The Multiple Roles of Glucose-6-Phosphate Dehydrogenase in Tumorigenesis and Cancer Chemoresistance
Abstract
1. Introduction
2. Historical Perspective of G6PD
3. Characteristics of G6PD
4. G6PD and Cancer
5. The Regulation of G6PD in Cancer
5.1. Impacts on Cancer at Transcriptional Level
5.2. Impacts on Cancer at Post-Transcriptional and Translational Level
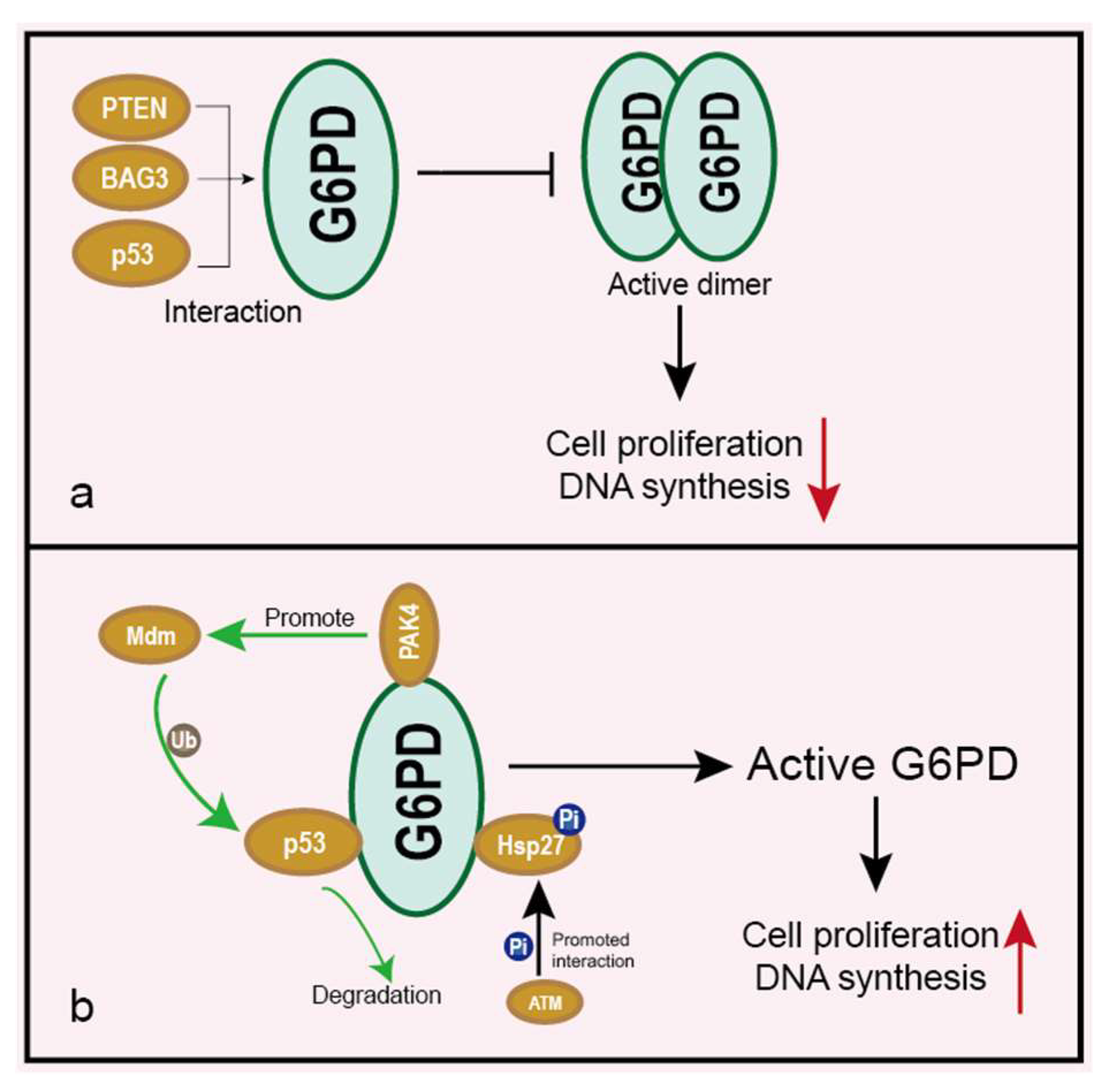
5.3. Impacts on Cancer at Post-Translational Modification Level
5.4. Impacts on Cancer under Interaction with Other Proteins
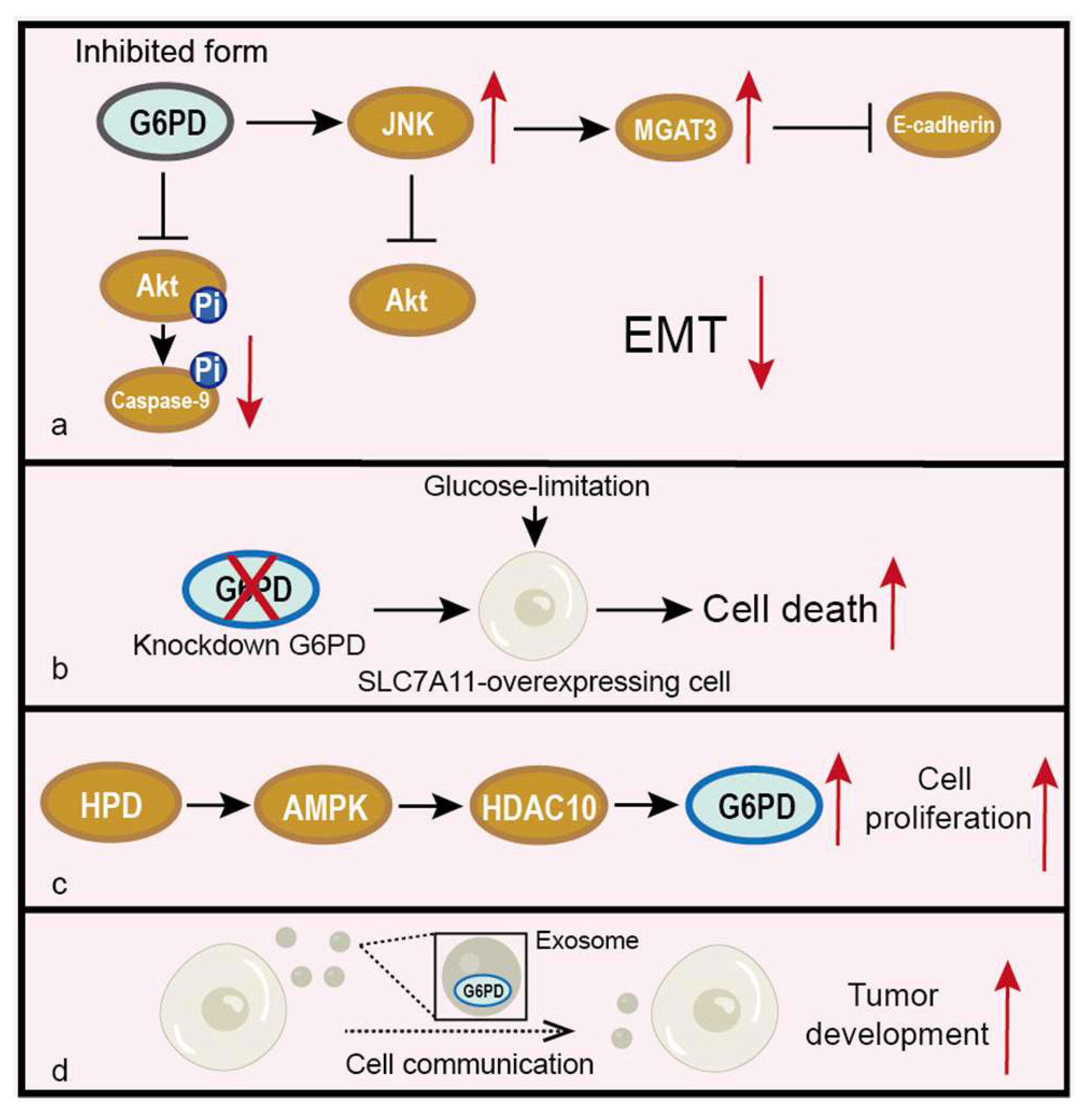
5.5. Other Pathways Related to G6PD in Cancer
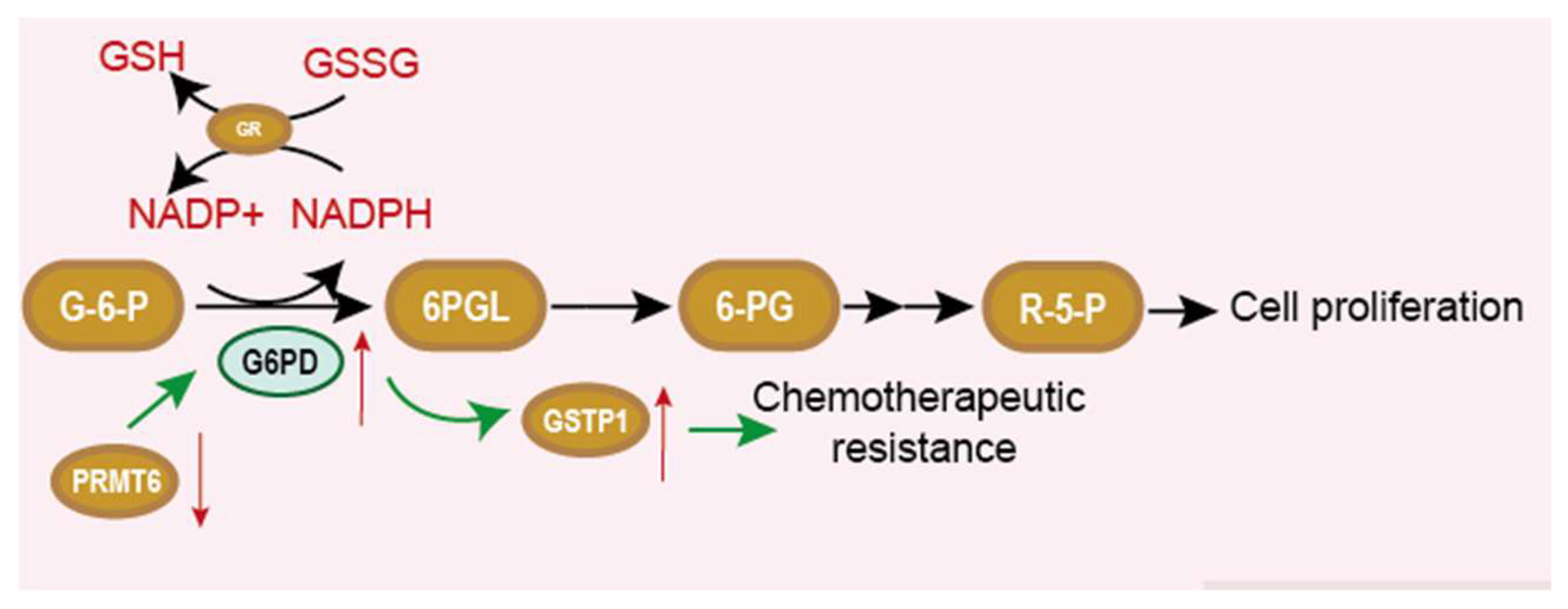
6. G6PD and Chemotherapy Resistance
7. Inhibitor of G6PD
7.1. 6-AN
7.2. DHEA
7.3. Polydatin
7.4. Zoledronic Acid
7.5. Aspirin
7.6. RRX-001
7.7. Phytol
7.8. Wedelolactone
7.9. Butyrate
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warburg, O.; Christian, W.; Griese, A. Wasserstoffübertragendes Co-Ferment, seine Zusammensetzung und Wirkungsweise. Biochem. Z. 1935, 282, 157–205. [Google Scholar]
- Horecker, B.L. The pentose phosphate pathway. J. Biol. Chem. 2002, 277, 47965–47971. [Google Scholar] [CrossRef]
- Alving, A.S.; Carson, P.E.; Flanagan, C.L.; Ickes, C.E. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science 1956, 124, 484–485. [Google Scholar] [PubMed]
- Cappellini, M.D.; Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008, 371, 64–74. [Google Scholar] [CrossRef]
- Beaconsfield, P.; Rainsbury, R.; Kalton, G. Glucose-6-Phosphate Dehydrogenase Deficiency and the Incidence of Cancer. Oncology 1965, 19, 11–19. [Google Scholar] [CrossRef]
- Persico, M.G.; Viglietto, G.; Martini, G.; Toniolo, D.; Paonessa, G.; Moscatelli, C.; Dono, R.; Vulliamy, T.; Luzzatto, L.; D’urso, M. Isolation of human glucose-6-phosphate dehydrogenase (G6PD) cDNA clones: Primary structure of the protein and unusual 5’ non-coding region. Nucleic Acids Res. 1986, 14, 2511–2522. [Google Scholar] [CrossRef]
- Rowland, P.; Basak, A.K.; Gover, S.; Levy, H.R.; Adams, M.J. The three-dimensional structure of glucose 6-phosphate dehydrogenase from Leuconostoc mesenteroides refined at 2.0 A resolution. Structure 1994, 2, 1073–1087. [Google Scholar] [CrossRef]
- Bandell, M.; Lhotte, M.E.; Marty-Teysset, C.; Veyrat, A.; Prévost, H.; Dartois, V.; Divies, C.; Konings, W.N.; Lolkema, J.S. Mechanism of the citrate transporters in carbohydrate and citrate cometabolism in Lactococcus and Leuconostoc species. Appl. Environ. Microbiol. 1998, 64, 1594–1600. [Google Scholar] [CrossRef]
- Marty-Teysset, C.; Lolkema, J.S.; Schmitt, P.; Diviès, C.; Konings, W.N. The citrate metabolic pathway in Leuconostoc mesenteroides: Expression, amino acid synthesis, and alpha-ketocarboxylate transport. J. Bacteriol. 1996, 178, 6209–6215. [Google Scholar] [CrossRef]
- Haghighi, B.; Levy, H.R. Glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides. Conformational transitions induced by nicotinamide adenine dinucleotide, nicotinamide adenine dinucleotide phosphate, and glucose 6-phosphate monitored by fluorescent probes. Biochemistry 1982, 21, 6421–6428. [Google Scholar] [CrossRef]
- Olive, C.; Geroch, M.E.; Levy, H.R. Glucose 6-phosphate dehydrogenase from Leuconostoc mesenteroides. Kinetic studies. J. Biol. Chem. 1971, 246, 2047–2057. [Google Scholar] [CrossRef]
- Bondar, R.J.; Mead, D.C. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin. Chem. 1974, 20, 586–590. [Google Scholar] [CrossRef]
- Ravera, S.; Calzia, D.; Morelli, A.; Panfoli, I. Oligomerization studies of Leuconostoc mesenteroides G6PD activity after SDS-PAGE and blotting. Mol. Biol. 2010, 44, 472–476. [Google Scholar] [CrossRef]
- Au, S.W.; Gover, S.; Lam, V.M.; Adams, M.J. Human glucose-6-phosphate dehydrogenase: The crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency. Structure 2000, 8, 293–303. [Google Scholar] [CrossRef]
- Spencer, N.Y.; Stanton, R.C. Glucose 6-phosphate dehydrogenase and the kidney. Curr. Opin. Nephrol. Hypertens. 2017, 26, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Bagirova, M.; Elcicek, S.; Koc, R.C.; Ates, S.C.; Baydar, S.Y.; Yaman, S.; Abamor, E.S.; Oztel, O.N. Glucose-6-Phosphate Dehydrogenase Deficiency and Malaria: A Method to Detect Primaquine-Induced Hemolysis in vitro. In Dehydrogenases; IntechOpen: London, UK, 2012. [Google Scholar]
- Kotaka, M.; Gover, S.; Vandeputte-Rutten, L.; Au, S.W.; Lam, V.M.; Adams, M.J. Structural studies of glucose-6-phosphate and NADP+ binding to human glucose-6-phosphate dehydrogenase. Acta Crystallogr. D Biol. Crystallogr. 2005, 61 Pt 5, 495–504. [Google Scholar] [CrossRef]
- Wang, X.T.; Chan, T.F.; Lam, V.M.; Engel, P.C. What is the role of the second “structural” NADP+-binding site in human glucose 6-phosphate dehydrogenase? Protein Sci. 2008, 17, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Hartman, K.; Gelbart, T.; Forman, L. G-6-PD Walter Reed: Possible insight into “structural” NADP in G-6-PD. Am. J. Hematol. 1986, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- American Association of Neurological Surgeons (AANS); American Society of Neuroradiology (ASNR); Cardiovascular and Interventional Radiology Society of Europe (CIRSE); Canadian Interventional Radiology Association (CIRA); Congress of Neurological Surgeons (CNS); European Society of Minimally Invasive Neurological Therapy (ESMINT); European Society of Neuroradiology (ESNR); European Stroke Organization (ESO); Society for Cardiovascular Angiography and Interventions (SCAI); Society of Interventional Radiology (SIR); et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar]
- National Organization for Rare Disorders. Available online: https://rarediseases.org/rare-diseases/glucose-6-phosphate-dehydrogenase-deficiency (accessed on 24 July 2019).
- Available online: https://emedicine.medscape.com/article/200390-treatment#d10 (accessed on 25 July 2020).
- Italian G6PD Deficiency Association. Drugs That Should Be Avoided: Offi Cial List. Available online: www.g6pd.org/en/G6PDDeficiency/SafeUnsafe/DaEvitare_ISS-it (accessed on 24 July 2020).
- Cohen, P.; Rosemeyer, M.A. Subunit interactions of glucose-6-phosphate dehydrogenase from human erythrocytes. Eur. J. Biochem. 1969, 8, 8–15. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, W.; Chen, Z.; Wu, S.; Chen, J.; Ge, J.; Hou, F.; Chen, Z. Overexpression of G6PD is associated with poor clinical outcome in gastric cancer. Tumour Biol. 2012, 33, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Gao, C.; Feng, X.; Jiang, M. miR-613 suppresses migration and invasion in esophageal squamous cell carcinoma via the targeting of G6PD. Exp. Ther. Med. 2020, 19, 3081–3089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Han, Q.; Yang, Z.; Ni, Y.; Agbana, Y.L.; Bai, H.; Yi, Z.; Yi, X.; Kuang, Y.; Zhu, Y. G6PD facilitates clear cell renal cell carcinoma invasion by enhancing MMP2 expression through ROS-MAPK axis pathway. Int. J. Oncol. 2020, 57, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Lu, Z.; Li, Z.; Sheng, H.; Fan, J.; Qi, Q.; Liu, S.; Zhang, S. 4-hydroxyphenylpyruvate dioxygenase promotes lung cancer growth via pentose phosphate pathway (PPP) flux mediated by LKB1-AMPK/HDAC10/G6PD axis. Cell Death Dis. 2019, 10, 525. [Google Scholar] [CrossRef]
- Du, W.; Jiang, P.; Mancuso, A.; Stonestrom, A.; Brewer, M.D.; Minn, A.J.; Mak, T.W.; Wu, M.; Yang, X. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat. Cell Biol. 2013, 15, 991–1000. [Google Scholar] [CrossRef]
- Ye, H.; Huang, H.; Cao, F.; Chen, M.; Zheng, X.; Zhan, R. HSPB1 Enhances SIRT2-Mediated G6PD Activation and Promotes Glioma Cell Proliferation. PLoS ONE 2016, 11, e0164285. [Google Scholar] [CrossRef]
- Lu, M.; Lu, L.; Dong, Q.; Yu, G.; Chen, J.; Qin, L.; Wang, L.; Zhu, W.; Jia, H. Elevated G6PD expression contributes to migration and invasion of hepatocellular carcinoma cells by inducing epithelial-mesenchymal transition. Acta Biochim. Biophys. Sin. 2018, 50, 70–380. [Google Scholar] [CrossRef]
- Nagashio, R.; Oikawa, S.; Yanagita, K.; Hagiuda, D.; Kuchitsu, Y.; Igawa, S.; Naoki, K.; Satoh, Y.; Ichinoe, M.; Murakumo, Y.; et al. Prognostic significance of G6PD expression and localization in lung adenocarcinoma. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 38–46. [Google Scholar] [CrossRef]
- Yang, C.A.; Huang, H.Y.; Lin, C.L.; Chang, J.G. G6PD as a predictive marker for glioma risk, prognosis and chemosensitivity. J. Neurooncol. 2018, 139, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Q.; Lu, Y.X.; Wu, Q.N.; Liu, J.; Zeng, Z.L.; Mo, H.Y.; Chen, Y.; Tian, T.; Wang, Y.; Kang, T.B.; et al. Disrupting G6PD-mediated Redox homeostasis enhances chemosensitivity in colorectal cancer. Oncogene 2017, 36, 6282–6292. [Google Scholar] [CrossRef]
- Poulain, L.; Sujobert, P.; Zylbersztejn, F.; Barreau, S.; Stuani, L.; Lambert, M.; Palama, T.L.; Chesnais, V.; Birsen, R.; Vergez, F.; et al. High mTORC1 activity drives glycolysis addiction and sensitivity to G6PD inhibition in acute myeloid leukemia cells. Leukemia 2017, 31, 2326–2335. [Google Scholar] [CrossRef] [PubMed]
- Mele, L.; la Noce, M.; Paino, F.; Regad, T.; Wagner, S.; Liccardo, D.; Papaccio, G.; Lombardi, A.; Caraglia, M.; Tirino, V.; et al. Glucose-6-phosphate dehydrogenase blockade potentiates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation. J. Exp. Clin. Cancer Res. 2019, 38, 160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Zhang, X.; Fan, R.; Gu, H.; Shi, Y.; Liu, H. Glucose-6-phosphate dehydrogenase expression is correlated with poor clinical prognosis in esophageal squamous cell carcinoma. Eur. J. Surg. Oncol. 2015, 41, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Li, S.; Du, Z.X.; Liu, C.; Liu, B.Q.; Li, C.; Zong, Z.H.; Wang, H.Q. BAG3 elevation inhibits cell proliferation via direct interaction with G6PD in hepatocellular carcinomas. Oncotarget 2016, 7, 700–711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, D.; Zhu, Y.; Tang, Q.; Lu, H.; Li, H.; Yang, Y.; Li, Z.; Tong, S. A new G6PD knockdown tumor-cell line with reduced proliferation and increased susceptibility to oxidative stress. Cancer Biother. Radiopharm. 2009, 24, 81–90. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Yang, J.; Ding, J.; Li, S.; Wu, H.; Xiong, Y.; Zhou, F.; Jiang, Y.; Teng, L.; Yang, J. Downregulation of glucose-6-phosphate dehydrogenase by microRNA-1 inhibits the growth of pituitary tumor cells. Oncol. Rep. 2018, 40, 3533–3542. [Google Scholar] [CrossRef]
- Bao, B.Y.; Ting, H.J.; Hsu, J.W.; Lee, Y.F. Protective role of 1 alpha, 25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. Int. J. Cancer 2008, 122, 2699–2706. [Google Scholar] [CrossRef]
- Wu, S.; Wang, H.; Li, Y.; Xie, Y.; Huang, C.; Zhao, H.; Miyagishi, M.; Kasim, V. Transcription Factor YY1 Promotes Cell Proliferation by Directly Activating the Pentose Phosphate Pathway. Cancer Res. 2018, 78, 4549–4562. [Google Scholar] [CrossRef]
- Yin, X.; Tang, B.; Li, J.H.; Wang, Y.; Zhang, L.; Xie, X.Y.; Zhang, B.H.; Qiu, S.J.; Wu, W.Z.; Ren, Z.G. ID1 promotes hepatocellular carcinoma proliferation and confers chemoresistance to oxaliplatin by activating pentose phosphate pathway. J. Exp. Clin. Cancer Res. 2017, 36, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, Z.; Han, Q.; Bai, H.; Wang, Y.; Yi, X.; Yi, Z.; Yang, L.; Jiang, L.; Song, X.; et al. G6PD promotes renal cell carcinoma proliferation through positive feedback regulation of p-STAT3. Oncotarget 2017, 8, 109043–109060. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sheng, H.; Wu, T.; Song, J.; Sun, H.; Wang, Y.; Wang, J.; Li, Z.; Zhao, H.; Tan, J.; et al. PIKE-A promotes glioblastoma growth by driving PPP flux through increasing G6PD expression mediated by phosphorylation of STAT3. Biochem. Pharmacol. 2021, 192, 114736. [Google Scholar] [CrossRef] [PubMed]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Amemiya-Kudo, M.; Shimano, H.; Hasty, A.H.; Yahagi, N.; Yoshikawa, T.; Matsuzaka, T.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K.; et al. Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J. Lipid Res. 2002, 43, 1220–1235. [Google Scholar] [CrossRef]
- Jiang, P.; Du, W.; Yang, X. A critical role of glucose-6-phosphate dehydrogenase in TAp73-mediated cell proliferation. Cell Cycle 2013, 12, 3720–3726. [Google Scholar] [CrossRef]
- Wang, E.; Aifantis, I. RNA Splicing and Cancer. Trends Cancer 2020, 6, 631–644. [Google Scholar] [CrossRef]
- Hong, X.; Song, R.; Song, H.; Zheng, T.; Wang, J.; Liang, Y.; Qi, S.; Lu, Z.; Song, X.; Jiang, H.; et al. PTEN antagonises Tcl1/hnRNPK-mediated G6PD pre-mRNA splicing which contributes to hepatocarcinogenesis. Gut 2014, 63, 1635–1647. [Google Scholar] [CrossRef]
- Hu, T.; Chang, Y.F.; Xiao, Z.; Mao, R.; Tong, J.; Chen, B.; Liu, G.C.; Hong, Y.; Chen, H.L.; Kong, S.Y.; et al. miR-1 inhibits progression of high-risk papillomavirus-associated human cervical cancer by targeting G6PD. Oncotarget 2016, 7, 86103–86116. [Google Scholar] [CrossRef]
- Barajas, J.M.; Reyes, R.; Guerrero, M.J.; Jacob, S.T.; Motiwala, T.; Ghoshal, K. The role of miR-122 in the dysregulation of glucose-6-phosphate dehydrogenase (G6PD) expression in hepatocellular cancer. Sci. Rep. 2018, 8, 9105. [Google Scholar] [CrossRef]
- Cui, J.; Pan, Y.; Wang, J.; Liu, Y.; Wang, H.; Li, H. MicroRNA-206 suppresses proliferation and predicts poor prognosis of HR-HPV-positive cervical cancer cells by targeting G6PD. Oncol. Lett. 2018, 16, 5946–5952. [Google Scholar] [CrossRef] [PubMed]
- Coda, D.M.; Lingua, M.F.; Morena, D.; Foglizzo, V.; Bersani, F.; Ala, U.; Ponzetto, C.; Taulli, R. SMYD1 and G6PD modulation are critical events for miR-206-mediated differentiation of rhabdomyosarcoma. Cell Cycle 2015, 14, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Ai, G.; Dachineni, R.; Kumar, D.R.; Alfonso, L.F.; Marimuthu, S.; Bhat, G.J. Aspirin inhibits glucose-6-phosphate dehydrogenase activity in HCT 116 cells through acetylation: Identification of aspirin-acetylated sites. Mol. Med. Rep. 2016, 14, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.N.; Wang, T.S.; Li, X.; Wang, Y.P. SIRT2 activates G6PD to enhance NADPH production and promote leukaemia cell proliferation. Sci. Rep. 2016, 6, 32734. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Zhou, L.S.; Zhao, Y.Z.; Wang, S.W.; Chen, L.L.; Liu, L.X.; Ling, Z.Q.; Hu, F.J.; Sun, Y.P.; Zhang, J.Y.; et al. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J. 2014, 33, 1304–1320. [Google Scholar] [CrossRef]
- Rao, X.; Duan, X.; Mao, W.; Li, X.; Li, Z.; Li, Q.; Zheng, Z.; Xu, H.; Chen, M.; Wang, P.G.; et al. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat. Commun. 2015, 6, 8468. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, L.; Huang, D.; Li, Y.; Yang, D.; Li, T.; Li, F.; Sun, L.; Wei, H.; He, K.; et al. Polo-like kinase 1 coordinates biosynthesis during cell cycle progression by directly activating pentose phosphate pathway. Nat. Commun. 2017, 8, 1506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Du, W.; Wang, X.; Mancuso, A.; Gao, X.; Wu, M.; Yang, X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 2011, 13, 310–316. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, Y.; Shao, Y.; Xiao, J.; Zhu, G.; Li, F. PAK4 regulates G6PD activity by p53 degradation involving colon cancer cell growth. Cell Death Dis. 2017, 8, e2820. [Google Scholar] [CrossRef]
- Cosentino, C.; Grieco, D.; Costanzo, V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011, 30, 546–555. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Niu, L.; Xu, L.; Guo, Y.; Wang, L.; Guo, C. Suppression of G6PD induces the expression and bisecting GlcNAc-branched N-glycosylation of E-Cadherin to block epithelial-mesenchymal transition and lymphatic metastasis. Br. J. Cancer 2020, 123, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Z.; Zhu, Z.; Chen, A.; Fu, G.; Wang, Y.; Pan, H.; Jin, B. Modulation of G6PD affects bladder cancer via ROS accumulation and the AKT pathway in vitro. Int. J. Oncol. 2018, 53, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Olszewski, K.; Zhang, Y.; Lim, E.W.; Shi, J.; Zhang, X.; Zhang, J.; Lee, H.; Koppula, P.; Lei, G.; et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat. Cell Biol. 2020, 22, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zheng, X.; Song, J.; Shen, R.; Su, Y.; Lin, D. Exosomes mediated pentose phosphate pathway in ovarian cancer metastasis: A proteomics analysis. Int. J. Clin. Exp. Pathol. 2015, 8, 15719–15728. [Google Scholar] [PubMed]
- Polimeni, M.; Voena, C.; Kopecka, J.; Riganti, C.; Pescarmona, G.; Bosia, A.; Ghigo, D. Modulation of doxorubicin resistance by the glucose-6-phosphate dehydrogenase activity. Biochem. J. 2011, 439, 141–149. [Google Scholar] [CrossRef]
- Yamawaki, K.; Mori, Y.; Sakai, H.; Kanda, Y.; Shiokawa, D.; Ueda, H.; Ishiguro, T.; Yoshihara, K.; Nagasaka, K.; Onda, T.; et al. Integrative analyses of gene expression and chemosensitivity of patient-derived ovarian cancer spheroids link G6PD-driven redox metabolism to cisplatin chemoresistance. Cancer Lett. 2021, 521, 29–38. [Google Scholar] [CrossRef]
- Feng, Q.; Li, X.; Sun, W.; Sun, M.; Li, Z.; Sheng, H.; Xie, F.; Zhang, S.; Shan, C. Targeting G6PD reverses paclitaxel resistance in ovarian cancer by suppressing GSTP1. Biochem. Pharmacol. 2020, 178, 114092. [Google Scholar] [CrossRef]
- Arbe, M.F.; Agnetti, L.; Breininger, E.; Glikin, G.C.; Finocchiaro, L.M.E.; Villaverde, M.S. Glucose 6-phosphate dehydrogenase inhibition sensitizes melanoma cells to metformin treatment. Transl. Oncol. 2020, 13, 100842. [Google Scholar] [CrossRef]
- Varshney, R.; Dwarakanath, B.; Jain, V. Radiosensitization by 6-aminonicotinamide and 2-deoxy-D-glucose in human cancer cells. Int. J. Radiat. Biol. 2005, 81, 397–408. [Google Scholar] [CrossRef]
- Schwartz, A.G.; Pashko, L.L. Dehydroepiandrosterone, glucose-6-phosphate dehydrogenase, and longevity. Ageing Res. Rev. 2004, 3, 171–187. [Google Scholar] [CrossRef]
- Fang, Z.; Jiang, C.; Feng, Y.; Chen, R.; Lin, X.; Zhang, Z.; Han, L.; Chen, X.; Li, H.; Guo, Y.; et al. Effects of G6PD activity inhibition on the viability, ROS generation and mechanical properties of cervical cancer cells. Biochim. Biophys. Acta 2016, 1863, 2245–2254. [Google Scholar] [CrossRef]
- Mele, L.; Paino, F.; Papaccio, F.; Regad, T.; Boocock, D.; Stiuso, P.; Lombardi, A.; Liccardo, D.; Aquino, G.; Barbieri, A.; et al. A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death Dis. 2018, 9, 572. [Google Scholar] [CrossRef]
- Chen, P.; Wang, L.; Sun, S.; Zhou, Q.; Zeng, Z.; Hu, M.; Hussain, M.; Lu, C.; Du, H. High-throughput screening suggests glutathione synthetase as an anti-tumor target of polydatin using human proteome chip. Int. J. Biol. Macromol. 2020, 161, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, B.; Li, Y.; Yin, F. Polydatin inhibits cell proliferation and induces apoptosis in laryngeal cancer and HeLa cells via suppression of the PDGF/AKT signaling pathway. J. Biochem. Mol. Toxicol. 2017, 31, e21900. [Google Scholar] [CrossRef] [PubMed]
- Finianos, A.; Aragon-Ching, J.B. Zoledronic acid for the treatment of prostate cancer. Expert Opin. Pharmacother. 2019, 20, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Chen, F.K.; Liu, C.; Liu, P.J.; Feng, Z.P.; Jia, L.; Yang, Z.X.; Hou, F.; Deng, Z.Y. Zoledronic acid inhibits thyroid cancer stemness and metastasis by repressing M2-like tumor-associated macrophages induced Wnt/β-catenin pathway. Life Sci. 2020, 256, 117925. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Cao, G.; Yang, L.; Xu, H.; Huang, J.; Hou, J. Zoledronic acid inhibits the pentose phosphate pathway through attenuating the Ras-TAp73-G6PD axis in bladder cancer cells. Mol. Med. Rep. 2015, 12, 4620–4625. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y. Complex roles of the old drug aspirin in cancer chemoprevention and therapy. Med. Res. Rev. 2019, 39, 114–145. [Google Scholar] [CrossRef]
- Ai, G.; Hagen, F.K.; Gunaje, J.B. Aspirin acetylates glucose 6 phosphate dehydrogenase and inhibits its activity in colon cancer cells. Cancer Res. 2013, 73 (Suppl. 8), 3681. [Google Scholar]
- Oronsky, B.; Reid, T.R.; Oronsky, A.; Caroen, S.; Carter, C.A.; Cabrales, P. Brief report: RRx-001 is a c-Myc inhibitor that targets cancer stem cells. Oncotarget 2018, 9, 23439–23442. [Google Scholar] [CrossRef]
- Alencar, M.; Islam, M.T.; Ali, E.S.; Santos, J.; Paz, M.; Sousa, J.; Dantas, S.; Mishra, S.K.; Cavalcante, A. Association of Phytol with Toxic and Cytotoxic Activities in an Antitumoral Perspective: A Meta-Analysis and Systemic Review. Anticancer Agents Med. Chem. 2018, 18, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- de Alencar, M.; Islam, M.T.; de Lima, R.; Paz, M.; Dos Reis, A.C.; da Mata, A.; Filho, J.; Cerqueira, G.S.; Ferreira, P.; E Sousa, J.; et al. Phytol as an anticarcinogenic and antitumoral agent: An in vivo study in swiss mice with DMBA-Induced breast cancer. IUBMB Life 2019, 71, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Nehybová, T.; Šmarda, J.; Daniel, L.; Stiborek, M.; Kanický, V.; Spasojevič, I.; Preisler, J.; Damborský, J.; Beneš, P. Wedelolactone Acts as Proteasome Inhibitor in Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18, 729. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Du, D.; Liu, Y.; Lu, T.; Liu, L.; Jiang, H.; Chen, K.; Shan, C.; Luo, C. Discovery and characterization of a novel glucose-6-phosphate dehydrogenase (G6PD) inhibitor via high-throughput screening. Bioorg. Med. Chem. Lett. 2021, 40, 127905. [Google Scholar] [CrossRef]
- Torun, A.; Enayat, S.; Sheraj, I.; Tunçer, S.; Ülgen, D.H.; Banerjee, S. Butyrate mediated regulation of RNA binding proteins in the post-transcriptional regulation of inflammatory gene expression. Cell Signal. 2019, 64, 109410. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Park, S.Y.; Chang, S.Y.; Kim, S.H. Regulation of Butyrate-Induced Resistance through AMPK Signaling Pathway in Human Colon Cancer Cells. Biomedicines 2021, 9, 1604. [Google Scholar] [CrossRef]
- Magrin, G.L.; Di Summa, F.; Strauss, F.J.; Panahipour, L.; Mildner, M.; Magalhães Benfatti, C.A.; Gruber, R. Butyrate Decreases ICAM-1 Expression in Human Oral Squamous Cell Carcinoma Cells. Int. J. Mol. Sci. 2020, 21, 1679. [Google Scholar] [CrossRef]
- Geng, H.W.; Yin, F.Y.; Zhang, Z.F.; Gong, X.; Yang, Y. Butyrate Suppresses Glucose Metabolism of Colorectal Cancer Cells via GPR109a-AKT Signaling Pathway and Enhances Chemotherapy. Front. Mol. Biosci. 2021, 8, 634874. [Google Scholar] [CrossRef]
- Preuss, J.; Richardson, A.D.; Pinkerton, A.; Hedrick, M.; Sergienko, E.; Rahlfs, S.; Becker, K.; Bode, L. Identification and characterization of novel human glucose-6-phosphate dehydrogenase inhibitors. J. Biomol. Screen. 2013, 18, 286–297. [Google Scholar] [CrossRef]
- Zhao, Z.B.; Liu, Y.; Yao, Y. Computational determination of binding structures and free energies of glucose 6-phosphate dehydrogenase with novel steroid inhibitors. J. Mol. Graph. Model. 2014, 51, 168–172. [Google Scholar] [CrossRef]
- Yang, H.C.; Stern, A.; Chiu, D.T. G6PD: A hub for metabolic reprogramming and redox signaling in cancer. Biomed. J. 2020, 44, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; He, Z.; Yao, J.; Tan, R.; Zhu, Y.; Li, Z.; Guo, Q.; Wei, L. Regulation of AMPK-related glycolipid metabolism imbalances redox homeostasis and inhibits anchorage independent growth in human breast cancer cells. Redox Biol. 2018, 17, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.; Hassan, S.S.; Ramaraj, P. Differential biological effects of dehydroepiandrosterone (DHEA) between mouse (B16F10) and human melanoma (BLM) cell lines. Dermatoendocrinology 2017, 9, e1389360. [Google Scholar] [CrossRef]
- Hakkak, R.; Bell, A.; Korourian, S. Dehydroepiandrosterone (DHEA) Feeding Protects Liver Steatosis in Obese Breast Cancer Rat Model. Sci. Pharm. 2017, 85, 13. [Google Scholar] [CrossRef]
- Chen, Y.; Niu, J.; Li, L.; Li, Z.; Jiang, J.; Zhu, M.; Dong, T.; Zhang, J.; Shi, C.; Xu, P.; et al. Polydatin executes anticancer effects against glioblastoma multiforme by inhibiting the EGFR-AKT/ERK1/2/STAT3-SOX2/Snail signaling pathway. Life Sci. 2020, 258, 118158. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, B.Y.; Nie, J.; Zhao, G.H.; Zhuo, J.Y.; Yuan, J.; Li, Y.C.; Wang, L.L.; Chen, Z.W. Polydatin prevents bleomycin-induced pulmonary fibrosis by inhibiting the TGF-β/Smad/ERK signaling pathway. Exp. Ther. Med. 2020, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Boran, G.; Tavakoli, S.; Dierking, I.; Kamali, A.R.; Ege, D. Synergistic effect of graphene oxide and zoledronic acid for osteoporosis and cancer treatment. Sci. Rep. 2020, 10, 7827. [Google Scholar] [CrossRef]
- Hao, W.; Shen, Y.; Feng, M.; Wang, H.; Lin, M.; Fang, Y.; Tan, L. Aspirin acts in esophageal cancer: A brief review. J. Thorac. Dis. 2018, 10, 2490–2497. [Google Scholar] [CrossRef]
- Song, Y.; Zhong, X.; Gao, P.; Zhou, C.; Shi, J.; Wu, Z.; Guo, Z.; Wang, Z. Aspirin and Its Potential Preventive Role in Cancer: An Umbrella Review. Front. Endocrinol. 2020, 11, 3. [Google Scholar] [CrossRef]
- Singh, A.K.; Trotman, B.W. Use and safety of aspirin in the chemoprevention of colorectal cancer. J. Assoc. Acad. Minor. Phys. 1998, 9, 40–44. [Google Scholar]
- Morgensztern, D.; Rose, M.; Waqar, S.N.; Morris, J.; Ma, P.C.; Reid, T.; Brzezniak, C.E.; Zeman, K.G.; Padmanabhan, A.; Hirth, J.; et al. RRx-001 followed by platinum plus etoposide in patients with previously treated small-cell lung cancer. Br. J. Cancer 2019, 121, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Scicinski, J.; Reid, T.; Oronsky, A.; Carter, C.; Oronsky, N.; Cabrales, P. RRx-001, a novel clinical-stage chemosensitizer, radiosensitizer, and immunosensitizer, inhibits glucose 6-phosphate dehydrogenase in human tumor cells. Discov. Med. 2016, 21, 251–265. [Google Scholar] [PubMed]
- Cirrik, S.; Ugurel, E.; Aksu, A.C.; Oronsky, B.; Cabrales, P.; Yalcin, O. Nitrite may serve as a combination partner and a biomarker for the anti-cancer activity of RRx-001. Biorheology 2019, 56, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Thakor, P.; Subramanian, R.B.; Thakkar, S.S.; Ray, A.; Thakkar, V.R. Phytol induces ROS mediated apoptosis by induction of caspase 9 and 3 through activation of TRAIL, FAS and TNF receptors and inhibits tumor progression factor Glucose 6 phosphate dehydrogenase in lung carcinoma cell line (A549). Biomed. Pharmacother. 2017, 92, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Soltanian, S.; Sheikhbahaei, M.; Ziasistani, M. Phytol Down-Regulates Expression of Some Cancer Stem Cell Markers and Decreases Side Population Proportion in Human Embryonic Carcinoma NCCIT Cells. Nutr. Cancer 2020, 73, 1520–1533. [Google Scholar] [CrossRef]
- Pan, H.; Lin, Y.; Dou, J.; Fu, Z.; Yao, Y.; Ye, S.; Zhang, S.; Wang, N.; Liu, A.; Li, X.; et al. Wedelolactone facilitates Ser/Thr phosphorylation of NLRP3 dependent on PKA signalling to block inflammasome activation and pyroptosis. Cell Prolif. 2020, 53, e12868. [Google Scholar] [CrossRef]
- Zhu, M.M.; Wang, L.; Yang, D.; Li, C.; Pang, S.T.; Li, X.H.; Li, R.; Yang, B.; Lian, Y.P.; Ma, L.; et al. Wedelolactone alleviates doxorubicin-induced inflammation and oxidative stress damage of podocytes by IκK/IκB/NF-κB pathway. Biomed. Pharmacother. 2019, 117, 109088. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Han, X.F.; Bo, J.X.; Ma, H.P. Wedelolactone Enhances Osteoblastogenesis but Inhibits Osteoclastogenesis through Sema3A/NRP1/PlexinA1 Pathway. Front. Pharmacol. 2016, 7, 375. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Günther, C.; Bischoff, S.C. Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front. Immunol. 2021, 12, 678360. [Google Scholar] [CrossRef]
- Torun, A.; Enayat, S.; Sheraj, I.; Tunçer, S.; Ülgen, D.H.; Banerjee, S. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar]
- Sukhatme, V.P.; Chan, B. Glycolytic cancer cells lacking 6-phosphogluconate dehydrogenase metabolize glucose to induce senescence. FEBS Lett. 2012, 586, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; VanderLaan, P.A.; Sukhatme, V.P. 6-Phosphogluconate dehydrogenase regulates tumor cell migration in vitro by regulating receptor tyrosine kinase c-Met. Biochem. Biophys. Res. Commun. 2013, 439, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; DePinho, R.A.; Cantley, L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar] [CrossRef]
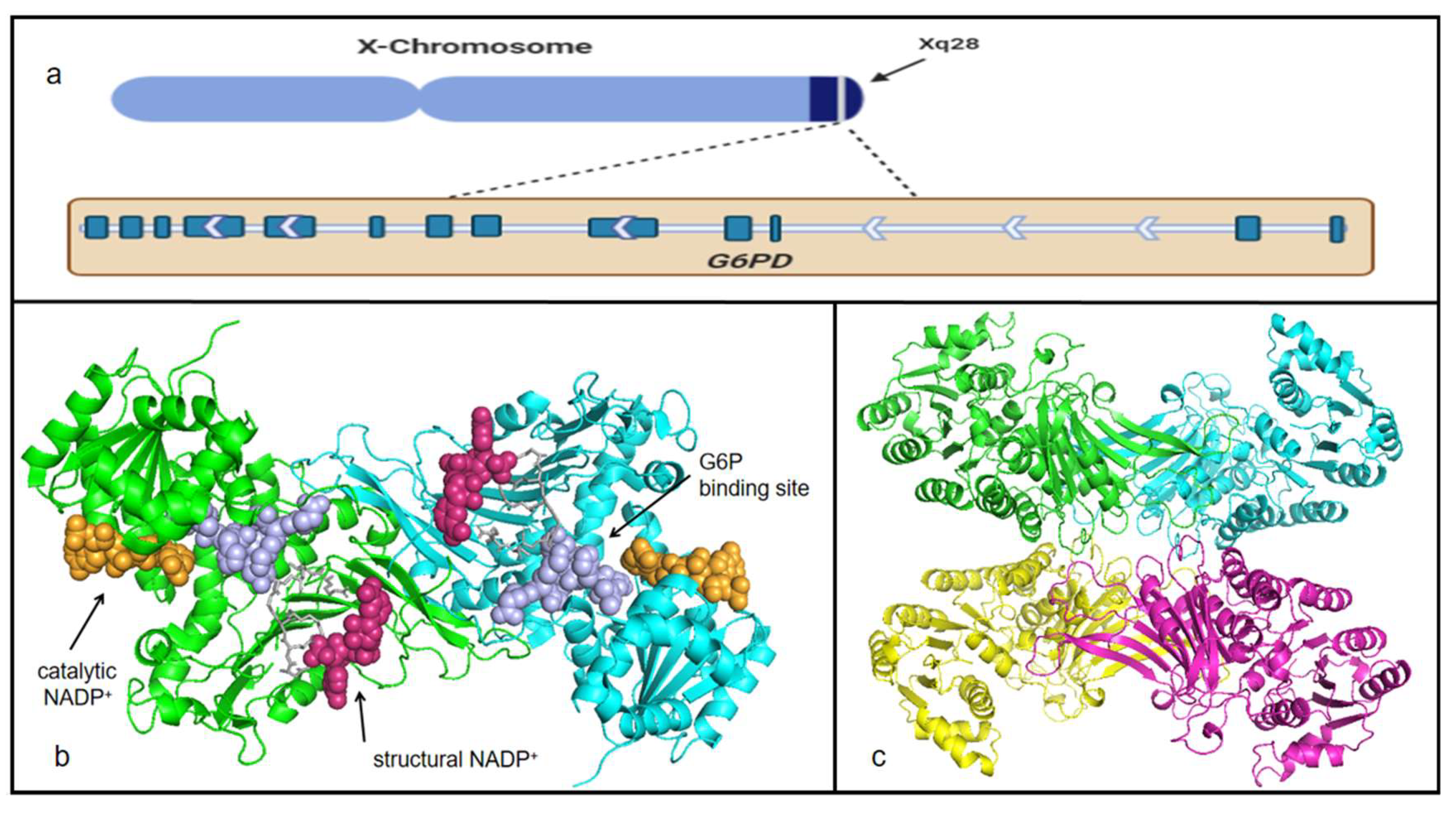
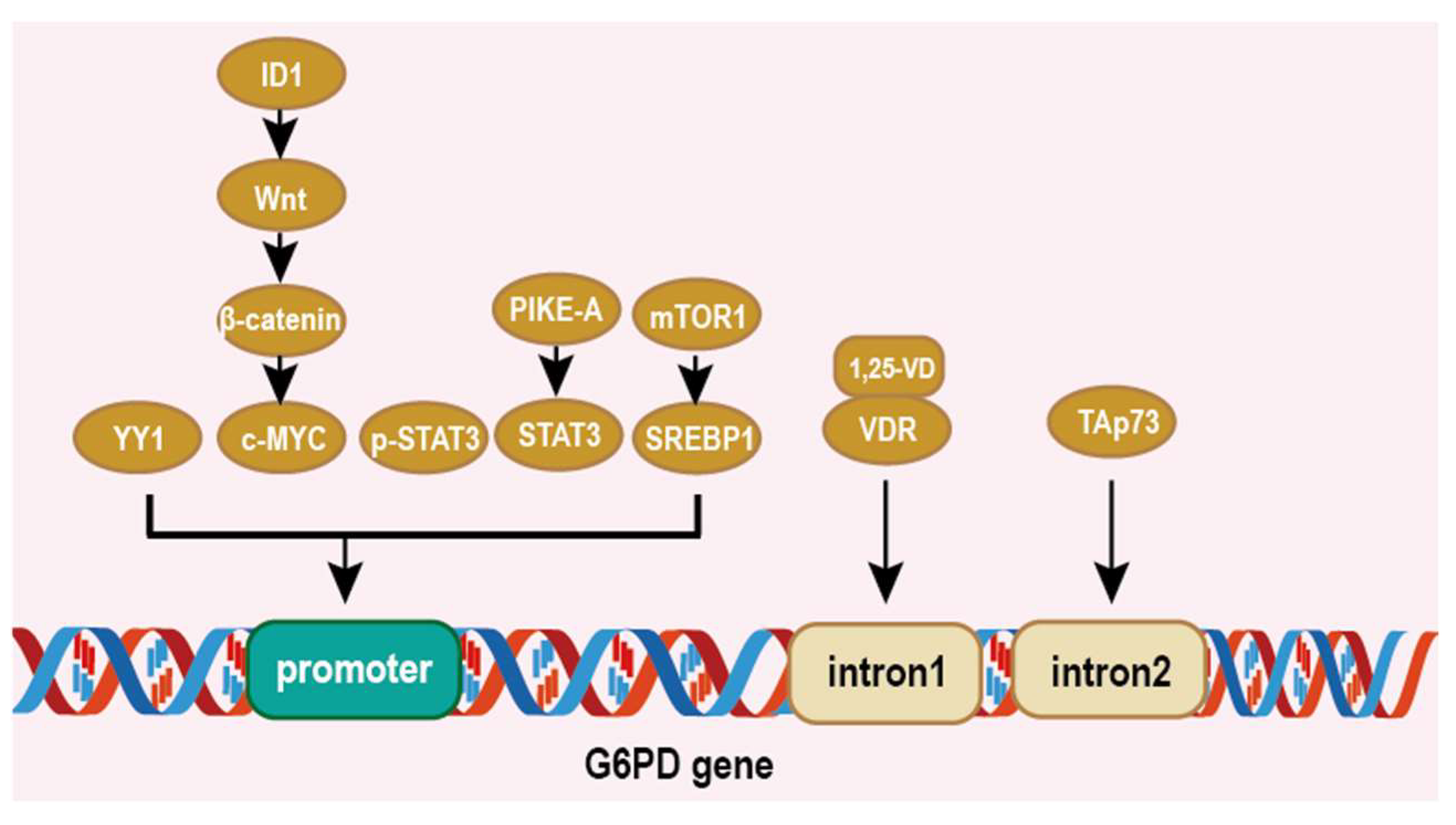
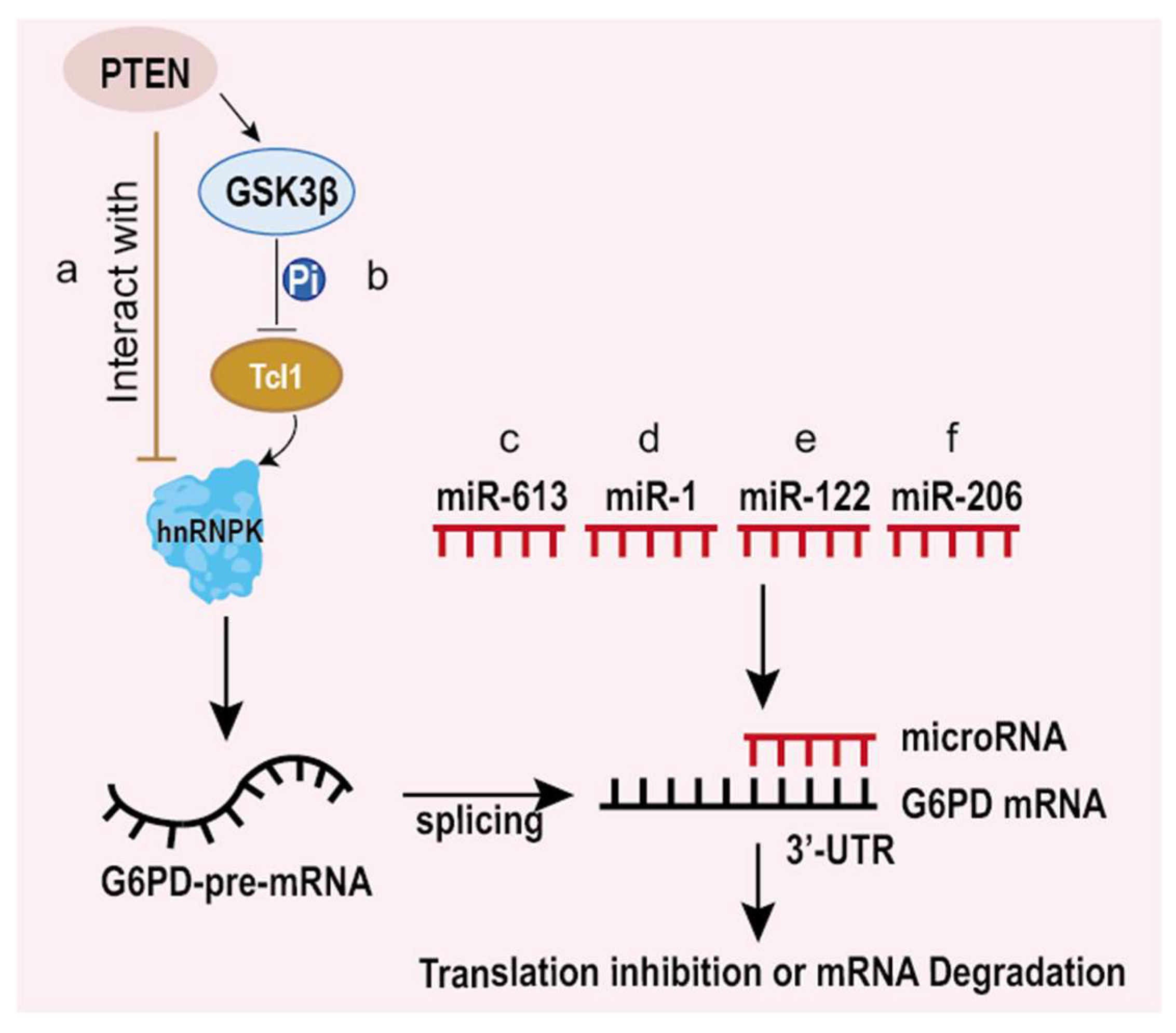
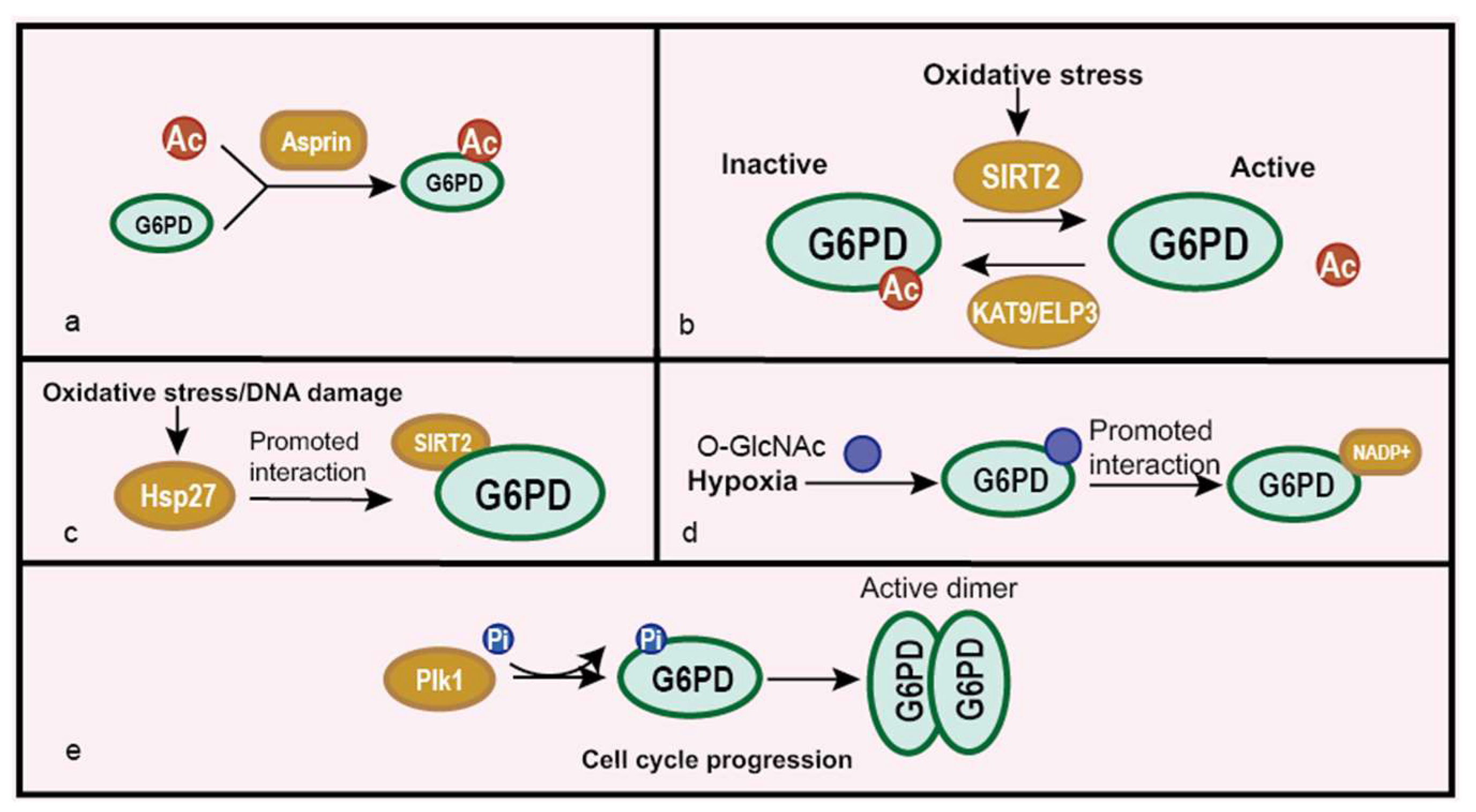

| Cancer Type | G6PD Expression | Effects of High/Low Expression |
|---|---|---|
| Lung adenocarcinoma [34] | High | Advanced stage, Metastasis, Invasion |
| Glioma [35] | High | Poor survival |
| Colorectal cancer [36] | High | Poor prognosis, Poor outcome of chemotherapy |
| Acute myeloid leukemia [37] | Low | Reduced chemotherapy-resistance |
| Clear cell renal cell carcinoma [29] | High | Invasion and ECM |
| Breast carcinoma [38] | High | Chemotherapy-resistance |
| Esophageal squamous cell carcinoma [39] | High | Metastasis, Poor survival |
| Hepatocellularcarcinoma [33,40] | High | Growth, Invasion, ECM, Migration |
| Gastric cancer [27] | High | Invasion, ECM, Migration, Poor survival |
| Melanoma [41] | Low | Decreased proliferation, Enhanced apoptosis |
| Pituitary cancer [42] | Low | Decreased glycolysis |
| Compounds | Cancer (Cell) Type | Reference |
|---|---|---|
| 6-aminonicotinamide (6-AN) | Acute myeloid leukemia cells, melanoma cells, bladder cancer cells | [66,72,73] |
| Dehydroepiandrosterone (DHEA) | Cervical cancer cells, breast cancer cells | [74,75] |
| Polygonin | Lung cancer cells (A549 and NCI-H1975), nasopharyngeal carcinoma cells, epidermal carcinoma cells (A-431), breast cancer cells (MCF-7), ovarian cancer cells (OVCAR-8), cervical carcinoma cells (HeLa), osteosarcoma cells, orthotopic and metastatic model of oral cancer (mice) | [76,77,78] |
| Zoledronic acid | Lung cancer (patients), orthotopic implantation model of pancreatic cancer (mice), mammary cancer (mice), pancreatic cancer cells, bladder cancer cells | [79,80,81] |
| Aspirin | Hepatobiliary and colorectal carcinoma (patients), colorectal cancer cells | [36,82,83] |
| RRX-001 | Colorectal cancer cells (CACO-2, HT-29), hepatoma cells (HepG2) | [84] |
| Phytol | Hepatoma cells (HepG2), breast cancer cells (MCF-7, MDA-MB-231), prostate cancer cells (PC-3), colorectal cancer cells (HT-29), lung cancer cells (A-549), melanoma cells (Hs294T) | [85,86] |
| Wedelolactone | Ovarian cancer cells (Ovcar3), breast cancer cells (MDA-MB-231), colon cancer cells (WiDr), prostate cancer cells | [87,88] |
| Butyrate | Colon cancer cells (HCT116, LoVo, SW480, HT29) | [89,90,91,92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Sun, H.; Zhang, S.; Shan, C. The Multiple Roles of Glucose-6-Phosphate Dehydrogenase in Tumorigenesis and Cancer Chemoresistance. Life 2022, 12, 271. https://doi.org/10.3390/life12020271
Song J, Sun H, Zhang S, Shan C. The Multiple Roles of Glucose-6-Phosphate Dehydrogenase in Tumorigenesis and Cancer Chemoresistance. Life. 2022; 12(2):271. https://doi.org/10.3390/life12020271
Chicago/Turabian StyleSong, Jiaqi, Huanran Sun, Shuai Zhang, and Changliang Shan. 2022. "The Multiple Roles of Glucose-6-Phosphate Dehydrogenase in Tumorigenesis and Cancer Chemoresistance" Life 12, no. 2: 271. https://doi.org/10.3390/life12020271
APA StyleSong, J., Sun, H., Zhang, S., & Shan, C. (2022). The Multiple Roles of Glucose-6-Phosphate Dehydrogenase in Tumorigenesis and Cancer Chemoresistance. Life, 12(2), 271. https://doi.org/10.3390/life12020271






