A Fast-Growing Oleaginous Strain of Coelastrella Capable of Astaxanthin and Canthaxanthin Accumulation in Phototrophy and Heterotrophy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalga Strain, Medium and Culture Conditions
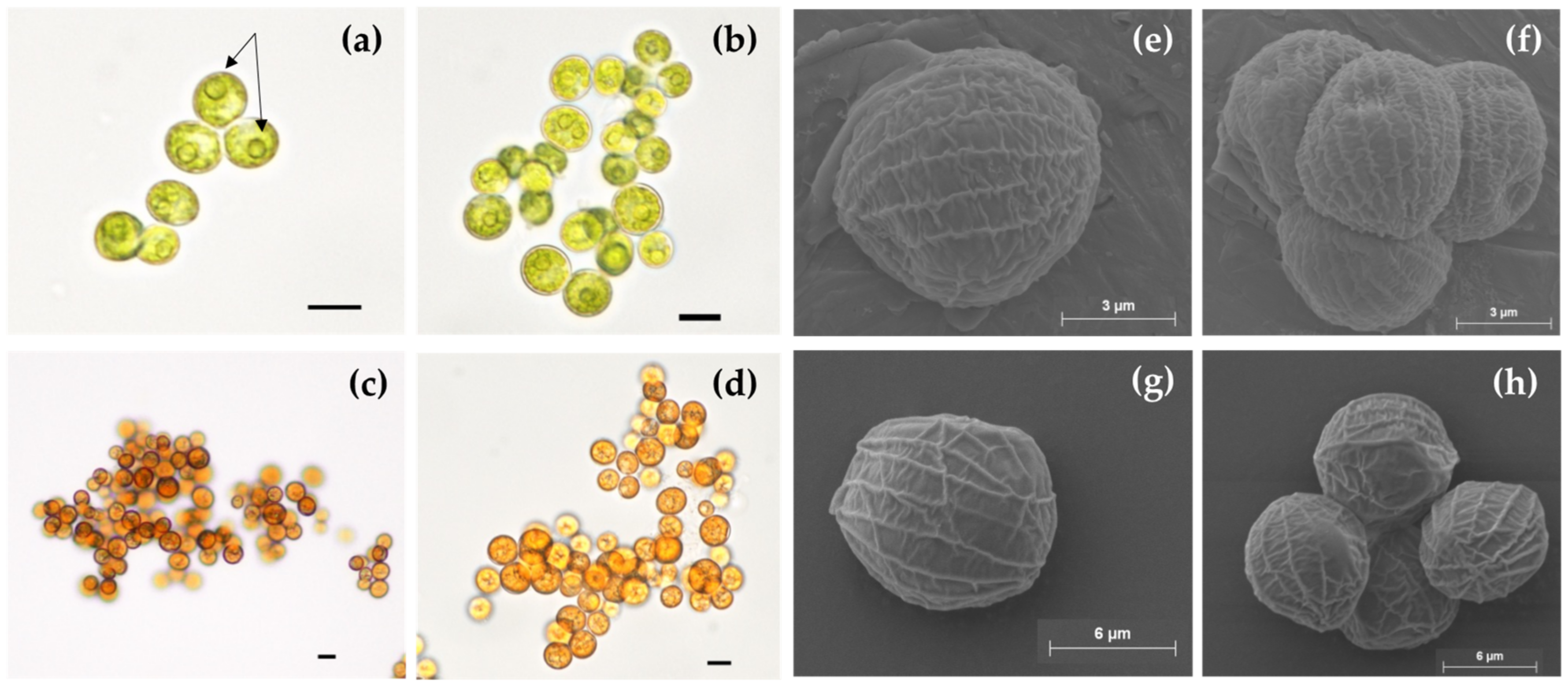
2.2. Optical Microscopy and SEM Analysis
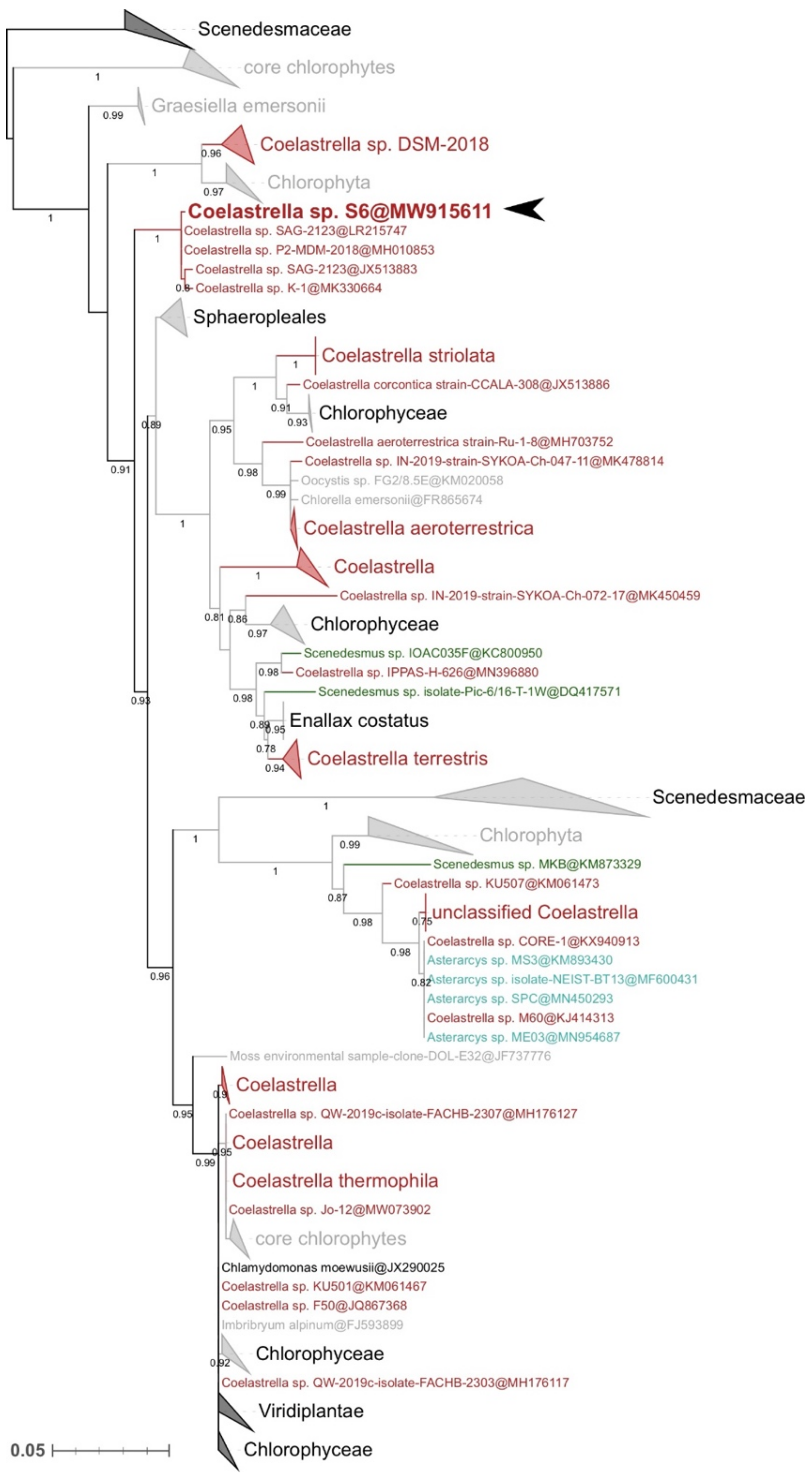
2.3. Molecular Identification and Phylogenetic Analyses
2.4. Growth and Carotenogenesis Conditions
2.5. Algal Growth Measurements
2.6. Nitrate and Glucose Quantification
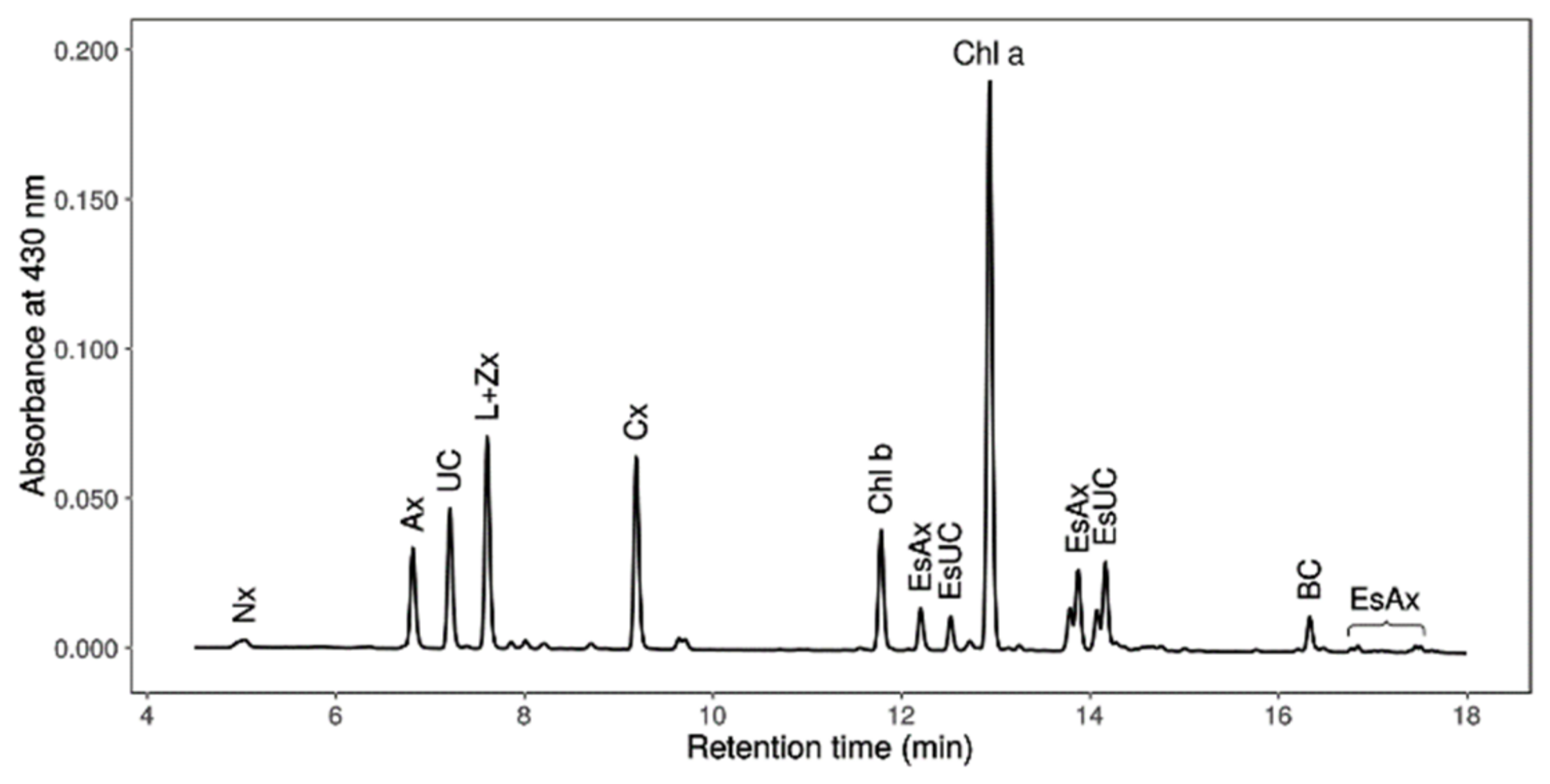
2.7. Chlorophylls, Primary Carotenoids (Neoxanthin, Violaxanthin, Antheraxanthin, Lutein, Zeaxanthin and β-Carotene), Astaxanthin and Canthaxanthin Determination
2.8. Lipids and FAMEs Determination
3. Results
3.1. Strain Identification
3.2. Characterization of the Coelastrella sp. S6 Isolate in Terms of Growth, Pigments and Fatty Acids Profiles and Accumulation
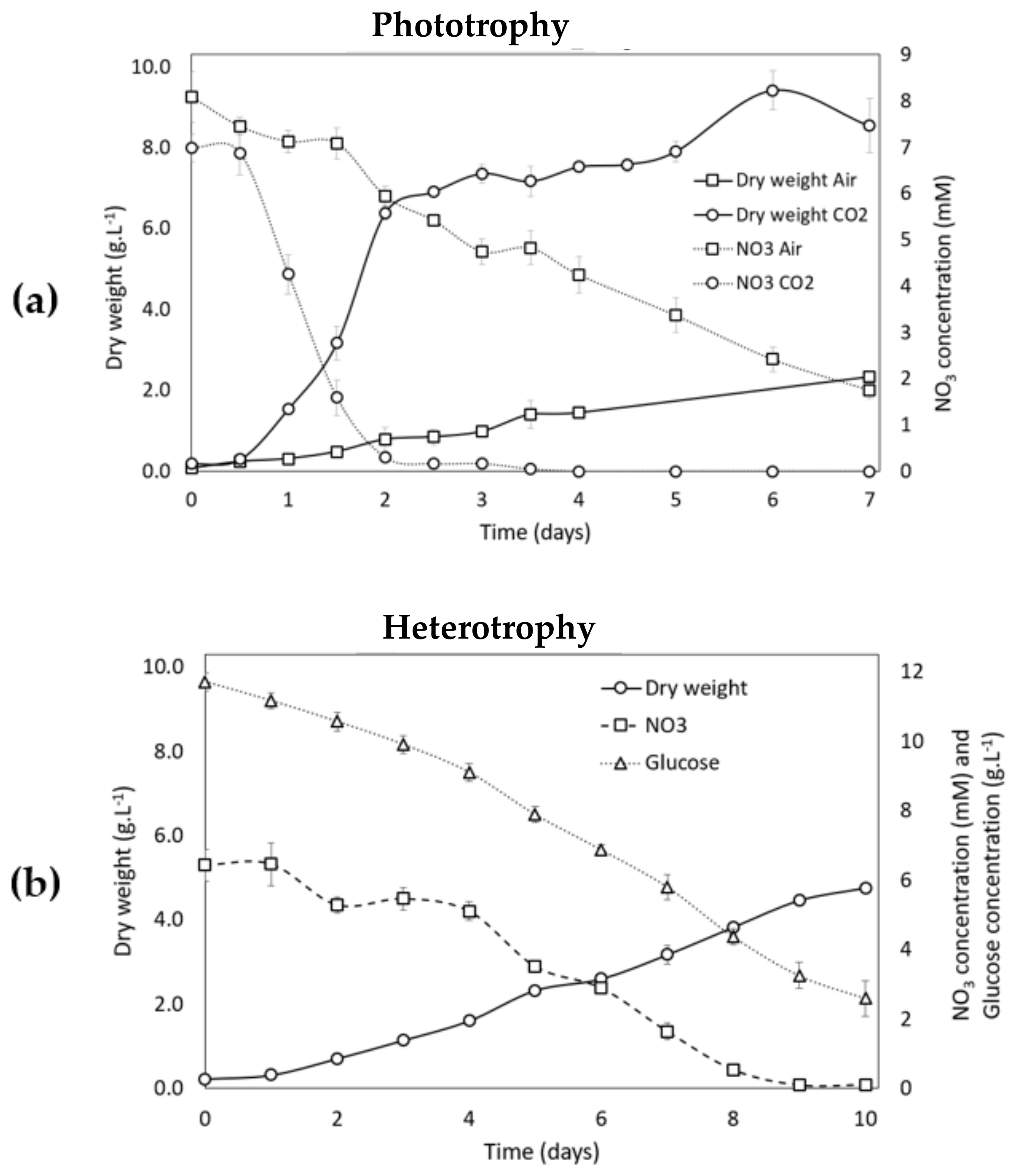
3.2.1. Growth Analysis during the Growth Phase (Phase 1) in Phototrophic and Heterotrophic Conditions
3.2.2. Growth Analysis during Carotenogenesis in Phototrophic Conditions
3.2.3. Growth Analysis during Carotenogenesis in Heterotrophic Conditions
3.2.4. Chlorophyll and Primary Carotenoid Contents of Coelastrella sp. S6 in Phototrophic Conditions
3.2.5. Chlorophyll and Primary Carotenoid Contents of Coelastrella sp. S6 in Heterotrophic Conditions
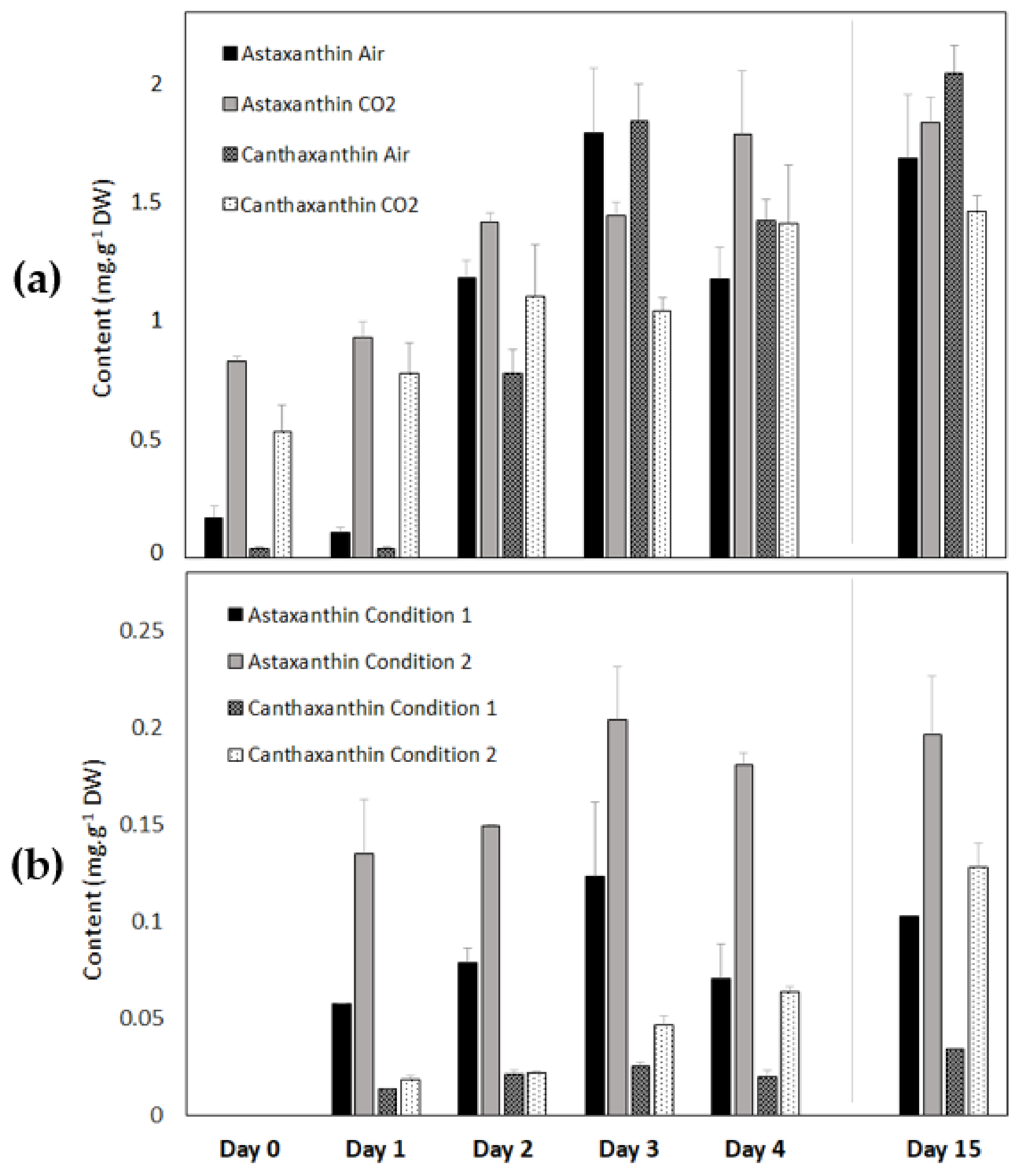
3.2.6. Astaxanthin and Canthaxanthin Contents of Coelastrella sp. S6 during Carotenogenesis in the Light and in the Dark
3.2.7. Fatty Acid and Lipid Contents of Coelastrella sp. S6 in the Light
3.2.8. Fatty Acid and Lipid Contents of Coelastrella sp. S6 under Heterotrophic Conditions
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Haro, A.; Verdín, J.; Kirchmayr, M.R.; Arellano-Plaza, M. Metabolic Engineering for High Yield Synthesis of Astaxanthin in Xanthophyllomyces dendrorhous. Microb. Cell Fact. 2021, 20, 175. [Google Scholar] [CrossRef]
- Mota, G.C.P.; de Moraes, L.B.S.; Oliveira, C.Y.B.; Oliveira, D.W.S.; de Abreu, J.L.; Dantas, D.M.M.; Gálvez, A.O. Astaxanthin from Haematococcus pluvialis: Processes, Applications, and Market. Prep. Biochem. Biotechnol. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.T.; Cysewski, G.R. Commercial Potential for Haematococcus Microalgae as a Natural Source of Astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Sun, X.-B.; Xu, Y.-X.; Zhao, H.; Zhu, Q.-Y.; Zhu, C.-Q. Astaxanthin Upregulates Heme Oxygenase-1 Expression through ERK1/2 Pathway and Its Protective Effect against Beta-Amyloid-Induced Cytotoxicity in SH-SY5Y Cells. Brain Res. 2010, 1360, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, B.A.; Farrona, S.; Ventura, M.R.; Abranches, R. Canthaxanthin, a Red-Hot Carotenoid: Applications, Synthesis, and Biosynthetic Evolution. Plants 2020, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, Y.; Schoefs, B. Secondary Ketocarotenoid Astaxanthin Biosynthesis in Algae: A Multifunctional Response to Stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef]
- Mariam, I.; Kareya, M.S.; Rehmanji, M.; Nesamma, A.A.; Jutur, P.P. Channeling of Carbon Flux Towards Carotenogenesis in Botryococcus braunii: A Media Engineering Perspective. Front. Microbiol. 2021, 12, 693106. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. Two-Phase Method of Cultivating Coelastrella Species for Increased Production of Lipids and Carotenoids. Bioresour. Technol. Rep. 2020, 9, 100366. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Nagai, S. Enhanced Carotenoid Biosynthesis by Oxidative Stress in Acetate-Induced Cyst Cells of a Green Unicellular Alga, Haematococcus pluvialis. Appl. Environ. Microbiol. 1993, 59, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Fučíková, K.; Lewis, L.A. Intersection of Chlorella, Muriella and Bracteacoccus: Resurrecting the Genus Chromochloris Kol et Chodat (Chlorophyceae, Chlorophyta). Fottea 2012, 12, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an Alternative Microalgal Producer of Astaxanthin: Biology and Industrial Potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef] [Green Version]
- Maltsev, Y.; Krivova, Z.; Maltseva, S.; Maltseva, K.; Gorshkova, E.; Kulikovskiy, M. Lipid Accumulation by Coelastrella multistriata (Scenedesmaceae, Sphaeropleales) during Nitrogen and Phosphorus Starvation. Sci. Rep. 2021, 11, 19818. [Google Scholar] [CrossRef] [PubMed]
- Minyuk, G.; Chelebieva, E.; Chubchikova, I.; Dantsyuk, N.; Drobetskaya, I.; Sakhon, E.; Chekanov, K.; Solovchenko, A. Stress-Induced Secondary Carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a Producer of Value-Added Keto-Carotenoids. Algae 2017, 32, 245–259. [Google Scholar] [CrossRef]
- Ip, P.-F.; Chen, F. Production of Astaxanthin by the Green Microalga Chlorella zofingiensis in the Dark. Process Biochem. 2005, 40, 733–738. [Google Scholar] [CrossRef]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.S.; Lee, D.J. Heterotrophic Cultivation of Microalgae for Pigment Production: A Review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef]
- Goecke, F.; Noda, J.; Paliocha, M.; Gislerød, H.R. Revision of Coelastrella (Scenedesmaceae, Chlorophyta) and First Register of This Green Coccoid Microalga for Continental Norway. World J. Microbiol. Biotechnol. 2020, 36, 149. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Liu, X.; Zhu, H.; Hu, Z.; Liu, G. Deep Genomic Analysis of Coelastrella saipanensis (Scenedesmaceae, Chlorophyta): Comparative Chloroplast Genomics of Scenedesmaceae. Eur. J. Phycol. 2019, 54, 52–65. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Liu, X.; Liu, B.; Hu, Z.; Liu, G. Morphology and Molecular Phylogeny of Coccoid Green Algae Coelastrella Sensu Lato (Scenedesmaceae, Sphaeropeales), Including the Description of Three New Species and Two New Varieties. J. Phycol. 2019, 55, 1290–1305. [Google Scholar] [CrossRef]
- Kawasaki, S.; Yamazaki, K.; Nishikata, T.; Ishige, T.; Toyoshima, H.; Miyata, A. Photooxidative Stress-Inducible Orange and Pink Water-Soluble Astaxanthin-Binding Proteins in Eukaryotic Microalga. Commun. Biol. 2020, 3, 490. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Sashidhar, B.; Adholeya, A. The Isolation and Identification of New Microalgal Strains Producing Oil and Carotenoid Simultaneously with Biofuel Potential. Bioresour. Technol. 2016, 211, 556–565. [Google Scholar] [CrossRef]
- Zaytseva, A.; Chekanov, K.; Zaytsev, P.; Bakhareva, D.; Gorelova, O.; Kochkin, D.; Lobakova, E. Sunscreen Effect Exerted by Secondary Carotenoids and Mycosporine-like Amino Acids in the Aeroterrestrial Chlorophyte Coelastrella rubescens under High Light and UV-A Irradiation. Plants 2021, 10, 2601. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A Review on the Assessment of Stress Conditions for Simultaneous Production of Microalgal Lipids and Carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasio, M.; Balzano, S. Fatty Acids Derivatives from Eukaryotic Microalgae, Pathways and Potential Applications. Front. Microbiol. 2021, 12, 718933. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Comparison of the Photoautotrophic Growth Regimens of Chlorella sorokiniana in a Photobioreactor for Enhanced Biomass Productivity. Biology 2020, 9, 169. [Google Scholar] [CrossRef]
- Bold, H.C. The Cultivation of Algae. Bot. Rev. 1942, 8, 69–138. [Google Scholar] [CrossRef]
- Minyuk, G.S.; Dantsyuk, N.V.; Chelebieva, E.S.; Chubchikova, I.N.; Drobetskaya, I.V.; Solovchenko, A.E. The Effect of Diverse Nitrogen Sources in the Nutrient Medium on the Growth of the Green Microalgae Chromochloris zofingiensis in the Batch Culture. Mar. Biol. J. 2019, 4, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Hulatt, C.J.; Wijffels, R.H.; Kiron, V. Growth and LC-PUFA Production of the Cold-Adapted Microalga Koliella antarctica in Photobioreactors. J. Appl. Phycol. 2019, 31, 981–997. [Google Scholar] [CrossRef] [Green Version]
- Newman, S.M.; Boynton, J.E.; Gillham, N.W.; Randolph-Anderson, B.L.; Johnson, A.M.; Harris, E.H. Transformation of Chloroplast Ribosomal RNA Genes in Chlamydomonas: Molecular and Genetic Characterization of Integration Events. Genetics 1990, 126, 875–888. [Google Scholar] [CrossRef]
- Ferrigo, D.; Galla, G.; Sforza, E.; Morosinotto, T.; Barcaccia, G.; Ceschi Berrini, C. Biochemical Characterization and Genetic Identity of an Oil-Rich Acutodesmus obliquus Isolate. J. Appl. Phycol. 2015, 27, 149–161. [Google Scholar] [CrossRef]
- Huang, X.; Madan, A. CAP3: A DNA Sequence Assembly Program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Benson, D.A. GenBank. Nucleic Acids Res. 2000, 28, 15–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. A Simple Method to Control Over-Alignment in the MAFFT Multiple Sequence Alignment Program. Bioinformatics 2016, 32, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gascuel, O. BIONJ: An Improved Version of the NJ Algorithm Based on a Simple Model of Sequence Data. Mol. Biol. Evol. 1997, 14, 685–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavaré, S. Some Probabilistic and Statistical Problems in the Analysis of DNA Sequences. In Some Mathematical Questions in Biology: DNA Sequence Analysis; Lectures on Mathematics in the Life Sciences; American Mathematical Society: Providence, RI, USA, 1986; Volume 17, pp. 57–86. [Google Scholar]
- Anisimova, M.; Gascuel, O. Approximate Likelihood-Ratio Test for Branches: A Fast, Accurate, and Powerful Alternative. Syst. Biol. 2006, 55, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v4: Recent Updates and New Developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [Green Version]
- Chi, N.T.L.; Duc, P.A.; Mathimani, T.; Pugazhendhi, A. Evaluating the Potential of Green Alga Chlorella Sp. for High Biomass and Lipid Production in Biodiesel Viewpoint. Biocatal. Agric. Biotechnol. 2019, 17, 184–188. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Beckers, L.; Hiligsmann, S.; Hamilton, C.; Masset, J.; Thonart, P. Fermentative Hydrogen Production by Clostridium butyricum CWBI1009 and Citrobacter Freundii CWBI952 in Pure and Mixed Cultures. Biotechnol. Agron. Soc. Environ. 2010, 14, 541–548. [Google Scholar]
- Gérin, S.; Leprince, P.; Sluse, F.E.; Franck, F.; Mathy, G. New Features on the Environmental Regulation of Metabolism Revealed by Modeling the Cellular Proteomic Adaptations Induced by Light, Carbon, and Inorganic Nitrogen in Chlamydomonas Reinhardtii. Front. Plant Sci. 2016, 7, 1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Gérin, S.; Delhez, T.; Corato, A.; Remacle, C.; Franck, F. A Novel Culture Medium for Freshwater Diatoms Promotes Efficient Photoautotrophic Batch Production of Biomass, Fucoxanthin, and Eicosapentaenoic Acid. J. Appl. Phycol. 2020, 32, 1581–1596. [Google Scholar] [CrossRef]
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguin, O.; Polle, J.E.W. Characterization and Classification of Highly Productive Microalgae Strains Discovered for Biofuel and Bioproduct Generation. Algal Res. 2016, 15, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.-W.; Chuang, L.-T.; Yu, P.-C.; Chen, C.-N.N. Pigment Production by a New Thermotolerant Microalga Coelastrella Sp. F50. Food Chem. 2013, 138, 2071–2078. [Google Scholar] [CrossRef]
- Kaufnerová, V.; Eliáš, M. The Demise of the Genus Scotiellopsis Vinatzer (Chlorophyta). Nova Hedwig. 2013, 97, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Ho, S.-H.; Chen, C.-N.N.; Chen, C.-Y.; Ng, I.-S.; Jing, K.-J.; Chang, J.-S.; Lu, Y. Phototrophic Cultivation of a Thermo-Tolerant Desmodesmus sp. for Lutein Production: Effects of Nitrate Concentration, Light Intensity and Fed-Batch Operation. Bioresour. Technol. 2013, 144, 435–444. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.-Y.; Chang, J.-S. Effect of Light Intensity and Nitrogen Starvation on CO2 Fixation and Lipid/Carbohydrate Production of an Indigenous Microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef]
- Chekanov, K.; Schastnaya, E.; Solovchenko, A.; Lobakova, E. Effects of CO2 Enrichment on Primary Photochemistry, Growth and Astaxanthin Accumulation in the Chlorophyte Haematococcus pluvialis. J. Photochem. Photobiol. B Biol. 2017, 171, 58–66. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Comparison of Heterotrophic and Photoautotrophic Induction on Astaxanthin Production by Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2005, 68, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kaewkannetra, P.; Enmak, P.; Chiu, T. The Effect of CO2 and Salinity on the Cultivation of Scenedesmus obliquus for Biodiesel Production. Biotechnol. Bioprocess Eng. 2012, 17, 591–597. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of CO2 Concentration on Algal Growth: A Review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- De Marchin, T.; Erpicum, M.; Franck, F. Photosynthesis of Scenedesmus Obliquus in Outdoor Open Thin-Layer Cascade System in High and Low CO2 in Belgium. J. Biotechnol. 2015, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.L. Effects of Carbon Trophic Mode on Growth, Pigments and Fatty Acids in Green Microalgae Analyzed on the Basis of Well-Defined Growth Kinetics of Batch Cultures. Ph.D. Thesis, Université de Liège, Liège, Belgium, 2019. [Google Scholar]
- Aburai, N.; Sumida, D.; Abe, K. Effect of Light Level and Salinity on the Composition and Accumulation of Free and Ester-Type Carotenoids in the Aerial Microalga Scenedesmus sp. (Chlorophyceae). Algal Res. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Chekanov, K.; Lukyanov, A.; Boussiba, S.; Aflalo, C.; Solovchenko, A. Modulation of Photosynthetic Activity and Photoprotection in Haematococcus pluvialis Cells during Their Conversion into Haematocysts and Back. Photosynth. Res. 2016, 128, 313–323. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in Microalgae: Pathways, Functions and Biotechnological Implications. Algae 2013, 28, 131. [Google Scholar] [CrossRef]
- Liu, B.-H.; Lee, Y.-K. Secondary Carotenoids Formation by the Green Alga Chlorococcum sp. J. Appl. Phycol. 2000, 12, 301–307. [Google Scholar] [CrossRef]
- Abe, K.; Hattori, H.; Hirano, M. Accumulation and Antioxidant Activity of Secondary Carotenoids in the Aerial Microalga Coelastrella striolata Var. multistriata. Food Chem. 2007, 100, 656–661. [Google Scholar] [CrossRef]
- Abe, K.; Takizawa, H.; Kimura, S.; Hirano, M. Characteristics of Chlorophyll Formation of the Aerial Microalga Coelastrella striolata Var. multistriata and Its Application for Environmental Biomonitoring. J. Biosci. Bioeng. 2004, 98, 34–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Y.; Ding, W.; Mao, X.; Li, Y.; Gerken, H.; Liu, J. Astaxanthin Is Ketolated from Zeaxanthin Independent of Fatty Acid Synthesis in Chromochloris zofingiensis. Plant Physiol. 2020, 183, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Minyuk, G.; Sidorov, R.; Solovchenko, A. Effect of Nitrogen Source on the Growth, Lipid, and Valuable Carotenoid Production in the Green Microalga Chromochloris zofingiensis. J. Appl. Phycol. 2020, 32, 923–935. [Google Scholar] [CrossRef]
- Minyuk, G.S.; Chelebieva, E.S.; Chubchikova, I.N.; Dantsyuk, N.V.; Drobetskaya, I.V.; Sakhon, E.G.; Chivkunova, O.B.; Chekanov, K.A.; Lobakova, E.S.; Sidorov, R.A.; et al. PH and CO2 Effects on Coelastrella (Scotiellopsis) Rubescens Growth and Metabolism. Russ. J. Plant Physiol. 2016, 63, 566–574. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the Production of Lipid and Carotenoids: A Review with Focus on Stress Regulation and Adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal Triacylglycerols as Feedstocks for Biofuel Production: Perspectives and Advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Aburai, N.; Ohkubo, S.; Miyashita, H.; Abe, K. Composition of Carotenoids and Identification of Aerial Microalgae Isolated from the Surface of Rocks in Mountainous Districts of Japan. Algal Res. 2013, 2, 237–243. [Google Scholar] [CrossRef]
- Tocquin, P.; Fratamico, A.; Franck, F. Screening for a Low-Cost Haematococcus pluvialis Medium Reveals an Unexpected Impact of a Low N/P Ratio on Vegetative Growth. J. Appl. Phycol. 2012, 24, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Chen, F. Optimization of Culture Medium for Growth of Haematococcus pluvialis. J. Appl. Phycol. 1997, 9, 437–444. [Google Scholar] [CrossRef]
- Boussiba, S. Carotenogenesis in the Green Alga Haematococcus pluvialis: Cellular Physiology and Stress Response. Physiol. Plant 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Wei, D. Enhanced Production of Astaxanthin by Chromochloris zofingiensis in a Microplate-Based Culture System under High Light Irradiation. Bioresour. Technol. 2017, 245, 518–529. [Google Scholar] [CrossRef]
- Orosa, M.; Valero, J.F.; Herrero, C.; Abalde, J. Comparison of the Accumulation of Astaxanthin in Haematococcus pluvialis and Other Green Microalgae under N-Starvation and High Light Conditions. Biotechnol. Lett. 2001, 23, 1079–1085. [Google Scholar] [CrossRef] [Green Version]
- Loor, G.; Kondapalli, J.; Schriewer, J.M.; Chandel, N.S.; Vanden Hoek, T.L.; Schumacker, P.T. Menadione Triggers Cell Death through ROS-Dependent Mechanisms Involving PARP Activation without Requiring Apoptosis. Free Radic. Biol. Med. 2010, 49, 1925–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, F.A.V.; Mariani, D.; Panek, A.D.; Eleutherio, E.C.A.; Pereira, M.D. Cytotoxicity Mechanism of Two Naphthoquinones (Menadione and Plumbagin) in Saccharomyces cerevisiae. PLoS ONE 2008, 3, e3999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criddle, D.N.; Gillies, S.; Baumgartner-Wilson, H.K.; Jaffar, M.; Chinje, E.C.; Passmore, S.; Chvanov, M.; Barrow, S.; Gerasimenko, O.V.; Tepikin, A.V.; et al. Menadione-Induced Reactive Oxygen Species Generation via Redox Cycling Promotes Apoptosis of Murine Pancreatic Acinar Cells. J. Biol. Chem. 2006, 281, 40485–40492. [Google Scholar] [CrossRef] [Green Version]
- Chen, F. Methods for Production of Astaxanthin from the Green Microalgae Chlorella in Dark-Heterotrophic Cultures. U.S. Patent 7,063,957, 20 June 2006. [Google Scholar]
- Paliwal, C.; Jutur, P.P. Dynamic Allocation of Carbon Flux Triggered by Task-Specific Chemicals Is an Effective Non-Gene Disruptive Strategy for Sustainable and Cost-Effective Algal Biorefineries. Chem. Eng. J. 2021, 418, 129413. [Google Scholar] [CrossRef]
- Umbach, A.L.; Fiorani, F.; Siedow, J.N. Characterization of Transformed Arabidopsis with Altered Alternative Oxidase Levels and Analysis of Effects on Reactive Oxygen Species in Tissue. Plant Physiol. 2005, 139, 1806–1820. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, J.; Sandmann, G.; Chen, F. Glucose Sensing and the Mitochondrial Alternative Pathway Are Involved in the Regulation of Astaxanthin Biosynthesis in the Dark-Grown Chlorella zofingiensis (Chlorophyceae). Planta 2008, 228, 735–743. [Google Scholar] [CrossRef]
- Suriya Narayanan, G.; Kumar, G.; Seepana, S.; Elankovan, R.; Arumugan, S.; Premalatha, M. Isolation, Identification and Outdoor Cultivation of Thermophilic Freshwater Microalgae Coelastrella Sp. FI69 in Bubble Column Reactor for the Application of Biofuel Production. Biocatal. Agric. Biotechnol. 2018, 14, 357–365. [Google Scholar] [CrossRef]
- Shao, H.; Tu, Y.; Wang, Y.; Jiang, C.; Ma, L.; Hu, Z.; Wang, J.; Zeng, B.; He, B. Oxidative Stress Response of Aspergillus Oryzae Induced by Hydrogen Peroxide and Menadione Sodium Bisulfite. Microorganisms 2019, 7, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanklin, J.; Cahoon, E.B. Desaturation and Related Modifications of Fatty Acids1. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 611–641. [Google Scholar] [CrossRef] [Green Version]


| Phase 1: Growth | Phase 2: Carotenogenesis | |||||||
|---|---|---|---|---|---|---|---|---|
| Phototrophy | High Light | Air | Condition “Air” | 7 days | High Light + 5% CO2 + Nitrate starvation | 15 days | ||
| 5% CO2 | Condition “CO2” | |||||||
| Heterotrophy | Dark + Glucose + Air | / | 10 days | Dark + Glucose + Air + Nitrate starvation | / | Condition 1 | ||
| + Acetate + MSB + Fe2+ | Condition 2 | |||||||
| Content (mg·g−1 DW) | Yield (mg·L−1) | ||
|---|---|---|---|
| Astaxanthin | |||
| Phototrophy | Air | 1.68 ± 0.27 | 12.19 ± 1.78 |
| CO2 | 1.83 ± 0.11 | 3.99 ± 0.35 | |
| Heterotrophy | Condition 1 | 0.120 ± 0.020 | 0.102 ± 0.040 |
| Condition 2 | 0.200 ± 0.030 | 0.180 ± 0.030 | |
| Canthaxanthin | |||
| Phototrophy | Air | 2.04 ± 0.12 | 14.81 ± 1.11 |
| CO2 | 1.46 ± 0.07 | 3.18 ± 0.31 | |
| Heterotrophy | Condition 1 | 0.031 ± 0.003 | 0.030 ± 0.001 |
| Condition 2 | 0.130 ± 0.010 | 0.114 ± 0.010 |
| Day 0 | Day 4 | Day 15 | ||||
|---|---|---|---|---|---|---|
| Air | CO2 | Air | CO2 | Air | CO2 | |
| C16:0 | 8.3 ± 1.1 | 37.8 ± 4 | 81.2 ± 5.6 | 70.8 ± 1.9 | 94.4 ± 3.6 | 103 ± 6.1 |
| C18:0 | 1.7 ± 0.2 | 6.6 ± 0.5 | 7.3 ± 0.5 | 6.4 ± 0.8 | 7.2 ± 0.3 | 7.8 ± 0.4 |
| C18:1n9c | 7.9 ± 1.1 | 46.3 ± 5.1 | 121.9 ± 8.3 | 72.6 ± 3.8 | 120.8 ± 4.4 | 91.9 ± 4.7 |
| C18:2n6c | 4.3 ± 0.5 | 13.8 ± 1.8 | 23.4 ± 1.4 | 17.9 ± 0.2 | 27.5 ± 1 | 23.8 ± 1.2 |
| C18:3n3 | 16.9 ± 1.8 | 29.4 ± 4.4 | 53.1 ± 3.8 | 54.7 ± 0.4 | 73.2 ± 3 | 75.6 ± 3.6 |
| C20:0 | 1.1 ± 0.1 | 1.9 ± 0.2 | 2.9 ± 0.1 | 2.8 ± 0.1 | 3.3 ± 0.1 | 3.5 ± 0.1 |
| SFAs | 29.9 ± 1.9 | 36.1 ± 0.3 | 31.8 ± 0.1 | 36.4 ± 0.4 | 32.5 ± 0.1 | 38.2 ± 0.3 |
| MUFAs | 24.5 ± 0.5 | 34.4 ± 1.4 | 42.7 ± 0.1 | 32.7 ± 0.8 | 37.6 ± 0.2 | 30.5 ± 0.8 |
| PUFAs | 45.6 ± 1.9 | 29.5 ± 1.6 | 25.6 ± 0 | 30.9 ± 0.8 | 30 ± 0.2 | 31.4 ± 0.6 |
| TFAs | 4.6 ± 0.5 | 14.9 ± 1.6 | 29.9 ± 2 | 23.8 ± 0.6 | 33.6 ± 1.3 | 32 ± 1.6 |
| Total Lipids | 24.8 ± 3 | 35.3 ± 2.2 | - | - | 44.2 ± 0.7 | 42.6 ± 0.7 |
| Day 0 | Day 4 | Day 15 | |||
|---|---|---|---|---|---|
| Heterotrophy | Condition 1 | Condition 2 | Condition 1 | Condition 2 | |
| C16:0 | 18.8 ± 0.7 | 18.5 ± 1 | 26.2 ± 1.5 | 20.3 ± 0.3 | 35.2 ± 1.6 |
| C16:1 | - | 1.6 ± 0.3 | 2.3 ± 0.2 | 1.6 ± 0.3 | 3.7 ± 0.4 |
| C18:0 | 10.2 ± 0.4 | 6.2 ± 0.3 | 8.4 ± 0.6 | 5.7 ± 0.1 | 10.4 ± 0.8 |
| C18:1n9c | 16.4 ± 0.7 | 22 ± 0.9 | 39.3 ± 2.8 | 23.8 ± 0.3 | 62.2 ± 6.4 |
| C18:2n6c | 6.9 ± 0.1 | 6.9 ± 0.9 | 7.7 ± 0.5 | 6.5 ± 0 | 10.8 ± 0.9 |
| C18:3n3 | 4.1 ± 0.3 | 4.9 ± 0.5 | 6.4 ± 0.3 | 4.4 ± 0.4 | 6.2 ± 0.3 |
| C20:0 | 2.2 ± 0.3 | 1.2 ± 0.2 | 1.4 ± 0.2 | 1.1 ± 0.1 | 1.6 ± 0.1 |
| SFAs | 51.9 ± 0.9 | 42.2 ± 0.5 | 38.1 ± 2.7 | 43.8 ± 0.3 | 36.9 ± 0.9 |
| MUFAs | 28.6 ± 0.6 | 38.6 ± 0.5 | 44.2 ± 4.2 | 39.5 ± 0.3 | 50.3 ± 1.1 |
| PUFAs | 19.5 ± 0.3 | 19.2 ± 0.9 | 14.9 ± 1.1 | 16.8 ± 0.6 | 12.9 ± 0.2 |
| TFAs | 6.2 ± 0.2 | 6.2 ± 0.4 | 9.2 ± 0.6 | 6.5 ± 0 | 13.2 ± 1.1 |
| Total Lipids | 22 ± 0.6 | - | - | 27.8 ± 0 | 39.5 ± 0.9 |
| Strain | Growth Phase | Stress Condition | |||||
|---|---|---|---|---|---|---|---|
| Nutrient Stress and Addition | Light Intensity (µmol·m−2·s−1) | Duration (Days) | Astaxanthin | Canthaxanthin | References | ||
| Coelastrella sp. S6 | Phototrophy—5% CO2 | N starvation + 5% CO2 | 400 | 15 | 1.83 ± 0.11 | 1.46 ± 0.07 | This manuscript |
| Phototrophy (Air) | N starvation + 5% CO2 | 400 | 15 | 1.68 ± 0.27 | 2.04 ± 0.12 | ||
| Heterotrophy | N starvation + MSB + Fe2+ + Acetate | 0 | 15 | 0.20 ± 0.03 | 0.13 ± 0.01 | ||
| Coelastrella striolata var. multistriata | Phototrophy | Aging | 40 | 21 | 1 | 35 | [62] |
| Aging | 65 | 50 | 1.5 | 47.5 | [61] | ||
| Aging | 214 | 21 | 0.99 ± 0.21 | 0.42 ± 0.05 | [68] | ||
| Coelastrella sp. F50 | Phototrophy | 1.5% NaCl | 400 | 12 | 18 | 1.8 | [47] |
| Coelastrella rubescens | Phototrophy | N and P starvation, low pH | 240 | 8 | 7.95 ± 0.85 | 12.73 ± 0.56 | [13] a |
| C. zofingiensis | Phototrophy—High Light | High C:N | > 80 | 12 | 1.80 ± 0.10 | - | [72] |
| Phototrophy—Low light | High C:N | > 80 | 12 | 2.19 ± 0.19 | - | ||
| H. pluvialis | Phototrophy | N starvation | 350 | 12 | 21.8 ± 0.4 | - | [73] |
| Strain | Stress Condition | Light Intensity (µmol·m−2·s−1) | CO2 Addition (%) or Glucose (g·L−1) | Duration | Lipids (% DW) | References |
|---|---|---|---|---|---|---|
| Green cells | ||||||
| Coelastrella sp. S6 | - | 400 (Air) | - | 7 | 24.8 ± 0.3 | This manuscript |
| - | 400 (CO2) | 5% | 7 | 35.3 ± 2.2 | ||
| - | 0 | 10 g·L−1 | 10 | 22.0 ± 0.6 | ||
| Coelastrella striolata MZ-Ch23 | - | 100 (16:8 L:D) | - | 25 | 27.0 ± 3.64 | [12] |
| Red cells | ||||||
| Coelastrella sp. S6 | N-starvation | 400 (Air) | 5% | 15 | 44.2 ± 0.7 | This manuscript |
| N-starvation | 400 (CO2) | 5% | 15 | 42.6 ± 0.7 | ||
| N-starvation | - | 10 g·L−1 | 15 | 27.8 ± 0 | ||
| N-starvation + MSB + acetate + Fe2+ | - | 10 g·L−1 | 15 | 39.5 ± 0.9 | ||
| Coelastrella striolata MZ-Ch23 | N-starvation | 100 (16:8 L:D) | - | 25 | 45.90 ± 1.67 | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corato, A.; Le, T.T.; Baurain, D.; Jacques, P.; Remacle, C.; Franck, F. A Fast-Growing Oleaginous Strain of Coelastrella Capable of Astaxanthin and Canthaxanthin Accumulation in Phototrophy and Heterotrophy. Life 2022, 12, 334. https://doi.org/10.3390/life12030334
Corato A, Le TT, Baurain D, Jacques P, Remacle C, Franck F. A Fast-Growing Oleaginous Strain of Coelastrella Capable of Astaxanthin and Canthaxanthin Accumulation in Phototrophy and Heterotrophy. Life. 2022; 12(3):334. https://doi.org/10.3390/life12030334
Chicago/Turabian StyleCorato, Amélie, Thanh Tung Le, Denis Baurain, Philippe Jacques, Claire Remacle, and Fabrice Franck. 2022. "A Fast-Growing Oleaginous Strain of Coelastrella Capable of Astaxanthin and Canthaxanthin Accumulation in Phototrophy and Heterotrophy" Life 12, no. 3: 334. https://doi.org/10.3390/life12030334
APA StyleCorato, A., Le, T. T., Baurain, D., Jacques, P., Remacle, C., & Franck, F. (2022). A Fast-Growing Oleaginous Strain of Coelastrella Capable of Astaxanthin and Canthaxanthin Accumulation in Phototrophy and Heterotrophy. Life, 12(3), 334. https://doi.org/10.3390/life12030334







