B-Cell Activating Factor Increases Related to Adiposity, Insulin Resistance, and Endothelial Dysfunction in Overweight and Obese Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Anthropometric Measurements
2.3. Hematic and Biochemical Analyses; Leucoglycemic Index Calculation
2.4. Quantification of Serum BAFF, Insulin, Leptin, and Von Willebrand Factor
2.5. Quantification of Pro- and Anti-Inflammatory Cytokines
2.6. Statistical Analysis
3. Results
3.1. Characterization of the Metabolic, Inflammatory, and Endothelial Markers of Study Subjects
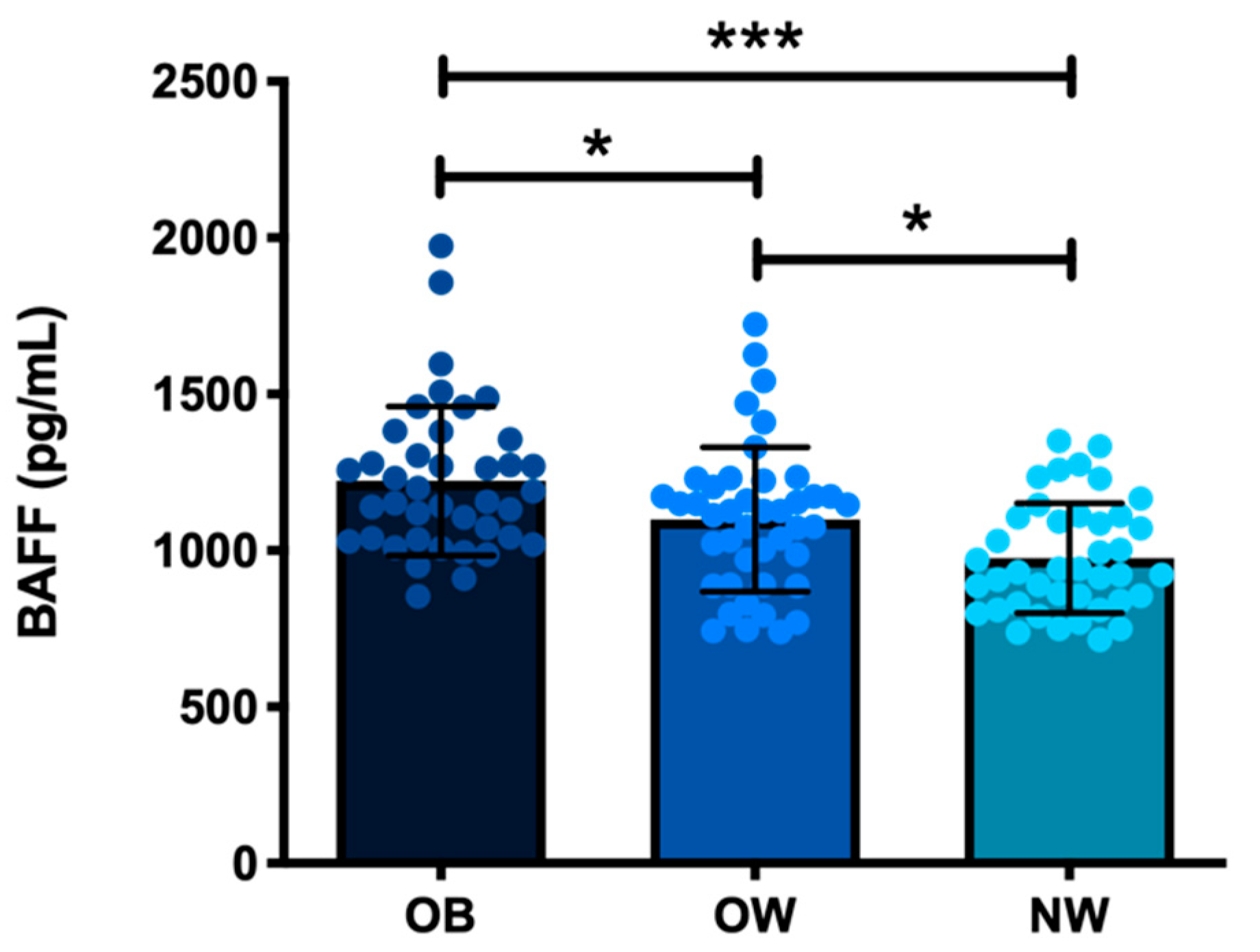
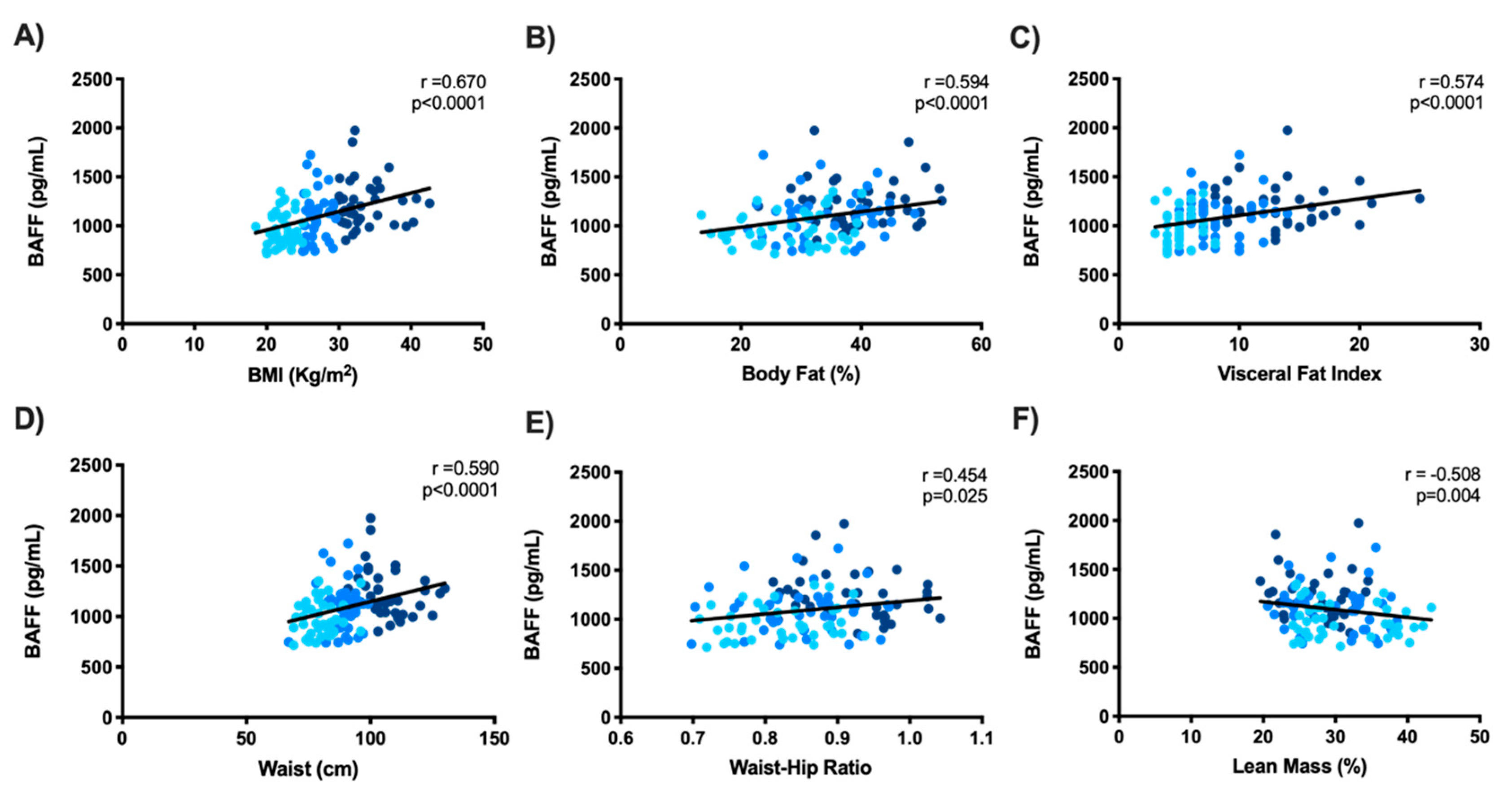
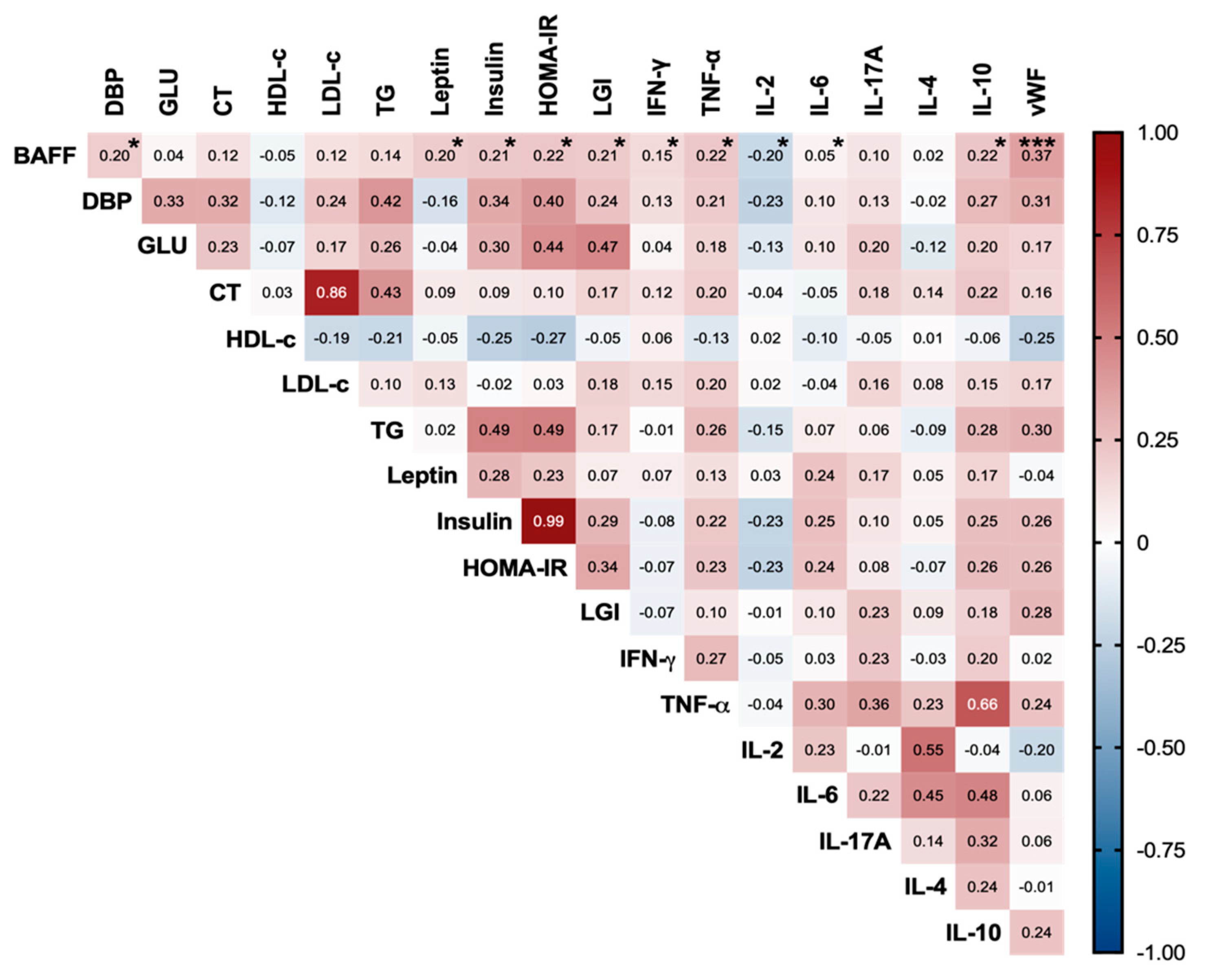
3.2. Serum BAFF’s Relationship with Metabolic, Inflammatory, and Endothelial Markers in the Study Subjects
4. Discussion
4.1. Low-Grade Systemic Inflammation, Insulin Resistance, and Endothelial Dysfunction in OB and OW Study Subjects
4.2. Increased Serum BAFF Related to Adiposity, Insulin Resistance, Systemic Inflammation, and Endothelial Dysfunction in the Study Subjects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grosso, G. Obesity during COVID-19: An underrated pandemic? eClinicalMedicine 2021, 39, 101062. [Google Scholar] [CrossRef]
- Barquera, S.; Hernandez-Barrera, L.; Trejo-Valdivia, B.; Shamah, T.; Campos-Nonato, I.; Rivera-Dommarco, J. Obesity in Mexico, prevalence andtrends in adults. Ensanut 2018-19. Salud Public. Mex. 2020, 62, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojta, I.; Chacinska, M.; Blachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.; Gorwood, J.; Olivo, A.; Le Pelletier, L.; Capeau, J.; Lambotte, O.; Bereziat, V.; Lagathu, C. Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated with HIV Infection and Its Treatment. Front. Immunol. 2021, 12, 670566. [Google Scholar] [CrossRef]
- Wernstedt Asterholm, I.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar] [CrossRef] [Green Version]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazzaa, H.M.; Albawardi, N.M. Obesity, Lifestyle Behaviors, and Dietary Habits of Saudi Adolescents Living in Riyadh (ATLS-2 Project): Revisited after a Ten-Year Period. Life 2021, 11, 1078. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, E.; Romani, A.; Marrone, G.; Di Lauro, M.; Cerulli, C.; Urciuoli, S.; Murri, A.; Guerriero, C.; Tranchita, E.; Tesauro, M.; et al. Impact of Physical Activity and Natural Bioactive Compounds on Endothelial Dysfunction in Chronic Kidney Disease. Life 2021, 11, 841. [Google Scholar] [CrossRef]
- Sun, Y.; Rawish, E.; Nording, H.M.; Langer, H.F. Inflammation in Metabolic and Cardiovascular Disorders-Role of Oxidative Stress. Life 2021, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Lejawa, M.; Osadnik, K.; Czuba, Z.; Osadnik, T.; Pawlas, N. Association of Metabolically Healthy and Unhealthy Obesity Phenotype with Markers Related to Obesity, Diabetes among Young, Healthy Adult Men. Analysis of MAGNETIC Study. Life 2021, 11, 1350. [Google Scholar] [CrossRef] [PubMed]
- Do, M.S.; Joing, H.S.; Choi, B.H.; Hunter, L.; Langley, S.; Pazmany, L.; Trayhurn, P. Inflammatory Gene Expression Patterns Revealed by DNA Microarray Analysis in TNF-α-treated SGBS Human Adipocytes. Yonsei Med. J. 2006, 47, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Pelekanou, V.; Kampa, M.; Kafousi, M.; Darivianaki, K.; Sanidas, E.; Tsiftsis, D.D.; Stathopoulos, E.N.; Tsapis, A.; Castanas, E. Expression of TNF-superfamily members BAFF and APRIL in breast cancer: Immunohistochemical study in 52 invasive ductal breast carcinomas. BMC Cancer 2008, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, B.H.; Cheon, H.G.; Do, M.S. B cell activation factor (BAFF) is a novel adipokine that links obesity and inflammation. Exp. Mol. Med. 2009, 41, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Alexaki, V.I.; Notas, G.; Pelekanou, V.; Kampa, M.; Valkanou, M.; Theodoropoulos, P.; Stathopoulos, E.N.; Tsapis, A.; Castanas, E. Adipocytes as immune cells: Differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J. Immunol. 2009, 183, 5948–5956. [Google Scholar] [CrossRef] [Green Version]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9, 2285. [Google Scholar] [CrossRef]
- Mackay, F.; Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 2009, 9, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Rowland, S.L.; Leahy, K.F.; Halverson, R.; Torres, R.M.; Pelanda, R. BAFF receptor signaling aids the differentiation of immature B cells into transitional B cells following tonic BCR signaling. J. Immunol. 2010, 185, 4570–4581. [Google Scholar] [CrossRef] [Green Version]
- Moisini, I.; Davidson, A. BAFF: A local and systemic target in autoimmune diseases. Clin. Exp. Immunol. 2009, 158, 155–163. [Google Scholar] [CrossRef]
- Szodoray, P.; Alex, P.; Jonsson, M.V.; Knowlton, N.; Dozmorov, I.; Nakken, B.; Delaleu, N.; Jonsson, R.; Centola, M. Distinct profiles of Sjogren’s syndrome patients with ectopic salivary gland germinal centers revealed by serum cytokines and BAFF. Clin. Immunol. 2005, 117, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Ballesteros, F.J.; Palafox-Sanchez, C.A.; Franco-Topete, R.A.; Munoz-Valle, J.F.; Orozco-Barocio, G.; Martinez-Bonilla, G.E.; Gomez-Lopez, C.E.; Marin-Rosales, M.; Lopez-Villalobos, E.F.; Luquin, S.; et al. Expression of BAFF and BAFF receptors in primary Sjogren’s syndrome patients with ectopic germinal center-like structures. Clin. Exp. Med. 2020, 20, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.B.; Kandane-Rathnayake, R.; Koelmeyer, R.; Hoi, A.Y.; Harris, J.; Mackay, F.; Morand, E.F. Analysis of serum B cell-activating factor from the tumor necrosis factor family (BAFF) and its soluble receptors in systemic lupus erythematosus. Clin. Transl. Immunol. 2019, 8, e01047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienertova-Vasku, J.; Bienert, P.; Zlamal, F.; Tomandl, J.; Forejt, M.; Tomandlova, M.; Vavrina, M.; Kudelková, J.; Splichal, Z.; Vasku, A. B-cell activating factor (BAFF)—A new factor linking immunity to diet? Open Med. 2012, 7, 275–283. [Google Scholar] [CrossRef]
- Miyake, T.; Abe, M.; Tokumoto, Y.; Hirooka, M.; Furukawa, S.; Kumagi, T.; Hamada, M.; Kawasaki, K.; Tada, F.; Ueda, T.; et al. B cell-activating factor is associated with the histological severity of nonalcoholic fatty liver disease. Hepatol. Int. 2013, 7, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Obesity: Preventing and Managing the Global Epidemic. In Report of a WHO Consultation; WHO: Geneva, Switzerland, 2000; pp. 1–253.
- Talavera, J.O.; Rivas-Ruiz, R.; Bernal-Rosales, L.P. Investigación clínica V. Tamaño de muestra. Rev. Med. Inst. Mex. Seguro. Soc. 2011, 49, 517–522. [Google Scholar] [PubMed]
- Quiroga-Castro, W.C.E.; Zelaya, F.; Isa, M.; Pacheco, G.; Sala, J.; Carlos, B. Estratificación del riesgo en el infarto agudo de miocardio según el índice leucoglucémico ¿El «Killip-Kimball» de laboratorio? Rev. Fed. Arg. Cardiol. 2010, 39, 29–34. [Google Scholar]
- Lelas, A.; Greinix, H.T.; Wolff, D.; Eissner, G.; Pavletic, S.Z.; Pulanic, D. Von Willebrand Factor, Factor VIII, and Other Acute Phase Reactants as Biomarkers of Inflammation and Endothelial Dysfunction in Chronic Graft-Versus-Host Disease. Front. Immunol. 2021, 12, 676756. [Google Scholar] [CrossRef]
- Chermiti, I.; Ghazal, H.; Dimassi, R.; Mahdhoui, S.; Gnena, H.; Chiboub, S.; Chkir, M.; Souissi, S. Prognostic Value of Leuko-glycemic Index in ST Segment Elevation Myocardial Infarction. J. Emerg. Med. 2017, 53, 435. [Google Scholar] [CrossRef]
- Leon-Aliz, E.; Moreno-Martinez, F.L.; Perez-Fernandez, G.A.; Vega-Fleites, L.F.; Rabassa-Lopez-Calleja, M.A. Leuko-glycemic index as an in-hospital prognostic marker in patients with ST-segment elevation myocardial infarction. Clin. Investig. Arterioscler. 2014, 26, 168–175. [Google Scholar]
- Saldaña-Martínez, A.; Mendoza, R.M.; López-González, A. Índice leucoglucémico como predictor de complicaciones en el síndrome coronario agudo. Med. Crit. 2018, 32, 27–33. [Google Scholar]
- Hernández-Sánchez, M.M.; Lozano-Nuevo, J.J.; Suárez-Cuenca, J.A.; Mendoza-Portillo, E.; Vergara-Orduña, F.I.; Palacios-Pérez, E.; González-Moreno, F.J.; Villanueva-Domínguez, J. Índice leuco-glucémico asociado con complicaciones en isquemia cerebral aterotrombótica. Med. Inter. Méx. 2019, 35, 39–44. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo-Salgado, A.L.F.; de Carvalho, L.; Oliveira, A.C.; Dos Santos, V.N.; Vieira, J.G.; Parise, E.R. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq. Gastroenterol. 2010, 47, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Bello-Chavolla, O.Y.; Vargas-Vazquez, A.; Antonio-Villa, N.E.; Del Razo-Olvera, F.M.; Elias-Lopez, D.; Aguilar-Salinas, C.A.; Metabolic Syndrome Study Group. A High Incidence of Metabolic Syndrome Traits in Mexicans Points at Obesity-Related Metabolic Dysfunction. Diabetes Metab. Syndr. Obes. 2021, 14, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Márquez, R.; Aguilar-Galarza, B.A.; Anaya-Loyola, M.A.; Moreno-Celis, U.; Elton-Puente, J.E.; Lloret, M.; Sosa-Ferreyra, C.F.; García-Gasca, T. Metabolic Alterations in Mexican Young Adults: Sensitivity and Specificity Approaches between Anthropometric Parameters and Biochemical Markers; Universidad Autónoma de Querétraro: Querétaro, México, 2017; Available online: https://fcn.uaq.mx/index.php/programas/posgrados/mnh/publicaciones-mcnh (accessed on 15 March 2022).
- Aguilar-Salinas, C.A.; Olaiz, G.; Valles, V.; Torres, J.M.R.; Pérez, F.J.G.; Rull, J.A.; Rojas, R.; Franco, A.; Sepulveda, J. High prevalence of low HDL cholesterol concentrations and mixed hyperlipidemia in a Mexican nationwide survey. J. Lipid Res. 2001, 42, 1298–1307. [Google Scholar] [CrossRef]
- Murguia-Romero, M.; Jimenez-Flores, J.R.; Sigrist-Flores, S.C.; Tapia-Pancardo, D.C.; Jimenez-Ramos, A.; Mendez-Cruz, A.R.; Villalobos-Molina, R. Prevalence of Metabolic Syndrome in Young Mexicans: A Sensitivity Analysis on Its Components. Nutr. Hosp. 2015, 32, 189–195. [Google Scholar] [PubMed]
- Moghbeli, M.; Khedmatgozar, H.; Yadegari, M.; Avan, A.; Ferns, G.A.; Ghayour Mobarhan, M. Cytokines and the immune response in obesity-related disorders. Adv. Clin. Chem. 2021, 101, 135–168. [Google Scholar] [PubMed]
- Pou, K.M.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Maurovich-Horvat, P.; Larson, M.G.; Keaney, J.F., Jr.; Meigs, J.B.; Lipinska, I.; Kathiresan, S.; et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation 2007, 116, 1234–1241. [Google Scholar] [CrossRef] [Green Version]
- Rakotoarivelo, V.; Lacraz, G.; Mayhue, M.; Brown, C.; Rottembourg, D.; Fradette, J.; Ilangumaran, S.; Menendez, A.; Langlois, M.F.; Ramanathan, S. Inflammatory Cytokine Profiles in Visceral and Subcutaneous Adipose Tissues of Obese Patients Undergoing Bariatric Surgery Reveal Lack of Correlation with Obesity or Diabetes. eBioMedicine 2018, 30, 237–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzamil, H. Elevated Serum TNF-alpha Is Related to Obesity in Type 2 Diabetes Mellitus and Is Associated with Glycemic Control and Insulin Resistance. J. Obes. 2020, 2020, 5076858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olszanecka-Glinianowicz, M.; Zahorska-Markiewicz, B.; Janowska, J.; Zurakowski, A. Serum concentrations of nitric oxide, tumor necrosis factor (TNF)-alpha and TNF soluble receptors in women with overweight and obesity. Metabolism 2004, 53, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Popko, K.G.E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory Cytokines IL-6 and TNF-α and the Development of Inflammation in Obese Subjects. Eur. J. Med. Res. 2010, 15, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Rosc, D.; Adamczyk, P.; Boinska, J.; Szafkowski, R.; Ponikowska, I.; Stankowska, K.; Goralczyk, B.; Ruszkowska-Ciastek, B. CRP, but not TNF-alpha or IL-6, decreases after weight loss in patients with morbid obesity exposed to intensive weight reduction and balneological treatment. J. Zhejiang Univ. Sci. B 2015, 16, 404–411. [Google Scholar] [CrossRef]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sola, E.; Jover, A.; Lopez-Ruiz, A.; Jarabo, M.; Vaya, A.; Morillas, C.; Gomez-Balaguer, M.; Hernandez-Mijares, A. Parameters of inflammation in morbid obesity: Lack of effect of moderate weight loss. Obes. Surg. 2009, 19, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Fasshauer, M.; Stumvoll, M.; et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef]
- Ferreira-Hermosillo, A.; Molina-Ayala, M.; Ramirez-Renteria, C.; Vargas, G.; Gonzalez, B.; Isibasi, A.; Archundia-Riveros, I.; Mendoza, V. Inflammatory Cytokine Profile Associated with Metabolic Syndrome in Adult Patients with Type 1 Diabetes. J. Diabetes Res. 2015, 2015, 972073. [Google Scholar] [CrossRef] [Green Version]
- Aradillas-García, C.; Monreal-Escalante, E.; Vargas-Morlaes, J.M.; Alegría-Torres, J.; Rosales-Méndoza, S.; Terán-García, M.; Portales-Pérez, D.P. Serum IL-17, obesity, and metabolic risk in Mexican young adults. RESPYN Rev. Salud Pública. Y Nutr. 2021, 20, 1–7. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Madry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.C.; Hsieh, P.S. The Chemokine Systems at the Crossroads of Inflammation and Energy Metabolism in the Development of Obesity. Int. J. Mol. Sci. 2021, 22, 13528. [Google Scholar] [CrossRef] [PubMed]
- Marchini, T.; Mitre, L.S.; Wolf, D. Inflammatory Cell Recruitment in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 635527. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.; Minshall, R.D. Endothelial Cell von Willebrand Factor Secretion in Health and Cardiovascular Disease. In Endothelial Dysfunction—Old Concepts and New Challenges; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/books/6209 (accessed on 21 March 2022).
- Berk, K.A.; Oudshoorn, T.P.; Verhoeven, A.J.M.; Mulder, M.T.; Roks, A.J.M.; Dik, W.A.; Timman, R.; Sijbrands, E.J.G. Diet-induced weight loss and markers of endothelial dysfunction and inflammation in treated patients with type 2 diabetes. Clin. Nutr. ESPEN 2016, 15, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Crocco, M.; d’Annunzio, G.; La Valle, A.; Piccolo, G.; Chiarenza, D.S.; Bigatti, C.; Molteni, M.; Milanaccio, C.; Garre, M.L.; Di Iorgi, N.; et al. Endothelial Dysfunction in Childhood Cancer Survivors: A Narrative Review. Life 2021, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Atiq, F.; Fijnvandraat, K.; van Galen, K.P.M.; Laros-Van Gorkom, B.A.P.; Meijer, K.; de Meris, J.; Coppens, M.; Mauser-Bunschoten, E.P.; Cnossen, M.H.; van der Bom, J.G.; et al. BMI is an important determinant of VWF and FVIII levels and bleeding phenotype in patients with von Willebrand disease. Am. J. Hematol. 2019, 94, E201–E205. [Google Scholar] [CrossRef] [Green Version]
- Mertens, I.V.G.; Van Gaal, L.F. Obesity, haemostasis and the fibrinolytic system. Obes. Rev. 2002, 3, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.; Harley, I.T.W.; Pfluger, P.T.; Trompette, A.; Stankiewicz, T.E.; Allen, J.L.; Moreno-Fernandez, M.E.; Damen, M.; Oates, J.R.; Alarcon, P.C.; et al. A BAFF/APRIL axis regulates obesogenic diet-driven weight gain. Nat. Commun. 2021, 12, 2911. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Abe, M.; Miyake, T.; Kawasaki, K.; Tada, F.; Furukawa, S.; Matsuura, B.; Hiasa, Y.; Onji, M. B cell-activating factor controls the production of adipokines and induces insulin resistance. Obesity 2011, 19, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Tada, F.A.M.; Kawasaki, K.; Miyake, T.; Shiyi, C.; Hiasa, Y.; Matsura, B.; Onji, M. B cell activating factor in obesity is regulated by oxidative stress in adipocytes. J. Clin. Biochem. Nutr. 2013, 52, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiantoulas, D.; Sage, A.P.; Goderle, L.; Ozsvar-Kozma, M.; Murphy, D.; Porsch, F.; Pasterkamp, G.; Menche, J.; Schneider, P.; Mallat, Z.; et al. B Cell-Activating Factor Neutralization Aggravates Atherosclerosis. Circulation 2018, 138, 2263–2273. [Google Scholar] [CrossRef] [PubMed]

| Obese n = 41 | Overweight n = 43 | Normal Weight n = 40 | p | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 35.5 ± 9.0 | 35.2 ± 9.0 | 33.8 ± 9.5 | 0.692 |
| SBP (mmHg) | 116.5 ± 8.8 ‡ | 110.5 ± 11.5 ϑ | 105.1 ± 10.4 | 0.0001 Φ |
| DBP (mmHg) | 77.9 ± 6.4 ‡ | 73.1 ± 9.2 ϑ | 68.1 ± 6.6 θ | 0.0001 Φ |
| Anthropometric measurements | ||||
| Weight (kg) | 96.9 ± 15.3 ‡ | 73.7 ± 8.0 ϑ | 61.5 ± 7.9 θ | 0.0001 Φ |
| Height (m) | 1.7 ± 0.09 | 1.7 ± 0.08 ϑ | 1.7 ± 0.08 | 0.037 * |
| BMI (kg/m2) | 33.5 ± 3.3 ‡ | 26.9 ± 1.4 ϑ | 22.3 ± 1.6 θ | 0.0001 Φ |
| Body fat (%) | 39.6 ± 7.4 ‡ | 34.4 ± 7.2 ϑ | 28.5 ± 7.4 θ | 0.0001 * |
| Lean mass (%) | 27.3 ± 4.3 ‡ | 29.5 ± 5.3 | 30.1 ± 6.2 | 0.012 * |
| Visceral fat index | 13.0 ± 4.5 ‡ | 8.5 ± 2.4 ϑ | 5.0 ± 1.3 θ | 0.0001 Φ |
| Waist (cm) | 106.6 ± 10.1 ‡ | 89.1 ± 5.9 ϑ | 79.6 ± 6.4 θ | 0.0001 * |
| WHR | 0.91 ± 0.07 ‡ | 0.85 ± 0.07 ϑ | 0.82 ± 0.06 | 0.0001 * |
| Biochemical parameters | ||||
| Glucose (mg/dL) | 93.7 ± 9.4 ‡ | 89.9 ± 8.5 | 85.9 ± 9.8 | 0.001 Φ |
| Cholesterol (mg/dL) | 182.0 ± 33.5 | 175.9 ± 32.6 | 164.5 ± 26.7 | 0.037 * |
| HDL-c (mg/dL) | 41.7 ± 7.1 ‡ | 44.4 ± 8.4 | 48.9 ± 10.2 | 0.001 * |
| LDL-c (mg/dL) | 110.9 ± 33.5 | 103.3 ± 31.2 | 97.8 ± 22.8 | 0.14 |
| Triglycerides (mg/dL) | 157.7 ± 58.5 ‡ | 140.8 ± 94.8 | 86.6 ± 42.5 θ | 0.0001 Φ |
| Hormones | ||||
| Leptin (ng/mL) | 11.3 ± 7.7 ‡ | 6.3 ± 7.3 ϑ | 7.6 ± 6.3 | 0.0001 Φ |
| Insulin (µUI/mL) | 27.0 ± 16.2 ‡ | 15.6 ± 11.0 ϑ | 8.9 ± 5.2 θ | 0.0001 Φ |
| HOMA-IR | 6.4 ± 4.1 ‡ | 3.6 ± 2.7 ϑ | 1.9 ± 1.1 θ | 0.0001 Φ |
| Hematic biometry parameters | ||||
| Leukocytes (103/µL) | 7.5 ± 1.6 ‡ | 7.1 ± 1.9 | 6.2 ± 1.2 | 0.025 Φ |
| Neutrophils (103/µL) | 4.2 ± 1.1 | 4.1 ± 1.6 | 3.5 ± 0.9 | 0.061 |
| Lymphocytes (103/µL) | 2.5 ± 0.7 | 2.3 ± 0.7 | 2.1 ± 0.6 | 0.115 |
| Monocytes (103/µL) | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.4 ± 0.1 | 0.132 |
| Eosinophils (103/µL) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.072 |
| Basophils (103/µL) | 0.05 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.081 |
| Platelets (103/µL) | 277.4 ± 62.5 | 274.5 ± 68.2 | 286.1 ± 72.1 | 0.794 |
| Obese n (%) | Overweight n (%) | Normal Weight n (%) | |
|---|---|---|---|
| Fasting glucose (≥100 mg/dL) | 11 (28%) | 4 (9%) | 0 (0%) |
| Triglycerides (≥150 mg/dL) | 20 (50%) | 11 (26%) | 4 (10%) |
| HDL-c (<40 mg/dL—men) (<50 mg/dL—women) | 23 (61%) | 18 (42%) | 16 (39%) |
| Blood pressure (≥130 mmHg—systolic) (≥85 mmHg—diastolic) | 3 (9%) | 5 (12%) | 0 (0%) |
| Waist circumference (≥102 cm—men) (≥88 cm—women) | 36 (95%) | 13 (31%) | 3 (8%) |
| Metabolic Syndrome (>3 above criteria) | 19 (49%) | 6 (14%) | 0 (0%) |
| Insulin Resistance (≥2.5 HOMA-IR) | 37 (89%) | 20 (47%) | 5 (14%) |
| Obese n = 41 | Overweight n = 43 | Normal Weight n = 40 | p | |
|---|---|---|---|---|
| Inflammatory indices | ||||
| LGI | 702.0 ±175.3 ‡ | 651.3 ± 184.5 | 548.3 ± 110.1 θ | 0.002 Φ |
| Pro-inflammatory cytokines | ||||
| IFN-γ (pg/mL) | 0.86 ± 0.06 | 0.86 ± 0.10 | 0.85 ± 0.07 | 0.217 |
| TNF-α (pg/mL) | 5.4 ± 1.0 ‡ | 4.8 ± 0.9 ϑ | 4.5 ± 1.0 | 0.001 * |
| IL-2 (pg/mL) | 2.7 ± 1.1 ‡ | 3.1 ± 1.2 | 3.5 ± 1.3 | 0.003 Φ |
| IL-6 (pg/mL) | 5.5 ± 3.0 ‡ | 4.3 ± 1.3 ϑ | 6.5 ± 7.6 | 0.019 Φ |
| IL-17A (pg/mL) | 13.1 ± 10.3 ‡ | 9.2 ± 5.1 ϑ | 8.2 ± 3.1 | 0.010 Φ |
| Anti-inflammatory cytokines | ||||
| IL-4 (pg/mL) | 6.4 ± 2.2 | 6.4 ± 1.3 | 6.5 ± 1.1 | 0.996 |
| IL-10 (pg/mL) | 6.0 ± 4.2 ‡ | 4.7 ± 0.7 ϑ | 4.8 ± 1.9 | 0.0001 Φ |
| Endothelial markers | ||||
| vWF (mU/mL) | 1292.8 ± 366.1 ‡ | 1290.9 ± 435.5 | 527.0 ± 314.4 θ | 0.0001 Φ |
| Variable | β | t | p |
|---|---|---|---|
| Included variables | |||
| BAFF | 0.801 | 2.984 | 0.004 ** |
| HDL-c | −14.181 | −2.409 | 0.018 * |
| Excluded variables | |||
| SBP | 0.086 | 0.774 | 0.441 |
| DBP | 0.080 | 0.717 | 0.476 |
| Cholesterol | −0.007 | −0.064 | 0.949 |
| LDL-c | −0.022 | −0.203 | 0.840 |
| Triglycerides | 0.027 | 0.247 | 0.806 |
| TNF-α | 0.093 | 0.850 | 0.398 |
| IFN-γ | −0.041 | −0.382 | 0.703 |
| IL-6 | −0.124 | −1.140 | 0.258 |
| LGI | 0.163 | 1.522 | 0.132 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, D.C.V.; Castellanos, S.G.; Sandoval, M.E.V.; García, A.G. B-Cell Activating Factor Increases Related to Adiposity, Insulin Resistance, and Endothelial Dysfunction in Overweight and Obese Subjects. Life 2022, 12, 634. https://doi.org/10.3390/life12050634
Sánchez DCV, Castellanos SG, Sandoval MEV, García AG. B-Cell Activating Factor Increases Related to Adiposity, Insulin Resistance, and Endothelial Dysfunction in Overweight and Obese Subjects. Life. 2022; 12(5):634. https://doi.org/10.3390/life12050634
Chicago/Turabian StyleSánchez, Diana Carolina Villalpando, Sergio Gutiérrez Castellanos, Martha Eva Viveros Sandoval, and Anel Gómez García. 2022. "B-Cell Activating Factor Increases Related to Adiposity, Insulin Resistance, and Endothelial Dysfunction in Overweight and Obese Subjects" Life 12, no. 5: 634. https://doi.org/10.3390/life12050634







