Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos
Abstract
:1. Introduction
1.1. Characteristics and Cargos of EV
1.1.1. EV Formation and Types
1.1.2. EV Membrane Components
1.1.3. EV Cargos
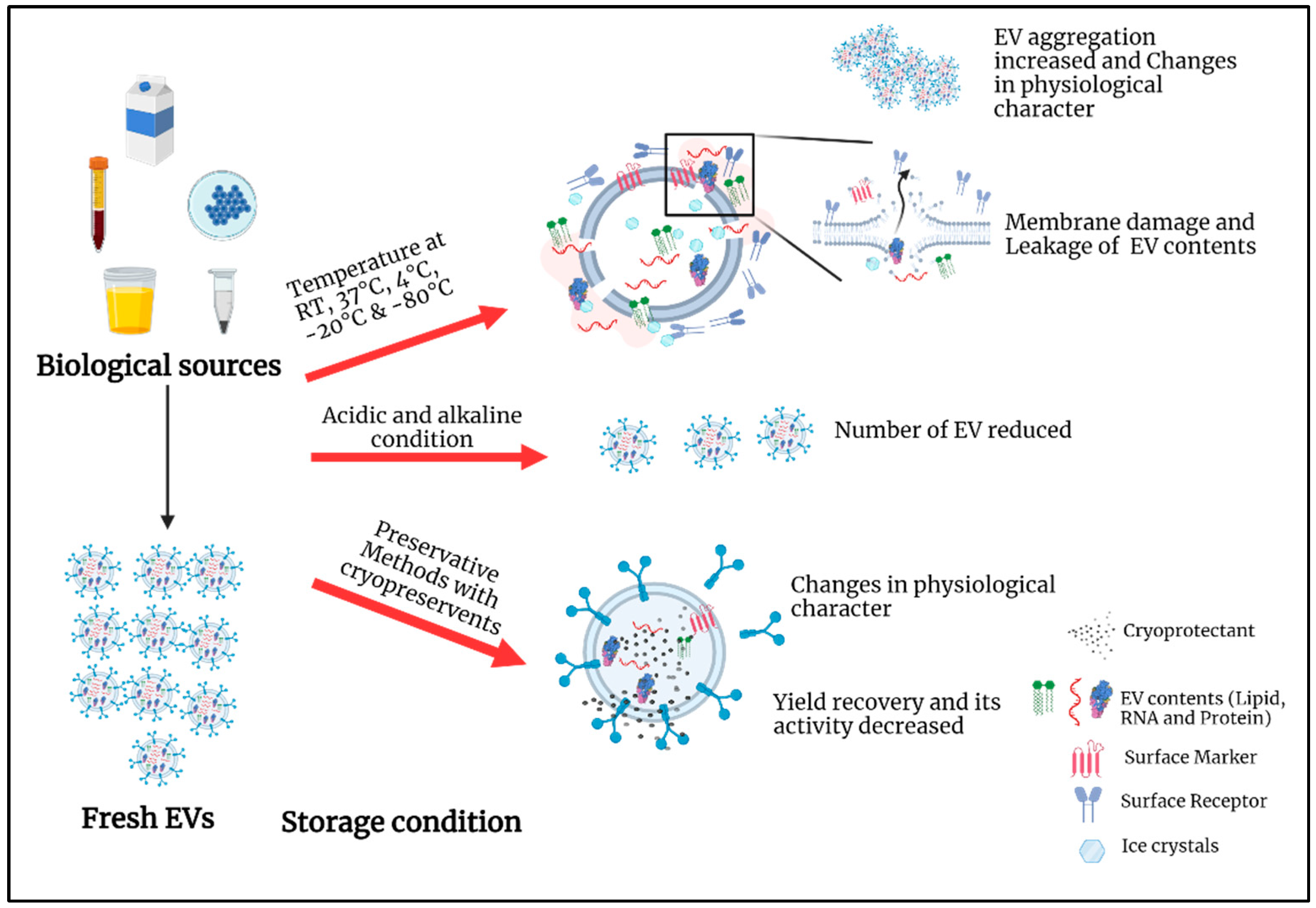
2. Current Storage Conditions
3. Biofluids and Extracellular Vesicles Characterization under Different Storage Conditions
3.1. Impact of Temperature on Biofluids and EV during Storage
3.1.1. Impact on Yield, Morphology, and Integrity
Storage of BALF and Their EVs
Storage of Urine, Sperm, and Their EVs
Storage of Milk and Their EVs
Storage of Blood, Plasma, Serum, and Their EVs
Storage of Saliva
Storage of EVs from Cell Culture Media
3.1.2. Impact on Functional Activities and Cargos Expression
3.2. Impact of pH
3.3. Impact of Preservation Techniques
4. Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef]
- Bazzan, E.; Tinè, M.; Casara, A.; Biondini, D.; Semenzato, U.; Cocconcelli, E.; Balestro, E.; Damin, M.; Radu, C.M.; Turato, G.; et al. Critical Review of the Evolution of Extracellular Vesicles’ Knowledge: From 1946 to Today. Int. J. Mol. Sci. 2021, 22, 6417. [Google Scholar] [CrossRef] [PubMed]
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Mishra, N.C.; Tatum, E.L. Non-Mendelian Inheritance of DNA-Induced Inositol Independence in Neurospora. Proc. Natl. Acad. Sci. USA 1973, 70, 3875–3879. [Google Scholar] [CrossRef] [Green Version]
- Fox, A.S.; Yoon, S.B.; Gelbart, W.M. DNA-Induced Transformation in Drosophila: Genetic Analysis of Transformed Stocks. Proc. Natl. Acad. Sci. USA 1971, 68, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Fox, A.S.; Yoon, S.B. DNA-Induced Transformation in Drosophila: Locus-Specificity and the Establishment of Transformed Stocks. Proc. Natl. Acad. Sci. USA 1970, 67, 1608–1615. [Google Scholar] [CrossRef] [Green Version]
- Aaronson, S.; Behrens, U.; Orner, R.; Haines, T.H. Ultrastructure of intracellular and extracellular vesicles, membranes, and myelin figures produced by Ochromonas danica. J. Ultrastruct. Res. 1971, 35, 418–430. [Google Scholar] [CrossRef]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Quynh, N.T.; Nhan, L.T.T.; Phuong, L.L.; Thao, B.P.; Linh, N.T.T.; Tho, L.T.; Thai, T.H. Mitochondrial A10398G Alteration in Plasma Exosome of Non-small Cell Lung Cancer Patients. VNU J. Sci. Med. Pharm. Sci. 2020, 36, 50–59. [Google Scholar] [CrossRef]
- Zomer, A.; Maynard, C.; Verweij, F.J.; Kamermans, A.; Schäfer, R.; Beerling, E.; Schiffelers, R.M.; de Wit, E.; Berenguer, J.; Ellenbroek, S.I.J.; et al. In Vivo Imaging Reveals Extracellular Vesicle-Mediated Phenocopying of Metastatic Behavior. Cell 2015, 161, 1046–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronisz, A.; Wang, Y.; Nowicki, M.O.; Peruzzi, P.; Ansari, K.I.; Ogawa, D.; Balaj, L.; De Rienzo, G.; Mineo, M.; Nakano, I.; et al. Extracellular Vesicles Modulate the Glioblastoma Microenvironment via a Tumor Suppression Signaling Network Directed by miR-1. Cancer Res. 2014, 74, 738–750. [Google Scholar] [CrossRef] [Green Version]
- Languino, L.R.; Singh, A.; Prisco, M.; Inman, G.J.; Luginbuhl, A.; Curry, J.M.; South, A.P. Exosome-mediated transfer from the tumor microenvironment increases TGFβ signaling in squamous cell carcinoma. Am. J. Transl. Res. 2016, 8, 2432–2437. [Google Scholar] [PubMed]
- Boelens, M.C.; Wu, T.J.; Nabet, B.Y.; Xu, B.; Qiu, Y.; Yoon, T.; Azzam, D.J.; Victor, C.T.-S.; Wiemann, B.Z.; Ishwaran, H.; et al. Exosome Transfer from Stromal to Breast Cancer Cells Regulates Therapy Resistance Pathways. Cell 2014, 159, 499–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, E.A.C.; Li, L.; Wang, L.; Meng, W.; Hao, Y.; Zhu, G. Exosome-mediated cellular crosstalk within the tumor microenvironment upon irradiation. Cancer Biol. Med. 2021, 18, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Chen, L.; Yuan, X.; Luo, Q.; Liu, Y.; Xie, G.; Ma, Y.; Shen, L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 53. [Google Scholar] [CrossRef] [Green Version]
- Su, T.; Zhang, P.; Zhao, F.; Zhang, S. Exosomal MicroRNAs Mediating Crosstalk Between Cancer Cells With Cancer-Associated Fibroblasts and Tumor-Associated Macrophages in the Tumor Microenvironment. Front. Oncol. 2021, 11, 631703. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Gao, W.; Chen, L.; Xu, W.; Zhu, X. Exosome-Mediated Crosstalk Between Tumor and Tumor-Associated Macrophages. Front. Mol. Biosci. 2021, 8, 977. [Google Scholar] [CrossRef]
- Li, C.-J.; Fang, Q.-H.; Liu, M.-L.; Lin, J.-N. Current understanding of the role of Adipose-derived Extracellular Vesicles in Metabolic Homeostasis and Diseases: Communication from the distance between cells/tissues. Theranostics 2020, 10, 7422–7435. [Google Scholar] [CrossRef]
- Akbar, N.; Azzimato, V.; Choudhury, R.P.; Aouadi, M. Extracellular vesicles in metabolic disease. Diabetologia 2019, 62, 2179–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayabalan, N.; Nair, S.; Nuzhat, Z.; Rice, G.E.; Zuniga, F.A.; Sobrevia, L.; Leiva, A.; Sanhueza, C.; Gutiérrez, J.A.; Lappas, M.; et al. Cross Talk between Adipose Tissue and Placenta in Obese and Gestational Diabetes Mellitus Pregnancies via Exosomes. Front. Endocrinol. 2017, 8, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Raftar, S.K.A.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Badi, S.A.; Khatami, S.; et al. Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef] [PubMed]
- Pardo, F.; Villalobos-Labra, R.; Sobrevia, B.; Toledo, F.; Sobrevia, L. Extracellular vesicles in obesity and diabetes mellitus. Mol. Asp. Med. 2018, 60, 81–91. [Google Scholar] [CrossRef] [PubMed]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Buzás, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, M.; Lu, Q. Extracellular Vesicles in Rheumatoid Arthritis and Systemic Lupus Erythematosus: Functions and Applications. Front. Immunol. 2021, 11, 575712. [Google Scholar] [CrossRef]
- Nielsen, T.B.; Nielsen, M.; Handberg, A. Third International Meeting of ISEV 2014: Rotterdam, The Netherlands, April 30th–May 3rd, 2014. J. Extracell. Vesicles 2014, 3, 24214. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular Vesicles: Structure, Function, and Potential Clinical Uses in Renal Diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chivero, E.T.; Dagur, R.S.; Peeples, E.S.; Sil, S.; Liao, K.; Ma, R.; Chen, L.; Gurumurthy, C.B.; Buch, S.; Hu, G. Biogenesis, physiological functions and potential applications of extracellular vesicles in substance use disorders. Cell. Mol. Life Sci. 2021, 78, 4849–4865. [Google Scholar] [CrossRef] [PubMed]
- Latifkar, A.; Hur, Y.H.; Sanchez, J.C.; Cerione, R.A.; Antonyak, M.A. New insights into extracellular vesicle biogenesis and function. J. Cell Sci. 2019, 132, jcs222406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juan, T.; Fürthauer, M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin. Cell Dev. Biol. 2018, 74, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Latifkar, A.; Cerione, R.A.; Antonyak, M.A. Probing the mechanisms of extracellular vesicle biogenesis and function in cancer. Biochem. Soc. Trans. 2018, 46, 1137–1146. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Soekmadji, C.; Hill, A.F.; Wauben, M.H.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Lötvall, J.; Sahoo, S.; Witwer, K.W. Towards mechanisms and standardization in extracellular vesicle and extracellular RNA studies: Results of a worldwide survey. J. Extracell. Vesicles 2018, 7, 1535745. [Google Scholar] [CrossRef] [Green Version]
- Pegtel, D.M.; Gould, S.J. Exosomes: Annual Review of Biochemistry. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Laulagnier, K.; Motta, C.; Hamdi, S.; Roy, S.; Fauvelle, F.; Pageaux, J.-F.; Kobayashi, T.; Salles, J.-P.; Perret, B.; Bonnerot, C.; et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 2004, 380, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Zhang, Q.; Hu, X.M.; Mi, T.Y.; Yu, H.Y.; Liu, S.S.; Zhang, B.; Tang, M.; Huang, J.F.; Xiong, K. How Does Temperature Play a Role in the Storage of Extracellular Vesicles? J. Cell. Physiol. 2020, 235, 7663–7680. [Google Scholar] [CrossRef] [PubMed]
- Llorente, A.; Skotland, T.; Sylvänne, T.; Kauhanen, D.; Róg, T.; Orłowski, A.; Vattulainen, I.; Ekroos, K.; Sandvig, K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Konishi, Y.; Kosaka, N.; Katsuda, T.; Kato, T.; Ochiya, T. Comparative marker analysis of extracellular vesicles in different human cancer types. J. Extracell. Vesicles 2013, 2, 20424. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A Common Pathway for a Specialized Function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.; König, A.-K.; Marmé, F.; Runz, S.; Wolterink, S.; Koensgen, D.; Mustea, A.; Sehouli, J.; Altevogt, P. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009, 278, 73–81. [Google Scholar] [CrossRef]
- Kwok, Z.H.; Ni, K.; Jin, Y. Extracellular Vesicle Associated Non-Coding RNAs in Lung Infections and Injury. Cells 2021, 10, 965. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Fang, L.; Bai, J.; Wang, Z. Characteristics and Changes of DNA in Extracellular Vesicles. DNA Cell Biol. 2020, 39, 1486–1493. [Google Scholar] [CrossRef]
- Bellingham, S.A.; Guo, B.B.; Coleman, B.M.; Hill, A.F. Exosomes: Vehicles for the Transfer of Toxic Proteins Associated with Neurodegenerative Diseases? Front. Physiol. 2012, 3, 124. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Zhao, W.-L.; Ye, Y.-Y.; Bai, X.-C.; Liu, R.-Q.; Chang, L.-F.; Zhou, Q.; Sui, S.-F. Cellular Internalization of Exosomes Occurs through Phagocytosis. Traffic 2010, 11, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2018, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Maroto, R.; Zhao, Y.; Jamaluddin, M.; Popov, V.L.; Wang, H.; Kalubowilage, M.; Zhang, Y.; Luisi, J.; Sun, H.; Culbertson, C.T.; et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles 2017, 6, 1359478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosthuyzen, W.; Sime, N.E.L.; Ivy, J.R.; Turtle, E.J.; Street, J.M.; Pound, J.; Bath, L.E.; Webb, D.J.; Gregory, C.D.; Bailey, M.A.; et al. Quantification of human urinary exosomes by nanoparticle tracking analysis. J. Physiol. 2013, 591, 5833–5842. [Google Scholar] [CrossRef]
- Madison, M.N.; Jones, P.H.; Okeoma, C.M. Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology 2015, 482, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Welch, J.L.; Madison, M.N.; Margolick, J.B.; Galvin, S.; Gupta, P.; Martínez-Maza, O.; Dash, C.; Okeoma, C.M. Effect of prolonged freezing of semen on exosome recovery and biologic activity. Sci. Rep. 2017, 7, 45034. [Google Scholar] [CrossRef] [Green Version]
- Yuana, Y.; Böing, A.N.; Grootemaat, A.E.; van der Pol, E.; Hau, C.M.; Cizmar, P.; Buhr, E.; Sturk, A.; Nieuwland, R. Handling and storage of human body fluids for analysis of extracellular vesicles. J. Extracell. Vesicles 2015, 4, 29260. [Google Scholar] [CrossRef]
- Zhou, H.; Yuen, P.S.T.; Pisitkun, T.; Gonzales, P.A.; Yasuda, H.; Dear, J.W.; Gross, P.; Knepper, M.A.; Star, R.A. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006, 69, 1471–1476. [Google Scholar] [CrossRef] [Green Version]
- Cheruvanky, A.; Zhou, H.; Pisitkun, T.; Kopp, J.B.; Knepper, M.A.; Yuen, P.S.T.; Star, R.A. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Ren. Physiol. 2007, 292, F1657–F1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2015, 371, 48–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Zonneveld, M.I.; Brisson, A.R.; van Herwijnen, M.J.C.; Tan, S.; van de Lest, C.H.A.; Redegeld, F.A.; Garssen, J.; Wauben, M.H.M.; Nolte-’t Hoen, E.N. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J. Extracell. Vesicles 2014, 3, 24215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Chen, K.; Wang, Z.; Wang, Y.; Liu, J.; Lin, L.; Shao, Y.; Gao, L.; Yin, H.; Cui, C.; et al. DNA in serum extracellular vesicles is stable under different storage conditions. BMC Cancer 2016, 16, 753. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, R.; Judicone, C.; Poncelet, P.; Robert, S.; Arnaud, L.; Sampol, J.; Dignat-George, F. Impact of pre-analytical parameters on the measurement of circulating microparticles: Towards standardization of protocol. J. Thromb. Haemost. 2012, 10, 437–446. [Google Scholar] [CrossRef]
- Bæk, R.; Søndergaard, E.K.L.; Varming, K.; Jørgensen, M.M. The impact of various preanalytical treatments on the phenotype of small extracellular vesicles in blood analyzed by protein microarray. J. Immunol. Methods 2016, 438, 11–20. [Google Scholar] [CrossRef] [Green Version]
- van Ierssel, S.H.; van Craenenbroeck, E.M.; Conraads, V.M.; van Tendeloo, V.F.; Vrints, C.J.; Jorens, P.G.; Hoymans, V.Y. Flow cytometric detection of endothelial microparticles (EMP): Effects of centrifugation and storage alter with the phenotype studied. Thromb. Res. 2010, 125, 332–339. [Google Scholar] [CrossRef]
- Tessier, S.N.; Bookstaver, L.D.; Angpraseuth, C.; Stannard, C.J.; Marques, B.; Ho, U.K.; Muzikansky, A.; Aldikacti, B.; Reátegui, E.; Rabe, D.C.; et al. Isolation of intact extracellular vesicles from cryopreserved samples. PLoS ONE 2021, 16, e0251290. [Google Scholar] [CrossRef]
- Schubert, P.; Johnson, L.; Culibrk, B.; Chen, Z.; Tan, S.; Marks, D.C.; Devine, D.V. Reconstituted cryopreserved platelets synthesize proteins during short-term storage and packaging a defined subset into microvesicles. Transfusion 2021, 61, 2549–2555. [Google Scholar] [CrossRef]
- Rubin, O.; Crettaz, D.; Canellini, G.; Tissot, J.-D.; Lion, N. Microparticles in stored red blood cells: An approach using flow cytometry and proteomic tools. Vox Sang. 2008, 95, 288–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The impact of storage on extracellular vesicles: A systematic study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef]
- Sokolova, V.; Ludwig, A.-K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, A.M.; Timar, C.I.; Marosvári, K.A.; Veres, D.S.; Otrokocsi, L.; Kittel, A.; Ligeti, E. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J. Extracell. Vesicles 2014, 3, 25465. [Google Scholar] [CrossRef]
- Deville, S.; Berckmans, P.; Van Hoof, R.; Lambrichts, I.; Salvati, A.; Nelissen, I. Comparison of extracellular vesicle isolation and storage methods using high-sensitivity flow cytometry. PLoS ONE 2021, 16, e0245835. [Google Scholar] [CrossRef] [PubMed]
- Kumeda, N.; Ogawa, Y.; Akimoto, Y.; Kawakami, H.; Tsujimoto, M.; Yanoshita, R. Characterization of Membrane Integrity and Morphological Stability of Human Salivary Exosomes. Biol. Pharm. Bull. 2017, 40, 1183–1191. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Ban, J.-J.; Im, W.; Kim, M. Influence of storage condition on exosome recovery. Biotechnol. Bioprocess Eng. 2016, 21, 299–304. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, L.; Lv, D.; Zhu, X.; Tang, H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J. Hematol. Oncol. 2019, 12, 53. [Google Scholar] [CrossRef]
- Park, S.J.; Jeon, H.; Yoo, S.-M.; Lee, M.-S. The effect of storage temperature on the biological activity of extracellular vesicles for the complement system. Vitr. Cell. Dev. Biol.-Anim. 2018, 54, 423–429. [Google Scholar] [CrossRef]
- Richter, M.; Fuhrmann, K.; Fuhrmann, G. Evaluation of the Storage Stability of Extracellular Vesicles. J. Vis. Exp. 2019, 2019, e59584. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Zeng, Q.; Han, Q.; Xia, W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell 2018, 10, 295–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köberle, V.; Pleli, T.; Schmithals, C.; Augusto Alonso, E.; Haupenthal, J.; Bönig, H.; Peveling-Oberhag, J.; Biondi, R.M.; Zeuzem, S.; Kronenberger, B.; et al. Differential Stability of Cell-Free Circulating microRNAs: Implications for Their Utilization as Biomarkers. PLoS ONE 2013, 8, e75184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Enderle, D.; Spiel, A.; Coticchia, C.M.; Berghoff, E.; Mueller, R.; Schlumpberger, M.; Sprenger-Haussels, M.; Shaffer, J.M.; Lader, E.; Skog, J.; et al. Characterization of RNA from Exosomes and Other Extracellular Vesicles Isolated by a Novel Spin Column-Based Method. PLoS ONE 2015, 10, e0136133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. miRNA in Plasma Exosome is Stable under Different Storage Conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef] [Green Version]
- Baddela, V.S.; Nayan, V.; Rani, P.; Onteru, S.K.; Singh, D. Physicochemical Biomolecular Insights into Buffalo Milk-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2016, 178, 544–557. [Google Scholar] [CrossRef]
- Sarker, S.; Scholz-Romero, K.; Perez, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E.; Salomon, C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014, 12, 204. [Google Scholar] [CrossRef] [Green Version]
- Ayers, L.; Kohler, M.; Harrison, P.; Sargent, I.; Dragovic, R.; Schaap, M.; Nieuwland, R.; Brooks, S.A.; Ferry, B. Measurement of circulating cell-derived microparticles by flow cytometry: Sources of variability within the assay. Thromb. Res. 2011, 127, 370–377. [Google Scholar] [CrossRef]
- Muller, L.; Hong, C.-S.; Stolz, D.B.; Watkins, S.C.; Whiteside, T.L. Isolation of biologically-active exosomes from human plasma. J. Immunol. Methods 2014, 411, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Nakase, I.; Ueno, N.; Matsuzawa, M.; Noguchi, K.; Hirano, M.; Omura, M.; Takenaka, T.; Sugiyama, A.; Kobayashi, N.B.; Hashimoto, T.; et al. Environmental pH stress influences cellular secretion and uptake of extracellular vesicles. FEBS Open Bio 2021, 11, 753–767. [Google Scholar] [CrossRef]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.K.; Wijaya, C.H. Plant-Derived Exosome-like Nanoparticles: A Concise Review on Its Extraction Methods, Content, Bioactivities, and Potential as Functional Food Ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef] [PubMed]
- Bahr, M.M.; Amer, M.S.; Abo-El-Sooud, K.; Abdallah, A.N.; El-Tookhy, O.S. Preservation techniques of stem cells extracellular vesicles: A gate for manufacturing of clinical grade therapeutic extracellular vesicles and long-term clinical trials. Int. J. Vet. Sci. Med. 2020, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Budgude, P.; Kale, V.; Vaidya, A. Cryopreservation of mesenchymal stromal cell-derived extracellular vesicles using trehalose maintains their ability to expand hematopoietic stem cells in vitro. Cryobiology 2021, 98, 152–163. [Google Scholar] [CrossRef]
- Tegegn, T.Z.; De Paoli, S.H.; Orecna, M.; Elhelu, O.K.; Woodle, S.A.; Tarandovskiy, I.D.; Ovanesov, M.V.; Simak, J. Characterization of procoagulant extracellular vesicles and platelet membrane disintegration in DMSO-cryopreserved platelets. J. Extracell. Vesicles 2016, 5, 30422. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Kreke, M.; Smith, R.; Hanscome, P.; Peck, K.; Ibrahim, A. Processes for Producing Stable Exosome Formulations. U.S. Patent US 2016/0158291 A1, 9 June 2016. [Google Scholar]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef]
- Akers, J.C.; Ramakrishnan, V.; Yang, I.; Hua, W.; Mao, Y.; Carter, B.S.; Chen, C.C. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomark. 2016, 17, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Frank, J.; Richter, M.; De Rossi, C.; Lehr, C.-M.; Fuhrmann, K.; Fuhrmann, G. Extracellular vesicles protect glucuronidase model enzymes during freeze-drying. Sci. Rep. 2018, 8, 12377. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, K.; Hirano, M.; Hashimoto, T.; Yuba, E.; Takatani-Nakase, T.; Nakase, I. Effects of Lyophilization of Arginine-rich Cell-penetrating Peptide-modified Extracellular Vesicles on Intracellular Delivery. Anticancer Res. 2019, 39, 6701–6709. [Google Scholar] [CrossRef]
- El Baradie, K.B.Y.; Nouh, M.; O’Brien, F.; Liu, Y.; Fulzele, S.; Eroglu, A.; Hamrick, M.W. Freeze-Dried Extracellular Vesicles From Adipose-Derived Stem Cells Prevent Hypoxia-Induced Muscle Cell Injury. Front. Cell Dev. Biol. 2020, 8, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusuma, G.D.; Barabadi, M.; Tan, J.L.; Morton, D.A.V.; Frith, J.E.; Lim, R. To Protect and to Preserve: Novel Preservation Strategies for Extracellular Vesicles. Front. Pharmacol. 2018, 9, 1199. [Google Scholar] [CrossRef] [Green Version]
- Evtushenko, E.G.; Bagrov, D.V.; Lazarev, V.N.; Livshits, M.A.; Khomyakova, E. Adsorption of extracellular vesicles onto the tube walls during storage in solution. PLoS ONE 2020, 15, e0243738. [Google Scholar] [CrossRef]
- van de Wakker, S.I.; van Oudheusden, J.; Mol, E.A.; Roefs, M.T.; Zheng, W.; Görgens, A.; El Andaloussi, S.; Sluijter, J.P.G.; Vader, P. Influence of short term storage conditions, concentration methods and excipients on extracellular vesicle recovery and function. Eur. J. Pharm. Biopharm. 2022, 170, 59–69. [Google Scholar] [CrossRef]
- Hermida-Nogueira, L.; Barrachina, M.N.; Izquierdo, I.; García-Vence, M.; Lacerenza, S.; Bravo, S.; Castrillo, A.; García, Á. Proteomic analysis of extracellular vesicles derived from platelet concentrates treated with Mirasol® identifies biomarkers of platelet storage lesion. J. Proteom. 2020, 210, 103529. [Google Scholar] [CrossRef] [PubMed]
- Ashwood-Smith, M.J. Mechanisms of cryoprotectant action. Symp. Soc. Exp. Biol. 1987, 41, 395–406. [Google Scholar] [PubMed]
- Bhattacharya, S. Cryopretectants and Their Usage in Cryopreservation Process. In Cryopreservation Biotechnology in Biomedical and Biological Sciences; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Buchanan, S.S.; Gross, S.A.; Acker, J.P.; Toner, M.; Carpenter, J.F.; Pyatt, D.W. Cryopreservation of Stem Cells Using Trehalose: Evaluation of the Method Using a Human Hematopoietic Cell Line. Stem Cells Dev. 2004, 13, 295–305. [Google Scholar] [CrossRef]
- Eroglu, A.; Russo, M.J.; Bieganski, R.; Fowler, A.; Cheley, S.; Bayley, H.; Toner, M. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol. 2000, 18, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; Avanzini, M.A.; Sorrenti, M.; Torre, M.L. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine 2019, 14, 753–765. [Google Scholar] [CrossRef]
- Yong, K.W.; Safwani, W.K.Z.W.; Xu, F.; Abas, W.A.B.W.; Choi, J.R.; Pingguan-Murphy, B. Cryopreservation of Human Mesenchymal Stem Cells for Clinical Applications: Current Methods and Challenges. Biopreserv. Biobank. 2015, 13, 231–239. [Google Scholar] [CrossRef]
- Mandawala, A.A.; Harvey, S.C.; Roy, T.K.; Fowler, K.E. Cryopreservation of animal oocytes and embryos: Current progress and future prospects. Theriogenology 2016, 86, 1637–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Features | Apoptotic Bodies | MVs | Exosomes |
|---|---|---|---|
| Shape | Heterogeneous | Heterogeneous | Spherical |
| Size (nm) | 50–5000 | 100–1000 | 30–150 |
| Formation mechanism | Nuclear chromatin condensation, followed by membrane blebbing | Plasma membrane direct outward budding and fission | Endosomal network fusion with the plasma membrane |
| Release or response | Apoptosis | Cell injury, proinflammatory stimulants, hypoxia, oxidative stress or shear stress | Cellular stress or activation signals |
| Surface markers | Apoptotic cell markers | Selectins, integrin, CD40, CD31+, CD235a+, CD42b−, CD45, CD61+, CD62E+, and CD144+, | Tetraspanins (CD9, CD63 CD81 and CD82) |
| Cargos and other markers | Intact chromatin, glycosylated proteins, Caspase 3, histones, HSP60, and GRP78 | Cytoskeletal proteins, heat shock proteins, integrins, and proteins containing post-translational modifications, such as glycosylation and phosphorylation | ALIX, TSG-101, PODXL, HSP70, and HSP90β |
| Source | Type of EVs | Storage Temperature/pH/Cryopreserves | Duration | Freeze-Thaw Cycles | Physical Changes | Functional Changes | References |
|---|---|---|---|---|---|---|---|
| BALF | EV | −80 °C | 4 days | - | Disruption in the surface and morphological characteristics and ↓ total protein content | - | [55] |
| Serum | MP | −80 °C | 1 week and 1 year | 1 | Microparticle counts are stable | - | [66] |
| EV | RT | 24 h | - | - | ↓ miR-21 and miR-142-3p | [82] | |
| EV | 4 and −70 °C | 96 h and 28 days | - | - | miR-21, miR-200b, and miR-205 expression was stable | [83] | |
| Blood | exosomes | RT, 4 °C, −20 °C, −80 °C and −160 °C | Days and Months | - | - | The stable expression in signals under storage at RT and 4 °C for long-term storage. ↓ signal intensities for long-term storage | [67] |
| Plasma | Microparticles | 4 °C and −80 °C | 7 h, 7 and 28 days | - | ↑ expression of CD31+, CD42b- and CD62E+ ↓ expression of CD144+ | - | [68] |
| EV | −80 °C | 10–12 days | - | ↓ EV concentration No changes in EV size | - | [69] | |
| EV | −80 °C | 6 months | - | ↓ Particle concentration ↑ Total protein content and particle size | - | [72] | |
| EV | −80 °C | 12 months | 1 | - | ↓ level of AnnV+ before thaw; and ↑ level of AnnV+ after a single freeze-thaw cycle | [88] | |
| Exosomes | RT | 0–48 h | - | - | Ct value of exosomal let-7a and miR-142-3p were stable | [84] | |
| −80 °C | 7 years | - | - | ↑ Amount of total protein and protein/nucleic acid aggregation | [89] | ||
| Platelets | MV | −80 °C | 24 h | - | ↑ MV secretion | - | [70] |
| EV | pathogen reduction technology (PRT) treatment with Mirasol® (vitamin B2plus UVB light) | 2 and 7 days | - | ↑151 proteins, including EV markers | [103] | ||
| EVs | Frozen with 6% DMSO | - | ↑ EV production | Procoagulant activity was stable | [95] | ||
| RBC | EV | 4 °C | 50 days | - | ↑ 20-folds Particle counts | - | [71] |
| Milk | EV | 4 °C and −80 °C | 2–8 weeks | - | - | No changes in CD63 and CD9 expression | [64] |
| Exosomes | −80 °C | 4 week | - | ↑ contamination by stress-induced exosomes | - | [63] | |
| 6 month | - | [62] | |||||
| EV | 4 °C | 24 h | - | - | ↓ 2-fold-miR-21 expression | [86] | |
| Urine | Exosomes | −20 °C & −80 °C with PI | 1 week | - | ↓ EV associated protein expression | - | [60] |
| 4 °C and −80 °C | 24 h | - | Stable expression of TSG101, AQP2, angiotensin-converting enzyme, and PODXL | - | [61] | ||
| RT, 4 °C and −80 °C | 2 h–7 days | - | ↓ EV yield | - | [56] | ||
| Semen | Exosomes | −80 °C | 2 and 30 years | - | Size, structure, or concentration are stable | ↓ Amount of protein, AChE, and anti-HIV activities on long-term freezing. But total RNA level is stable | [57,58] |
| Saliva | EV | −80 °C | 1 year | 1 | ↓ 2-fold EV concentration, ↑ 17% in size, Morphological characteristics are stable. | - | [59] |
| Exosomes | 4 °C | 7 days | - | No changes in total protein, dipeptidyl peptidase IV activity, morphology, and surface markers (CD9, ALIX, and TSG101) | Degradation in some functional proteins | [76] | |
| A431 cells (Culture media) | EVs | pH 5, 6 and 7 (cell culture condition) | 24 h | pH 5 cell culture condition increases its protein content and zeta potential. | ↑ EV uptake into recipient cells | [90] | |
| HEK293T cells (Culture media) | EV | RT, 4 °C, −20 °C and −80 °C | 10 days | - | ↓ CD63 expression under storage at RT and 4 °C. More stable in protein and RNA expression under storage at frozen condition | Exosome uptake efficiency and biodistribution were significantly decreased when stored at 4 °C and −20 °C | [77] |
| Exosomes | 60 °C, 37 °C, 4 °C, −20 °C, and −80 °C at pH 4, 7, or 10 | 1 day | 2 | No changes occur in ALIX, HSP70, and TSG101 at 4 °C. ↓ Exosome numbers in pH 4 and 10. | ↑ Cellular uptake of exosomes at pH4 and 10. ↓ Cellular uptake under stored at 4 °C | [81] | |
| HUVEC (Culture media) | EV | 37.4, −20, and −70 °C | 25 days | - | ↓ particle number and ↑ size on 37.4, and −20 °C | ↓ CD-63 and -81 expression under storage at 37 °C. ↓ Functional stability on 37 and −20 °C | [79] |
| THP-1 (Culture media) | EV | 4 °C and −80 °C | 1 week, 2 weeks, or 1 month | - | Stable EV concentration on all temperature | - | [75] |
| b. End.3 cells (Culture media) | Exosomes | 4 °C, −20 °C, and −80 °C | 0–28 days | 1–5 | ↓ particle number and ↑ size under all storage conditions. ↓ Number of exosomes for all freezing conditions | ↓ Amount of protein, RNA, and uptake efficiency at 4 °C. | [78] |
| CSF | EVs | Lyophilized and held at RT | 7 days | 2 | ↓37–43% in EV number. The shape of the EV is not stable | ↓ miRNAs abundance | [99] |
| Source | Type of EVs | Storage Temperature/pH/Cryopreserves | Duration | Freeze-Thaw Cycles | Physical Changes | Functional Changes | References |
|---|---|---|---|---|---|---|---|
| BALF | Exosomes | 4 °C, and −80 °C | 4 days | - | ↑ Size of exosome | ↓ protein concentration | [55] |
| Plasma | EV | 4 °C, −20 °C & −80 °C | 2 weeks–2 years | - | - | ↓ RNA or protein expression, storage at 4 °C for 2 weeks. No changes in RNA or protein expression storage at −80 °C | [85] |
| Serum | EV | RT and 4 °C | 6 h–1 week | 1, 3, and 5 | - | No changes occur in CD63, TSG101, expression, and DNA concentration at RT storage for 24 h; and 4 °C for 1 week. ↓ DNA concentration due to freeze-thaw cycles but no changes in CD63 and TSG101 expression | [65] |
| He-La cells (Culture media) | EV | Lyophilization | 48 h | - | Particle size and zeta potential stable | - | [101] |
| MSC (Culture media) | EV | −80 °C, 4 °C, RT, or lyophilized | 2–14 days | - | ↑ Particle size by −80 °C, 4 °C, RT ↑ Particle size non-significantly by lyophilization | - | [100] |
| A549 cells (Culture media) | EV | −80 °C, 4 °C, RT, or lyophilized | 2–14 days | - | ↓ Particle concentration and Particle size stable after lyophilization | - | |
| HUVEC (Culture media) | EV | −80 °C, 4 °C, RT, or lyophilized | 2–14 days | - | ↓ Particle concentration non-significantly after lyophilization | - | |
| HUVEC (Culture media) | EV | 4 °C, −80 °C and lyophilized with 4% trehalose | 14 days | - | ↑ size and % of particle recovery under all storage | ↓ Glucuronidase activity | [80] |
| Human Adipose-Derived Stem Cells (Culture media) | EV | lyophilized with trehalose or trehalose/PVP40 | 24 h | - | Particle number and size are stable | - | [102] |
| Plasma | EV | −80 °C | 6 months | 2 | ↓ Particle concentration ↑ Total protein content and particle size | - | [73] |
| Plasma | EV | −80 °C with trehalose 25 mM, DMSO 6 and 10%, glycerol 30%, PI and sodium azide at 4 °C or lyophilization with trehalose | 6 months | - | stable EV concentrations | - | [72] |
| Neutrophilic granulocytes | EV | 4 °C | 24 h | - | No changes in physiological characteristics | No changes in functional characteristics | [74] |
| Conditioned media/Urine | Exosomes | pH < 7 at RT | 30 min | - | ↑Yield and ↓ Degradation | ↑ Exosome-associated doxorubicin at pH 5 | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivanantham, A.; Jin, Y. Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos. Life 2022, 12, 697. https://doi.org/10.3390/life12050697
Sivanantham A, Jin Y. Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos. Life. 2022; 12(5):697. https://doi.org/10.3390/life12050697
Chicago/Turabian StyleSivanantham, Ayyanar, and Yang Jin. 2022. "Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos" Life 12, no. 5: 697. https://doi.org/10.3390/life12050697
APA StyleSivanantham, A., & Jin, Y. (2022). Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos. Life, 12(5), 697. https://doi.org/10.3390/life12050697






