Detection of Specific Immune Cell Subpopulation Changes Associated with Systemic Immune Inflammation–Index Level in Germ Cell Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. SII
2.3. Determination of Leukocyte Immunophenotypes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Correlation between the SII Level and Percentage of Different Innate Immune Cells in Chemotherapy-Naïve GCT Patients
3.3. Association between the SII Level and Selected Adaptive Immune Cell Percentages
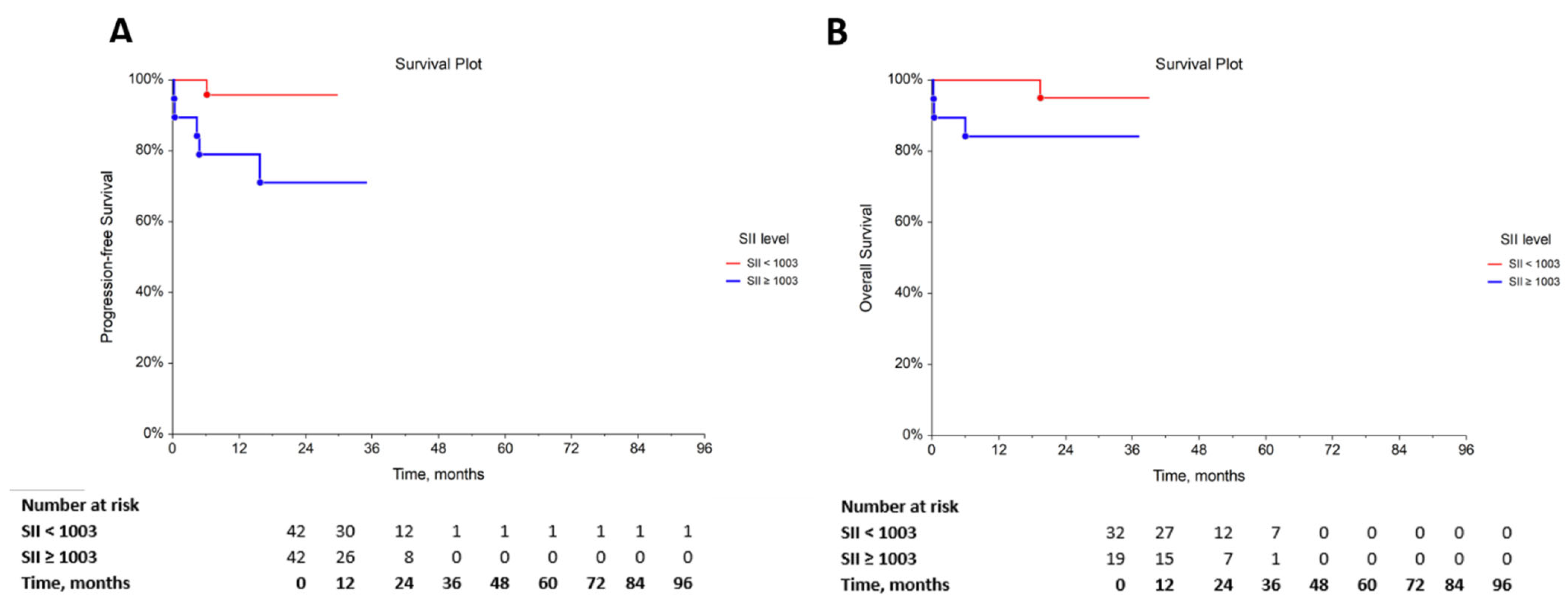
3.4. The Prognostic Role of the SII
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Liu, Q.; Zhu, L.; Zhang, Y.; Lu, X.; Wu, Y.; Liu, L. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer. Sci. Rep. 2019, 9, 3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Lippitz, B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013, 14, e218–e228. [Google Scholar] [CrossRef]
- Fankhauser, C.D.; Sander, S.; Roth, L.; Gross, O.; Eberli, D.; Sulser, T.; Seifert, B.; Beyer, J.; Hermanns, T. Systemic inflammatory markers have independent prognostic value in patients with metastatic testicular germ cell tumours undergoing first-line chemotherapy. Br. J. Cancer 2018, 118, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [Green Version]
- Murthy, P.; Zenati, M.S.; Al Abbas, A.I.; Rieser, C.J.; Bahary, N.; Lotze, M.T.; Zeh, H.J., 3rd; Zureikat, A.H.; Boone, B.A. Prognostic Value of the Systemic Immune-Inflammation Index (SII) After Neoadjuvant Therapy for Patients with Resected Pancreatic Cancer. Ann. Surg. Oncol. 2020, 27, 898–906. [Google Scholar] [CrossRef]
- Wang, C.; Jin, S.; Xu, S.; Cao, S. High Systemic Immune-Inflammation Index (SII) Represents an Unfavorable Prognostic Factor for Small Cell Lung Cancer Treated with Etoposide and Platinum-Based Chemotherapy. Lung 2020, 198, 405–414. [Google Scholar] [CrossRef]
- Zhang, K.; Hua, Y.Q.; Wang, D.; Chen, L.Y.; Wu, C.J.; Chen, Z.; Liu, L.M.; Chen, H. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J. Transl. Med. 2019, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.W.; Chong, S.Z.; Wong, F.H.; Evrard, M.; Tan, S.M.; Keeble, J.; Kemeny, D.M.; Ng, L.G.; Abastado, J.P.; Angeli, V. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood 2013, 122, 3666–3677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Hafner, M.; Echtenacher, B.; Männel, D.N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999, 59, 1295–1300. [Google Scholar] [PubMed]
- Chen, L.; Zhang, F.; Sheng, X.G.; Zhang, S.Q. Decreased pretreatment lymphocyte/monocyte ratio is associated with poor prognosis in stage Ib1-IIa cervical cancer patients who undergo radical surgery. Onco. Targets Ther. 2015, 8, 1355–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Guo, W.; Cai, S.; Zhang, F.; Shao, F.; Zhang, G.; Liu, T.; Tan, F.; Li, N.; Xue, Q.; et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected esophageal squamous cell carcinoma. J. Cancer 2019, 10, 3188–3196. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Hu, H.; Zhang, W.; Shao, Z. Prognostic value of prognostic nutritional index and systemic immune-inflammation index in patients with osteosarcoma. J. Cell Physiol. 2019, 234, 18408–18414. [Google Scholar] [CrossRef]
- Man, Y.N.; Chen, Y.F. Systemic immune-inflammation index, serum albumin, and fibrinogen impact prognosis in castration-resistant prostate cancer patients treated with first-line docetaxel. Int. Urol. Nephrol. 2019, 51, 2189–2199. [Google Scholar] [CrossRef]
- Mirili, C.; Paydas, S.; Kapukaya, T.K.; Yılmaz, A. Systemic immune-inflammation index predicting survival outcome in patients with classical Hodgkin lymphoma. Biomark Med. 2019, 13, 1565–1575. [Google Scholar] [CrossRef]
- Nie, D.; Gong, H.; Mao, X.; Li, Z. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: A retrospective study. Gynecol. Oncol. 2019, 152, 259–264. [Google Scholar] [CrossRef]
- Wang, D.; Guo, D.; Shi, F.; Zhu, Y.; Li, A.; Kong, L.; Teng, F.; Yu, J. The predictive effect of the systemic immune-inflammation index for patients with small-cell lung cancer. Future Oncol. 2019, 15, 3367–3379. [Google Scholar] [CrossRef] [PubMed]
- Merloni, F.; Pistelli, M.; Cantini, L.; Della Mora, A.; Bastianelli, L.; De Lisa, M.; Burattini, M.; Maccaroni, E.; Ballatore, Z.; Savini, A.; et al. Role of inflammation parameters in locally advanced breast cancer: The debate is still open. Ann. Oncol. 2017, 28, 37. [Google Scholar] [CrossRef]
- van der Willik, K.D.; Koppelmans, V.; Hauptmann, M.; Compter, A.; Ikram, M.A.; Schagen, S.B. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: A cohort study. Breast Cancer Res. 2018, 20, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fest, J.; Ruiter, R.; Mulder, M.; Groot Koerkamp, B.; Ikram, M.A.; Stricker, B.H.; van Eijck, C.H.J. The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int. J. Cancer 2020, 146, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Miklikova, S.; Minarik, G.; Sedlackova, T.; Plava, J.; Cihova, M.; Jurisova, S.; Kalavska, K.; Karaba, M.; Benca, J.; Smolkova, B.; et al. Inflammation-Based Scores Increase the Prognostic Value of Circulating Tumor Cells in Primary Breast Cancer. Cancers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Chovanec, M.; Cierna, Z.; Miskovska, V.; Machalekova, V.; Kalavska, K.; Rejlekova, K.; Svetlovska, D.; Macak, D.; Spanik, S.; Kajo, K.; et al. Systemic immune-inflammation index in germ-cell tumours. Br. J. Cancer 2018, 118, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Göger, Y.; Özkent, M.; Karaağaç, M.; Uçmak, H.; Artaç, M. Prognostic Value of Systemic Immune-Inflammation Index in Patients with Testicular Cancer: A Retrospective Case-Control Study. Bull. Urooncology 2021, 20, 252–257. [Google Scholar] [CrossRef]
- Cursano, M.C.; Kopf, B.; Scarpi, E.; Menna, C.; Casadei, C.; Schepisi, G.; Lolli, C.; Altavilla, A.; Gallà, V.; Santini, D.; et al. Prognostic Role of Systemic Inflammatory Indexes in Germ Cell Tumors Treated With High-Dose Chemotherapy. Front. Oncol. 2020, 10, 1325. [Google Scholar] [CrossRef]
- Chia, V.M.; Quraishi, S.M.; Devesa, S.S.; Purdue, M.P.; Cook, M.B.; McGlynn, K.A. International trends in the incidence of TC, 1973–2002. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1151–1159. [Google Scholar] [CrossRef] [Green Version]
- Stang, A.; Jansen, L.; Trabert, B.; Rusner, C.; Eberle, A.; Katalinic, A.; Emrich, K.; Holleczek, B.; Brenner, H. Survival after a diagnosis of testicular germ cell cancers in Germany and the United States, 2002–2006, a high resolution study by histology and age. Cancer Epidemiol. 2013, 37, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, S.; Theas, M.S.; Guazzone, V.A.; Jacobo, P.; Wang, M.; Fijak, M.; Meinhardt, A.; Lustig, L. Immune Cell Subtypes and Their Function in the Testis. Front. Immunol. 2020, 11, 583304. [Google Scholar] [CrossRef] [PubMed]
- Siska, P.J.; Johnpulle, R.A.N.; Zhou, A.; Bordeaux, J.; Kim, J.Y.; Dabbas, B.; Dakappagari, N.; Rathmell, J.C.; Rathmell, W.K.; Morgans, A.K.; et al. Deep exploration of the immune infiltrate and outcome prediction in testicular cancer by quantitative multiplexed immunohistochemistry and gene expression profiling. Oncoimmunology 2017, 6, e1305535. [Google Scholar] [CrossRef] [PubMed]
- Testis. In TNM Classification of Malignant Tumours, 8th ed.; Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) Wiley-Blackwell: Oxford, UK, 2017. [Google Scholar]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Ohno, Y. Role of systemic inflammatory response markers in urological malignancy. Int. J. Urol. 2019, 26, 31–47. [Google Scholar] [CrossRef] [Green Version]
- Pęksa, R.; Kunc, M.; Popęda, M.; Piątek, M.; Bieńkowski, M.; Żok, J.; Starzyńska, A.; Perdyan, A.; Sowa, M.; Duchnowska, R.; et al. Combined Assessment of Immune Checkpoint Regulator VISTA on Tumor-Associated Immune Cells and Platelet-to-Lymphocyte Ratio Identifies Advanced Germ Cell Tumors with Higher Risk of Unfavorable Outcomes. Cancers 2021, 13, 1750. [Google Scholar] [CrossRef]
- Dimitriou, N.; Felekouras, E.; Karavokyros, I.; Alexandrou, A.; Pikoulis, E.; Griniatsos, J. Neutrophils to lymphocytes ratio as a useful prognosticator for stage II colorectal cancer patients. BMC Cancer 2018, 18, 1202. [Google Scholar] [CrossRef]
- Pedrazzani, C.; Mantovani, G.; Fernandes, E.; Bagante, F.; Luca Salvagno, G.; Surci, N.; Campagnaro, T.; Ruzzenente, A.; Danese, E.; Lippi, G.; et al. Assessment of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and platelet count as predictors of long-term outcome after R0 resection for colorectal cancer. Sci. Rep. 2017, 7, 1494. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sun, Y.; Zhang, Q. Prognostic value of the systemic immune-inflammation index in patients with breast cancer: A meta-analysis. Cancer Cell Int. 2020, 20, 224. [Google Scholar] [CrossRef]
- Ma, M.; Yu, N.; Wu, B. High systemic immune-inflammation index represents an unfavorable prognosis of malignant pleural mesothelioma. Cancer Manag. Res. 2019, 11, 3973–3979. [Google Scholar] [CrossRef] [Green Version]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013, 123, 3446–3458. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Dovedi, S.J.; Thompson, C.; Lyons, J.; Kennedy, J.; Elliott, T.; West, C.M.; Choudhury, A. Pre-treatment lymphocytopaenia is an adverse prognostic biomarker in muscle-invasive and advanced bladder cancer. Ann. Oncol. 2016, 27, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorelik, L.; Flavell, R.A. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 2002, 2, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2017, 7, e1393134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef]
- Wong, D.; Winter, O.; Hartig, C.; Siebels, S.; Szyska, M.; Tiburzy, B.; Meng, L.; Kulkarni, U.; Fähnrich, A.; Bommert, K.; et al. Eosinophils and megakaryocytes support the early growth of murine MOPC315 myeloma cells in their bone marrow niches. PLoS ONE 2014, 9, e109018. [Google Scholar] [CrossRef] [Green Version]
- Zahoor, H.; Barata, P.C.; Jia, X.; Martin, A.; Allman, K.D.; Wood, L.S.; Gilligan, T.D.; Grivas, P.; Ornstein, M.C.; Garcia, J.A.; et al. Patterns, predictors and subsequent outcomes of disease progression in metastatic renal cell carcinoma patients treated with nivolumab. J. Immunother. Cancer 2018, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Rosner, S.; Kwong, E.; Shoushtari, A.N.; Friedman, C.F.; Betof, A.S.; Brady, M.S.; Coit, D.G.; Callahan, M.K.; Wolchok, J.D.; Chapman, P.B.; et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. 2018, 7, 690–697. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, X.; Wang, G.; Zhou, Y.; Luo, M.; Wang, S.; Hong, C. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage I-III colorectal cancer. Asia-Pac. J. Clin. Oncol. 2018, 14, e243–e251. [Google Scholar] [CrossRef]
- Tanizaki, J.; Haratani, K.; Hayashi, H.; Chiba, Y.; Nakamura, Y.; Yonesaka, K.; Kudo, K.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; et al. Peripheral Blood Biomarkers Associated with Clinical Outcome in Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holub, K.; Biete, A. Impact of systemic inflammation biomarkers on the survival outcomes of cervical cancer patients. Clin. Transl. Oncol. 2019, 21, 836–844. [Google Scholar] [CrossRef]
- Holub, K.; Conill, C. Unveiling the mechanisms of immune evasion in pancreatic cancer: May it be a systemic inflammation responsible for dismal survival? Clin. Transl. Oncol. 2020, 22, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ownby, H.E.; Roi, L.D.; Isenberg, R.R.; Brennan, M.J. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 1983, 52, 126–130. [Google Scholar] [CrossRef]
- Utsunomiya, A.; Ishida, T.; Inagaki, A.; Ishii, T.; Yano, H.; Komatsu, H.; Iida, S.; Yonekura, K.; Takeuchi, S.; Takatsuka, Y.; et al. Clinical significance of a blood eosinophilia in adult T-cell leukemia/lymphoma: A blood eosinophilia is a significant unfavorable prognostic factor. Leuk. Res. 2007, 31, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Hude, I.; Sasse, S.; Bröckelmann, P.J.; von Tresckow, B.; Momotow, J.; Engert, A.; Borchmann, S. Leucocyte and eosinophil counts predict progression-free survival in relapsed or refractory classical Hodgkin Lymphoma patients treated with PD1 inhibition. Br. J. Haematol. 2018, 181, 837–840. [Google Scholar] [CrossRef] [Green Version]
- Orsi, G.; Tovoli, F.; Dadduzio, V.; Vivaldi, C.; Brunetti, O.; Ielasi, L.; Conti, F.; Rovesti, G.; Gramantieri, L.; Rizzato, M.D.; et al. Prognostic role of blood eosinophil count in sorafenib-treated hepatocellular carcinoma patients: Time to reconsider the minorities. Target Oncol. 2020, 15, 773–785. [Google Scholar] [CrossRef]
- Sektioglu, I.M.; Carretero, R.; Bulbuc, N.; Bald, T.; Tüting, T.; Rudensky, A.Y.; Hämmerling, G.J. Basophils Promote Tumor Rejection via Chemotaxis and Infiltration of CD8+ T Cells. Cancer Res. 2017, 77, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.D.; Ward, S.; Crawford, G.; Seoane, R.C.; Jackson, W.D.; Kipling, D.; Voehringer, D.; Dunn-Walters, D.; Strid, J. Inflammation-induced IgE promotes epithelial hyperplasia and tumour growth. eLife 2020, 9, e51862. [Google Scholar] [CrossRef]
- De Monte, L.; Wörmann, S.; Brunetto, E.; Heltai, S.; Magliacane, G.; Reni, M.; Paganoni, A.M.; Recalde, H.; Mondino, A.; Falconi, M.; et al. Basophil Recruitment into Tumor-Draining Lymph Nodes Correlates with Th2 Inflammation and Reduced Survival in Pancreatic Cancer Patients. Cancer Res. 2016, 76, 1792–1803. [Google Scholar] [CrossRef] [Green Version]
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bax, H.J.; Chauhan, J.; Stavraka, C.; Khiabany, A.; Nakamura, M.; Pellizzari, G.; Ilieva, K.M.; Lombardi, S.; Gould, H.J.; Corrigan, C.J.; et al. Basophils from Cancer Patients Respond to Immune Stimuli and Predict Clinical Outcome. Cells 2020, 9, 1631. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Ameri, P.; Cadeddu, C.; Ghigo, A.; Madonna, R.; Marone, G.; Mercurio, V.; Monte, I.; Novo, G.; Parrella, P. Antineoplastic Drug-Induced Cardiotoxicity: A Redox Perspective. Front. Physiol. 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, D.; Cai, S.; Li, Q.; Li, X. Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer. Clin. Transl. Med. 2020, 9, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front. Immunol. 2020, 10, 3038. [Google Scholar] [CrossRef]
- Guerra, N.; Tan, Y.X.; Joncker, N.T.; Choy, A.; Gallardo, F.; Xiong, N.; Knoblaugh, S.; Cado, D.; Greenberg, N.M.; Raulet, D.H. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008, 28, 571–580, Erratum in Immunity 2008, 28, 723. [Google Scholar] [CrossRef] [Green Version]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Pernot, S.; Terme, M.; Radosevic-Robin, N.; Castan, F.; Badoual, C.; Marcheteau, E.; Penault-Llorca, F.; Bouche, O.; Bennouna, J.; Francois, E.; et al. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric. Cancer 2020, 23, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ménard, C.; Blay, J.Y.; Borg, C.; Michiels, S.; Ghiringhelli, F.; Robert, C.; Nonn, C.; Chaput, N.; Taïeb, J.; Delahaye, N.F.; et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009, 69, 3563–3569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delahaye, N.F.; Rusakiewicz, S.; Martins, I.; Ménard, C.; Roux, S.; Lyonnet, L.; Paul, P.; Sarabi, M.; Chaput, N.; Semeraro, M.; et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat. Med. 2011, 17, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, M.; Rusakiewicz, S.; Minard-Colin, V.; Delahaye, N.F.; Enot, D.; Vély, F.; Marabelle, A.; Papoular, B.; Piperoglou, C.; Ponzoni, M.; et al. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci. Transl. Med. 2015, 7, 283ra55. [Google Scholar] [CrossRef]
- Habif, G.; Crinier, A.; André, P.; Vivier, E.; Narni-Mancinelli, E. Targeting natural killer cells in solid tumors. Cell. Mol. Immunol. 2019, 16, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Pasero, C.; Gravis, G.; Guerin, M.; Granjeaud, S.; Thomassin-Piana, J.; Rocchi, P.; Paciencia-Gros, M.; Poizat, F.; Bentobji, M.; Azario-Cheillan, F.; et al. Inherent and Tumor-Driven Immune Tolerance in the Prostate Microenvironment Impairs Natural Killer Cell Antitumor Activity. Cancer Res. 2016, 76, 2153–2165. [Google Scholar] [CrossRef] [Green Version]
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014, 26, 638–652. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Paulete, A.R.; Cueto, F.J.; Martínez-López, M.; Labiano, S.; Morales-Kastresana, A.; Rodríguez-Ruiz, M.E.; Jure-Kunkel, M.; Azpilikueta, A.; Aznar, M.A.; Quetglas, J.I.; et al. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti-PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.B.; Huang, X.; Li, F.R. Impaired dendritic cell functions in lung cancer: A review of recent advances and future perspectives. Cancer Commun. 2019, 39, 43. [Google Scholar] [CrossRef] [Green Version]
- Adhikaree, J.; Franks, H.A.; Televantos, C.; Vaghela, P.; Kaur, A.P.; Walker, D.; Schmitz, M.; Jackson, A.M.; Patel, P.M. Impaired circulating myeloid CD1c+ dendritic cell function in human glioblastoma is restored by p38 inhibition—implications for the next generation of DC vaccines. Oncoimmunology 2019, 8, 1593803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legitimo, A.; Consolini, R.; Failli, A.; Orsini, G.; Spisni, R. Dendritic cell defects in the colorectal cancer. Hum. Vaccin Immunother. 2014, 10, 3224–3235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornwall, S.M.; Wikstrom, M.; Musk, A.W.; Alvarez, J.; Nowak, A.K.; Nelson, D.J. Human mesothelioma induces defects in dendritic cell numbers and antigen-processing function which predict survival outcomes. Oncoimmunology 2015, 5, e1082028. [Google Scholar] [CrossRef] [Green Version]
- Pinzon-Charry, A.; Maxwell, T.; McGuckin, M.A.; Schmidt, C.; Furnival, C.; López, J.A. Spontaneous apoptosis of blood dendritic cells in patients with breast cancer. Breast Cancer Res. 2006, 8, R5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiu, W.; Ma, J.; Lei, T.; Zhang, M.; Zhou, S. Immunosuppressive effect of bladder cancer on function of dendritic cells involving of Jak2/STAT3 pathway. Oncotarget 2016, 7, 63204–63214. [Google Scholar] [CrossRef] [Green Version]
- Kushwah, R.; Hu, J. Dendritic cell apoptosis: Regulation of tolerance versus immunity. J. Immunol. 2010, 185, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Krawczyk, P.; Wojas, K.; Milanowski, J.; Roliński, J. The influence of different culture microenvironments on the generation of dendritic cells from non-small-cell lung cancer patients. Arch. Immunol. Ther. Exp. 2007, 55, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Gardner, A.; de Mingo Pulido, Á.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Krishnan, I.; Maroni, G.; Meyerovitz, C.V.; Kerwin, C.M.; et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 2019, 50, 1317–1334.e10. [Google Scholar] [CrossRef]
- Treilleux, I.; Blay, J.Y.; Bendriss-Vermare, N.; Ray-Coquard, I.; Bachelot, T.; Guastalla, J.P.; Bremond, A.; Goddard, S.; Pin, J.J.; Barthelemy-Dubois, C.; et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin. Cancer Res. 2004, 10, 7466–7474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef] [PubMed]
| Variable | N | % |
|---|---|---|
| All patients | 51 | 100.0 |
| Histology | ||
| Seminoma | 12 | 23.5 |
| Nonseminoma | 39 | 76.5 |
| Primary tumor localization | ||
| Testicular | 48 | 94.1 |
| Extragonadal | 3 | 5.9 |
| IGCCCG risk group | ||
| Good risk | 33 | 64.7 |
| Intermediate risk | 5 | 9.8 |
| Poor risk | 13 | 25.5 |
| Stage IA and IB (adjuvant therapy) | 9 | 17.6 |
| Sites of metastases | ||
| Retroperitoneum | 38 | 74.5 |
| Mediastinum | 7 | 13.7 |
| Lungs | 15 | 29.4 |
| Liver | 7 | 13.7 |
| Brain | 2 | 3.9 |
| Other | 1 | 2.0 |
| Visceral nonpulmonary metastases | 9 | 17.6 |
| No. of metastatic site(s) | ||
| 0 | 10 | 19.6 |
| 1 to 2 | 29 | 56.9 |
| >3 | 12 | 23.5 |
| Staging (UICC) | ||
| IA | 2 | 3.9 |
| IB | 7 | 13.7 |
| IS | 1 | 2.0 |
| IIA | 4 | 7.8 |
| IIB | 9 | 17.6 |
| IIC | 1 | 2.0 |
| IIIA | 7 | 13.7 |
| IIIB | 6 | 11.8 |
| IIIC | 14 | 27.5 |
| Response to therapy * | ||
| Favorable response | 48 | 94.1 |
| Unfavorable response | 2 | 3.9 |
| Median age (range) | 34 | (22–59) |
| Median follow-up (range) | 21.1 | (0.2–39.1) |
| Total White Blood Cell Population (CD45+ Population) | % of Innate Immune Cell Subpopulations | ||||||
|---|---|---|---|---|---|---|---|
| Variable | N | Mean | SEM | Median | p-Value UNI | p-Value MVA | |
| Total leukocyte subpopulations (percentage) | Neutrophil percentage | ||||||
| SII < 1003 | 32 | 56.7 | 1.7 | 58.7 | 0.00000 | 0.00496 | |
| SII ≥ 1003 | 19 | 74.5 | 2.2 | 73.8 | |||
| Monocyte percentage | |||||||
| SII < 1003 | 32 | 10.0 | 0.6 | 10.2 | 0.11455 | ||
| SII ≥ 1003 | 19 | 8.7 | 0.7 | 7.9 | |||
| Monocyte subpopulations (percentage) | Classical monocyte percentage | ||||||
| SII < 1003 | 23 | 84.9 | 1.7 | 85.7 | 0.12215 | ||
| SII ≥ 1003 | 17 | 86.4 | 2.0 | 90.7 | |||
| Intermediate monocyte percentage | |||||||
| SII < 1003 | 17 | 5.3 | 0.7 | 5.3 | 0.84080 | ||
| SII ≥ 1003 | 10 | 5.1 | 0.9 | 5.2 | |||
| Nonclassical monocyte percentage | |||||||
| SII < 1003 | 21 | 5.8 | 0.9 | 4.7 | 0.61768 | ||
| SII ≥ 1003 | 17 | 5.2 | 1.0 | 5.0 | |||
| Total leukocyte subpopulations (percentage) | Polymorphonuclear monocyte (PNMs) percentage | ||||||
| SII < 1003 | 16 | 0.2 | 0.7 | 0.2 | 0.72347 | ||
| SII ≥ 1003 | 14 | 1.8 | 0.8 | 0.2 | |||
| Eosinophil percentage | |||||||
| SII < 1003 | 32 | 3.3 | 0.4 | 2.9 | 0.00431 | 0.63565 | |
| SII ≥ 1003 | 19 | 1.4 | 0.5 | 1.0 | |||
| Basophil percentage | |||||||
| SII < 1003 | 32 | 0.7 | 0.1 | 0.7 | 0.00852 | 0.92744 | |
| SII ≥ 1003 | 19 | 0.5 | 0.1 | 0.4 | |||
| Lymphocyte subpopulations (percentage) | NKT-cell percentage | ||||||
| SII < 1003 | 31 | 2.5 | 0.5 | 1.3 | 0.98405 | ||
| SII ≥ 1003 | 19 | 2.6 | 0.7 | 1.8 | |||
| CD4+ NKT-cell percentage | |||||||
| SII < 1003 | 18 | 0.3 | 0.1 | 0.2 | 0.24894 | ||
| SII ≥ 1003 | 10 | 0.6 | 0.2 | 0.3 | |||
| CD8+ NKT-cell percentage | |||||||
| SII < 1003 | 19 | 2.2 | 0.4 | 1.1 | 0.16867 | ||
| SII ≥ 1003 | 10 | 2.5 | 0.6 | 2.3 | |||
| NK-cell percentage | |||||||
| SII < 1003 | 32 | 10.3 | 1.6 | 9.6 | 0.02264 | 0.06767 | |
| SII ≥ 1003 | 19 | 18.3 | 2.1 | 15.8 | |||
| Total leukocyte subpopulations (percentage) | Dendritic cell (cDCs) percentage | ||||||
| SII < 1003 | 22 | 0.9 | 0.1 | 0.9 | 0.02728 | 0.76790 | |
| SII ≥ 1003 | 14 | 0.7 | 0.1 | 0.6 | |||
| Plasmocytoid dendritic cell (pDCs) percentage | |||||||
| SII < 1003 | 22 | 0.2 | 0.0 | 0.2 | 0.00310 | 0.84143 | |
| SII ≥ 1003 | 14 | 0.1 | 0.0 | 0.1 | |||
| Subpopulation of DCs (percentage) | CD16+ HLADR+ Lin- DC percentage | ||||||
| SII < 1003 | 13 | 48.9 | 5.3 | 46.1 | 1.00000 | ||
| SII ≥ 1003 | 12 | 46.9 | 5.5 | 47.4 | |||
| CD1c+ within DC percentage | |||||||
| SII < 1003 | 18 | 22.5 | 1.8 | 20.3 | 0.02497 | ||
| SII ≥ 1003 | 15 | 16.2 | 2.0 | 14.7 | |||
| Total White Blood Cell Population (CD45+ Population) | % of Adaptive Immune Cell Subpopulations | ||||||
|---|---|---|---|---|---|---|---|
| Variable | N | Mean | SEM | Median | p-Value UNI | p-Value MVA | |
| Total leukocyte subpopulations (percentage) | Lymphocyte percentage | ||||||
| SII < 1003 | 32 | 31.2 | 1.5 | 29.5 | 0.00000 | 0.42563 | |
| SII ≥ 1003 | 19 | 15.0 | 2.0 | 13.8 | |||
| Subpopulations of lymphocytes (percentage) | B-cell percentage (CD14+) | ||||||
| SII < 1003 | 32 | 11.0 | 0.8 | 10.7 | 0.60565 | ||
| SII ≥ 1003 | 19 | 10.7 | 1.0 | 8.8 | |||
| T-cell percentage (CD3+) | |||||||
| SII < 1003 | 32 | 76.3 | 1.7 | 77.3 | 0.01410 | 0.01385 | |
| SII ≥ 1003 | 19 | 68.1 | 2.2 | 72.3 | |||
| Helper T-cell percentage | |||||||
| SII < 1003 | 31 | 45.7 | 1.7 | 47.2 | 0.37378 | ||
| SII ≥ 1003 | 19 | 42.7 | 2.1 | 43.8 | |||
| Cytotoxic T-cell percentage | |||||||
| SII < 1003 | 32 | 27.7 | 1.0 | 27.9 | 0.02383 | 0.12797 | |
| SII ≥ 1003 | 19 | 23.4 | 1.3 | 23.4 | |||
| T-reg percentage | |||||||
| SII < 1003 | 32 | 4.1 | 0.2 | 3.8 | 0.53298 | ||
| SII ≥ 1003 | 19 | 3.9 | 0.3 | 3.9 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalavska, K.; Sestakova, Z.; Mlcakova, A.; Gronesova, P.; Miskovska, V.; Rejlekova, K.; Svetlovska, D.; Sycova-Mila, Z.; Obertova, J.; Palacka, P.; et al. Detection of Specific Immune Cell Subpopulation Changes Associated with Systemic Immune Inflammation–Index Level in Germ Cell Tumors. Life 2022, 12, 678. https://doi.org/10.3390/life12050678
Kalavska K, Sestakova Z, Mlcakova A, Gronesova P, Miskovska V, Rejlekova K, Svetlovska D, Sycova-Mila Z, Obertova J, Palacka P, et al. Detection of Specific Immune Cell Subpopulation Changes Associated with Systemic Immune Inflammation–Index Level in Germ Cell Tumors. Life. 2022; 12(5):678. https://doi.org/10.3390/life12050678
Chicago/Turabian StyleKalavska, Katarina, Zuzana Sestakova, Andrea Mlcakova, Paulina Gronesova, Viera Miskovska, Katarina Rejlekova, Daniela Svetlovska, Zuzana Sycova-Mila, Jana Obertova, Patrik Palacka, and et al. 2022. "Detection of Specific Immune Cell Subpopulation Changes Associated with Systemic Immune Inflammation–Index Level in Germ Cell Tumors" Life 12, no. 5: 678. https://doi.org/10.3390/life12050678






