Scholar] [CrossRef] [Green Version]
- Zhai, T.; Li, S.; Hu, W.; Li, D.; Leng, S. Potential Micronutrients and Phytochemicals against the Pathogenesis of Chronic Obstructive Pulmonary Disease and Lung Cancer. Nutrients 2018, 10, 813. [Google Scholar] [CrossRef] [Green Version]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Leng, S.; Picchi, M.A.; Tesfaigzi, Y.; Wu, G.; Gauderman, W.J.; Xu, F.; Gilliland, F.D.; Belinsky, S.A. Dietary nutrients associated with preservation of lung function in Hispanic and non-Hispanic white smokers from New Mexico. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 3171–3181. [Google Scholar] [CrossRef] [Green Version]
- Leng, S.; Picchi, M.A.; Kang, H.; Wu, G.; Filipczak, P.T.; Juri, D.E.; Zhang, X.; Gauderman, W.J.; Gilliland, F.D.; Belinsky, S.A. Dietary nutrient intake, ethnicity, and epigenetic silencing of lung cancer genes detected in sputum in New Mexican smokers. Cancer Prev. Res. 2018, 11, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Leung, A.; Trac, C.; Du, J.; Natarajan, R.; Schones, D.E. Persistent Chromatin Modifications Induced by High Fat Diet. J. Biol. Chem. 2016, 291, 10446–10455. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xu, G.B.; Zhou, D.; Pan, Y.-X. High-fat diet modifies expression of hepatic cellular senescence gene p16(INK4a) through chromatin modifications in adult male rats. Genes Nutr. 2018, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, P.V.; Ververis, K.; Karagiannis, T.C. Histone deacetylase inhibition and dietary short-chain Fatty acids. ISRN Allergy 2011, 2011, 869647. [Google Scholar] [CrossRef] [Green Version]
- Andersen, V.; Halekoh, U.; Tjønneland, A.; Vogel, U.; Kopp, T.I. Intake of Red and Processed Meat, Use of Non-Steroid Anti-Inflammatory Drugs, Genetic Variants and Risk of Colorectal Cancer: A Prospective Study of the Danish “Diet, Cancer and Health” Cohort. Int. J. Mol. Sci. 2019, 20, 1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skog, K. Procedures and food mutagens: A literature review. Food Chem. Toxicol. 1993, 31, 655–675. [Google Scholar] [CrossRef]
- Cross, A.J.; Pollock, J.R.; Bingham, S.A. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003, 63, 2358–2360. [Google Scholar]
- Roth, M.J.; Wei, W.Q.; Baer, J.; Abnet, C.C.; Wang, G.Q.; Sternberg, L.R.; Warner, A.C.; Johnson, L.L.; Lu, N.; Giffen, C.A. Aryl hydrocarbon receptor expression is associated with a family history of upper gastrointestinal tract cancer in a high-risk population exposed to aromatic hydrocarbons. Cancer Epidem. Biomar. 2009, 18, 2391–2396. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.; Kulldorff, M.; Curtin, J.; Brown, C.C.; Alavanja, M.C.R.; Swanson, C.A. Fried, well-done red meat and risk of lung cancer in women (United States). Cancer Causes Control 1998, 9, 621–630. [Google Scholar] [CrossRef]
- Tudek, B.; Swoboda, M.; Kowalczyk, P.; Olinski, R. Modulation of oxidative DNA damage repair by the diet, inflammation and neoplastic transformation. J. Physiol. Pharmacol. 2006, 57, 33. [Google Scholar]
- Brennan, P.; Fortes, C.; Butler, J.; Agudo, A.; Benhamou, S.; Darby, S.; Gerken, M.; Jökel, K.H.; Kreuzer, M.; Mallone, S.; et al. A multicenter case–control study of diet and lung cancer among non-smokers. Cancer Causes Control 2000, 11, 49–58. [Google Scholar] [CrossRef]
- Lampe, J.W. Dairy products and cancer. J. Am. Coll. Nutr. 2011, 30, 464S–470S. [Google Scholar] [CrossRef]
- Allen, B.G.; Bhatia, S.K.; Buatti, J.M.; Brandt, K.E.; Lindholm, K.E.; Button, A.M.; Szweda, L.I.; Smith, B.J.; Spitz, D.R.; Fath, M.A. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin. Cancer Res. 2013, 19, 3905–3913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aykin-Burns, N.; Ahmad, I.M.; Zhu, Y.; Oberley, L.W.; Spitz, D.R. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem. J. 2009, 418, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhong, Q. Histone deacetylase inhibitors and cell death. Cell Mol. Life Sci. 2014, 71, 3885–3901. [Google Scholar] [CrossRef] [PubMed]
- Rikiishi, H. Autophagic and apoptotic effects of HDAC inhibitors on cancer cells. J. Biomed. Biotechnol. 2011, 2011, 830260. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porro, C.; La Torre, M.E.; Tartaglia, N.; Benameur, T.; Santini, M.; Ambrosi, A.; Messina, G.; Cibelli, G.; Fiorelli, A.; Polito, R.; et al. The Potential Role of Nutrition in Lung Cancer Establishment and Progression. Life 2022, 12, 270. https://doi.org/10.3390/life12020270
Porro C, La Torre ME, Tartaglia N, Benameur T, Santini M, Ambrosi A, Messina G, Cibelli G, Fiorelli A, Polito R, et al. The Potential Role of Nutrition in Lung Cancer Establishment and Progression. Life. 2022; 12(2):270. https://doi.org/10.3390/life12020270
Chicago/Turabian StylePorro, Chiara, Maria Ester La Torre, Nicola Tartaglia, Tarek Benameur, Mario Santini, Antonio Ambrosi, Giovanni Messina, Giuseppe Cibelli, Alfonso Fiorelli, Rita Polito, and et al. 2022. "The Potential Role of Nutrition in Lung Cancer Establishment and Progression" Life 12, no. 2: 270. https://doi.org/10.3390/life12020270
APA StylePorro, C., La Torre, M. E., Tartaglia, N., Benameur, T., Santini, M., Ambrosi, A., Messina, G., Cibelli, G., Fiorelli, A., Polito, R., & Messina, G. (2022). The Potential Role of Nutrition in Lung Cancer Establishment and Progression. Life, 12(2), 270. https://doi.org/10.3390/life12020270










