Serum Osteopontin Level Is Positively Associated with Aortic Stiffness in Patients with Peritoneal Dialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Anthropometric Analysis
2.3. Biochemical Investigations
2.4. Carotid–Femoral Pulse Wave Velocity Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laurent, S.; Boutouyrie, P. Arterial stiffness: A new surrogate end point for cardiovascular disease? J. Nephrol. 2007, 20, S45–S50. [Google Scholar] [PubMed]
- Mahmud, A.; Feely, J. Arterial Stiffness Is Related to Systemic Inflammation in Essential. Hypertension 2005, 46, 1118–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, S.; Boutouyrie, P.; Lacolley, P. Structural and Genetic Bases of Arterial Stiffness. Hypertension 2005, 45, 1050–1055. [Google Scholar] [CrossRef]
- Karras, A.; Haymann, J.-P.; Bozec, E.; Metzger, M.; Jacquot, C.; Maruani, G.; Houillier, P.; Froissart, M.; Stengel, B.; Guardiola, P.; et al. Large Artery Stiffening and Remodeling Are Independently Associated with All-Cause Mortality and Cardiovascular Events in Chronic Kidney Disease. Hypertension 2012, 60, 1451–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of Cardiovascular Events and All-Cause Mortality with Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, R.R.; Anderson, A.H.; Chirinos, J.A.; Feldman, H.I.; Grunwald, J.E.; Nessel, L.; Roy, J.; Weir, M.R.; Wright, J.T., Jr.; Bansal, N.; et al. Association of pulse wave velocity with chronic kidney disease progression and mortality: Findings from the CRIC study (chronic renal insufficiency cohort). Hypertension 2018, 71, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [CrossRef] [PubMed]
- Franzen, A.; Heinegård, D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem. J. 1985, 232, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, J.M.; Hafer, C.; Faulhaber-Walter, R.; Kümpers, P.; Kielstein, J.T.; Haller, H.; Fliser, D. Osteopontin predicts survival in critically ill patients with acute kidney injury. Nephrol. Dial. Transplant. 2010, 26, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Vaschetto, R.; Nicola, S.; Olivieri, C.; Boggio, E.; Piccolella, F.; Mesturini, R.; Damnotti, F.; Colombo, D.; Navalesi, P.; Della Corte, F.; et al. Serum levels of osteopontin are increased in SIRS and sepsis. Intensiv. Care Med. 2008, 34, 2176–2184. [Google Scholar] [CrossRef]
- Lee, C.-J.; Wang, J.-H.; Chen, Y.-C.; Chen, M.-L.; Yang, C.-F.; Hsu, B.-G. Serum Osteopontin Level Correlates with Carotid-Femoral Pulse Wave Velocity in Geriatric Persons. BioMed Res. Int. 2014, 2014, 570698. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Huang, D.; Luo, X.; Zhang, Y. Relationship of osteopontin and renal function with severity of coronary artery lesions. Int. J. Clin. Exp. Med. 2014, 7, 1122–1127. [Google Scholar]
- Lorenzen, J.; Krämer, R.; Kliem, V.; Bode-Boeger, S.M.; Veldink, H.; Haller, H.; Fliser, D.; Kielstein, J.T. Circulating levels of osteopontin are closely related to glomerular filtration rate and cardiovascular risk markers in patients with chronic kidney disease. Eur. J. Clin. Investig. 2010, 40, 294–300. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Bae, N.; Almeida, M.; Denhardt, D.T.; Alpers, C.E.; Schwartz, S.M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J. Clin. Investig. 1993, 92, 1686–1696. [Google Scholar] [CrossRef] [Green Version]
- Steitz, S.A.; Speer, M.Y.; Curinga, G.; Yang, H.Y.; Haynes, P.; Aebersold, R.; Schinke, T.; Karsenty, G.; Giachelli, C.M. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001, 89, 1147–1154. [Google Scholar] [CrossRef]
- Maniatis, K.; Siasos, G.; Oikonomou, E.; Vavuranakis, M.; Zaromytidou, M.; Mourouzis, K.; Paraskevopoulos, T.; Charalambous, G.; Papavassiliou, A.G.; Tousoulis, D. Osteoprotegerin and Osteopontin Serum Levels are Associated with Vascular Function and Inflammation in Coronary Artery Disease Patients. Curr. Vasc. Pharmacol. 2020, 18, 523–530. [Google Scholar] [CrossRef]
- Rossi, S.H.; McQuarrie, E.P.; Miller, W.H.; MacKenzie, R.M.; A Dymott, J.; Moreno, M.U.; Taurino, C.; Miller, A.M.; Neisius, U.; Berg, G.A.; et al. Impaired renal function impacts negatively on vascular stiffness in patients with coronary artery disease. BMC Nephrol. 2013, 14, 173. [Google Scholar] [CrossRef] [Green Version]
- Tousoulis, D.; Siasos, G.; Maniatis, K.; Oikonomou, E.; Kioufis, S.; Zaromitidou, M.; Paraskevopoulos, T.; Michalea, S.; Kollia, C.; Miliou, A.; et al. Serum osteoprotegerin and osteopontin levels are associated with arterial stiffness and the presence and severity of coronary artery disease. Int. J. Cardiol. 2013, 167, 1924–1928. [Google Scholar] [CrossRef]
- Csiky, B.; Sági, B.; Peti, A.; Lakatos, O.; Prémusz, V.; Sulyok, E. The Impact of Osteocalcin, Osteoprotegerin and Osteopontin on Arterial Stiffness in Chronic Renal Failure Patients on Hemodialysis. Kidney Blood Press. Res. 2017, 42, 1312–1321. [Google Scholar] [CrossRef]
- Hsu, B.G.; Liou, H.H.; Lee, C.J.; Chen, Y.C.; Ho, G.J.; Lee, M.C. Serum sclerostin as an independent marker of peripheral arterial stiffness in renal transplantation recipients: A cross-sectional study. Medicine 2016, 95, e3300. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Lioufas, N.; Hawley, C.M.; Cameron, J.; Toussaint, N.D. Chronic Kidney Disease and Pulse Wave Velocity: A Narrative Review. Int. J. Hypertens. 2019, 2019, 9189362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- London, G.M.; Safar, M.E.; Pannier, B. Aortic Aging in ESRD: Structural, Hemodynamic, and Mortality Implications. J. Am. Soc. Nephrol. 2015, 27, 1837–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claes, K.J.; Viaene, L.; Heye, S.; Meijers, B.; D’Haese, P.; Evenepoel, P. Sclerostin: Another Vascular Calcification Inhibitor? J. Clin. Endocrinol. Metab. 2013, 98, 3221–3228. [Google Scholar] [CrossRef]
- Jean, G.; Chazot, C.; Bresson, E.; Zaoui, E.; Cavalier, E. High Serum Sclerostin Levels Are Associated with a Better Outcome in Haemodialysis Patients. Nephron Exp. Nephrol. 2016, 132, 181–190. [Google Scholar] [CrossRef]
- Grantham, C.E.; Hull, K.L.; Graham-Brown, M.; March, D.; Burton, J.O. The Potential Cardiovascular Benefits of Low-Glucose Degradation Product, Biocompatible Peritoneal Dialysis Fluids: A Review of the Literature. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2017, 37, 375–383. [Google Scholar] [CrossRef]
- Lu, Q.; Cheng, L.-T.; Wang, T.; Wan, J.; Liao, L.-L.; Zeng, J.; Qin, C.; Li, K.-J. Visceral Fat, Arterial Stiffness, and Endothelial Function in Peritoneal Dialysis Patients. J. Ren. Nutr. 2008, 18, 495–502. [Google Scholar] [CrossRef]
- Hsu, B.-G.; Chi, P.-J.; Lin, Y.-L.; Tasi, J.-P.; Wang, C.-H.; Hou, J.-S.; Lee, C.-J. Osteocalcin and carotid–femoral pulse wave velocity in patients on peritoneal dialysis. Tzu Chi Med. J. 2019, 31, 23. [Google Scholar] [CrossRef]
- Cho, H.-J.; Kim, H.-S. Osteopontin: A multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr. Atheroscler. Rep. 2009, 11, 206–213. [Google Scholar] [CrossRef]
- Wolak, T. Osteopontin—A multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis 2014, 236, 327–337. [Google Scholar] [CrossRef]
- Xie, Y.; Sakatsume, M.; Nishi, S.; Narita, I.; Arakawa, M.; Gejyo, F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001, 60, 1645–1657. [Google Scholar] [CrossRef] [Green Version]
- Filardi, T.; Carnevale, V.; Massoud, R.; Russo, C.; Nieddu, L.; Tavaglione, F.; Turinese, I.; Lenzi, A.; Romagnoli, E.; Morano, S. High serum osteopontin levels are associated with prevalent fractures and worse lipid profile in post-menopausal women with type 2 diabetes. J. Endocrinol. Investig. 2019, 42, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Barchetta, I.; Ceccarelli, V.; Cimini, F.A.; Bertoccini, L.; Fraioli, A.; Alessandri, C.; Lenzi, A.; Baroni, M.G.; Cavallo, M.G. Impaired bone matrix glycoprotein pattern is associated with increased cardio-metabolic risk profile in patients with type 2 diabetes mellitus. J. Endocrinol. Investig. 2019, 42, 513–520. [Google Scholar] [CrossRef]
- Abdalrhim, A.D.; Marroush, T.S.; Austin, E.E.; Gersh, B.J.; Solak, N.; Rizvi, S.A.; Bailey, K.R.; Kullo, I.J. Plasma Osteopontin Levels and Adverse Cardiovascular Outcomes in the PEACE Trial. PLoS ONE 2016, 11, e0156965. [Google Scholar] [CrossRef]
- Lin, J.-F.; Wu, S.; Juang, J.-M.J.; Chiang, F.-T.; Hsu, L.-A.; Teng, M.-S.; Cheng, S.-T.; Huang, H.-L.; Ko, Y.-L. Osteoprotegerin and osteopontin levels, but not gene polymorphisms, predict mortality in cardiovascular diseases. Biomark. Med. 2019, 13, 751–760. [Google Scholar] [CrossRef]
- Speer, M.Y.; McKee, M.D.; Guldberg, R.E.; Liaw, L.; Yang, H.Y.; Tung, E.; Karsenty, G.; Giachelli, C.M. Inactivation of the osteopontin gene enhances vascular calcification of matrix gla protein-deficient mice: Evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J. Exp. Med. 2002, 196, 1047–1055. [Google Scholar] [CrossRef]
- Sharif, S.; Bots, M.; Schalkwijk, C.; Stehouwer, C.; Visseren, F.; Westerink, J. Association between bone metabolism regulators and arterial stiffness in type 2 diabetes patients. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1245–1252. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Towler, D.; Slatopolsky, E. Vascular Calcification: The Killer of Patients with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2009, 20, 1453–1464. [Google Scholar] [CrossRef] [Green Version]

| Characteristic | All Participants (n = 70) | Control Group (n = 48) | Aortic Stiffness Group (n = 22) | p Value |
|---|---|---|---|---|
| Age (years) | 56.86 ± 15.11 | 52.96 ± 15.20 | 65.36 ± 11.10 | 0.001 * |
| Peritoneal dialysis vintage (months) | 50.26 ± 40.87 | 42.88 ± 38.93 | 66.36 ± 41.22 | 0.024 * |
| Height (cm) | 159.89 ± 8.58 | 160.71 ± 8.60 | 158.09 ± 8.47 | 0.239 |
| Body weight (kg) | 63.35 ± 13.64 | 63.50 ± 12.55 | 63.03 ± 16.07 | 0.895 |
| Body mass index (kg/m2) | 24.66 ± 4.14 | 24.57 ± 4.08 | 24.86 ± 4.41 | 0.785 |
| Carotid-femoral PWV (m/s) | 9.08 ± 3.15 | 7.38 ± 1.84 | 12.80 ± 1.95 | <0.001 * |
| Systolic blood pressure (mmHg) | 142.33 ± 23.69 | 140.31 ± 25.13 | 146.73 ± 20.01 | 0.296 |
| Diastolic blood pressure (mmHg) | 84.64 ± 12.94 | 82.96 ± 13.45 | 88.32 ± 11.16 | 0.108 |
| Total cholesterol (mg/dL) | 167.53 ± 38.47 | 164.35 ± 36.51 | 174.45 ± 42.49 | 0.311 |
| Triglyceride (mg/dL) | 178.66 ± 115.53 | 184.13 ± 129.86 | 166.73 ± 76.77 | 0.562 |
| Fasting glucose (mg/dL) | 106.00 (95.00–131.50) | 103.50 (93.25–124.25) | 114.00 (96.75–149.00) | 0.086 |
| Albumin (g/dL) | 3.73 ± 0.35 | 3.74 ± 0.38 | 3.70 ± 0.28 | 0.662 |
| Blood urea nitrogen (mg/dL) | 57.77 ± 18.27 | 58.17 ± 18.92 | 56.91 ± 17.16 | 0.791 |
| Creatinine (mg/dL) | 11.00 ± 3.01 | 11.07 ± 3.12 | 10.83 ± 2.85 | 0.756 |
| Total calcium (mg/dL) | 9.11 ± 0.79 | 9.08 ± 0.73 | 9.18 ± 0.90 | 0.617 |
| Phosphorus (mg/dL) | 5.21 ± 1.40 | 5.26 ± 1.42 | 5.10 ± 1.38 | 0.673 |
| Intact parathyroid hormone (pg/mL) | 248.63 (113.37–522.48) | 257.35 (121.64–546.23) | 221.77 (87.36–549.23) | 0.899 |
| C reactive protein (mg/dL) | 0.26 (0.07–0.73) | 0.14 (0.06–0.52) | 0.32 (0.25–1.18) | 0.005 * |
| Osteopontin (ng/mL) | 20.36 (9.85–56.95) | 13.36 (7.43–24.36) | 74.53 (41.77–141.66) | <0.001 * |
| Weekly Kt/V | 2.10 ± 0.39 | 2.15 ± 0.45 | 1.95 ± 0.33 | 0.070 |
| Peritoneal Kt/V | 1.74 ± 0.48 | 1.76 ± 0.46 | 1.71 ± 0.42 | 0.654 |
| Total clearance of creatinine (L/week) | 59.89 ± 24.85 | 61.54 ± 25.66 | 55.09 ± 20.20 | 0.308 |
| Peritoneal clearance of creatinine (L/week) | 41.15 ± 16.16 | 42.33 ± 16.88 | 42.35 ± 15.35 | 0.995 |
| Female, n (%) | 40 (57.1) | 28 (58.3) | 12 (54.5) | 0.766 |
| Diabetes, n (%) | 31 (44.3) | 22 (45.8) | 9 (40.9) | 0.700 |
| Hypertension, n (%) | 58 (82.9) | 39 (81.3) | 19 (86.4) | 0.598 |
| CAPD, n (%) | 50 (71.4) | 33 (68.8) | 17 (77.3) | 0.464 |
| ACE inhibitor use, n (%) | 5 (7.1) | 4 (8.3) | 1 (4.5) | 0.568 |
| ARB use, n (%) | 28 (40.0) | 19 (39.6) | 9 (40.9) | 0.916 |
| β-blocker use, n (%) | 27 (38.6) | 20 (41.7) | 7 (31.8) | 0.432 |
| CCB use, n (%) | 30 (42.9) | 20 (41.7) | 10 (45.5) | 0.766 |
| Statin use, n (%) | 22 (31.4) | 16 (33.3) | 6 (27.3) | 0.612 |
| Fibrate use, n (%) | 2 (2.9) | 1 (2.1) | 1 (4.5) | 0.566 |
| Calcium carbonate use, n (%) | 37 (52.9) | 27 (56.3) | 10 (45.5) | 0.401 |
| Calcitriol use, n (%) | 20 (28.6) | 14 (29.2) | 6 (27.3) | 0.871 |
| Icodextrin, n (%) | 48 (68.6) | 32 (66.7) | 16 (72.7) | 0.612 |
| Variables | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
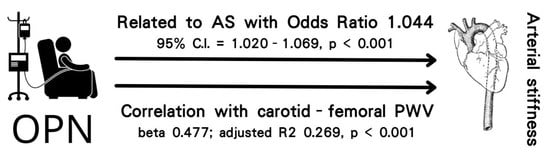
| Osteopontin, 1 ng/mL | 1.044 | 1.020–1.069 | <0.001 * |
| Peritoneal dialysis vintage, 1 month | 1.027 | 1.007–1.047 | 0.009 * |
| Age, 1 year | 1.063 | 0.983–1.150 | 0.127 |
| C reactive protein, 1 mg/dL | 1.762 | 0.720–4.313 | 0.215 |
| Variables | Central PWV (m/s) | ||||
|---|---|---|---|---|---|
| Simple Linear Regression | Multivariate Linear Regression | ||||
| r | p Value | Beta | Adjusted R2 Change | p Value | |
| Female | −0.071 | 0.557 | – | – | – |
| Diabetes mellitus | 0.016 | 0.896 | – | – | – |
| Hypertension | −0.083 | 0.497 | – | – | – |
| Age (years) | 0.394 | 0.001 * | – | – | – |
| Peritoneal dialysis vintage (months) | 0.221 | 0.066 | – | – | – |
| Height (cm) | −0.034 | 0.782 | – | – | – |
| Body weight (kg) | 0.038 | 0.754 | – | – | – |
| Body mass index (kg/m2) | 0.010 | 0.932 | – | – | – |
| Systolic blood pressure (mmHg) | 0.038 | 0.752 | – | – | – |
| Diastolic blood pressure (mmHg) | 0.037 | 0.762 | – | – | – |
| Total cholesterol (mg/dL) | 0.105 | 0.387 | – | – | – |
| Triglyceride (mg/dL) | −0.002 | 0.984 | – | – | – |
| Log-Glucose (mg/dL) | 0.095 | 0.435 | – | – | – |
| Albumin (g/dL) | −0.069 | 0.573 | – | – | – |
| Blood urea nitrogen (mg/dL) | 0.047 | 0.697 | – | – | – |
| Creatinine (mg/dL) | −0.099 | 0.415 | – | – | – |
| Total calcium (mg/dL) | 0.051 | 0.674 | – | – | – |
| Phosphorus (mg/dL) | −0.105 | 0.385 | – | – | – |
| Log-iPTH (pg/mL) | 0.004 | 0.971 | – | – | – |
| Log-CRP (mg/dL) | 0.375 | 0.001 * | 0.290 | 0.073 | 0.005 * |
| Log-Osteopontin (ng/mL) | 0.529 | <0.001 * | 0.477 | 0.269 | <0.001 * |
| Weekly Kt/V | −0.168 | 0.164 | – | – | – |
| Peritoneal Kt/V | −0.199 | 0.098 | – | – | – |
| Total clearance of creatinine (L/week) | 0.056 | 0.648 | – | – | – |
| Peritoneal clearance of creatinine (L/week) | −0.073 | 0.547 | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-H.; Wang, C.-H.; Hsu, B.-G.; Tsai, J.-P. Serum Osteopontin Level Is Positively Associated with Aortic Stiffness in Patients with Peritoneal Dialysis. Life 2022, 12, 397. https://doi.org/10.3390/life12030397
Chang K-H, Wang C-H, Hsu B-G, Tsai J-P. Serum Osteopontin Level Is Positively Associated with Aortic Stiffness in Patients with Peritoneal Dialysis. Life. 2022; 12(3):397. https://doi.org/10.3390/life12030397
Chicago/Turabian StyleChang, Kai-Hsiang, Chih-Hsien Wang, Bang-Gee Hsu, and Jen-Pi Tsai. 2022. "Serum Osteopontin Level Is Positively Associated with Aortic Stiffness in Patients with Peritoneal Dialysis" Life 12, no. 3: 397. https://doi.org/10.3390/life12030397
APA StyleChang, K.-H., Wang, C.-H., Hsu, B.-G., & Tsai, J.-P. (2022). Serum Osteopontin Level Is Positively Associated with Aortic Stiffness in Patients with Peritoneal Dialysis. Life, 12(3), 397. https://doi.org/10.3390/life12030397