No Reduction in Yield of Young Robusta Coffee When Grown under Shade Trees in Ecuadorian Amazonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. General Results
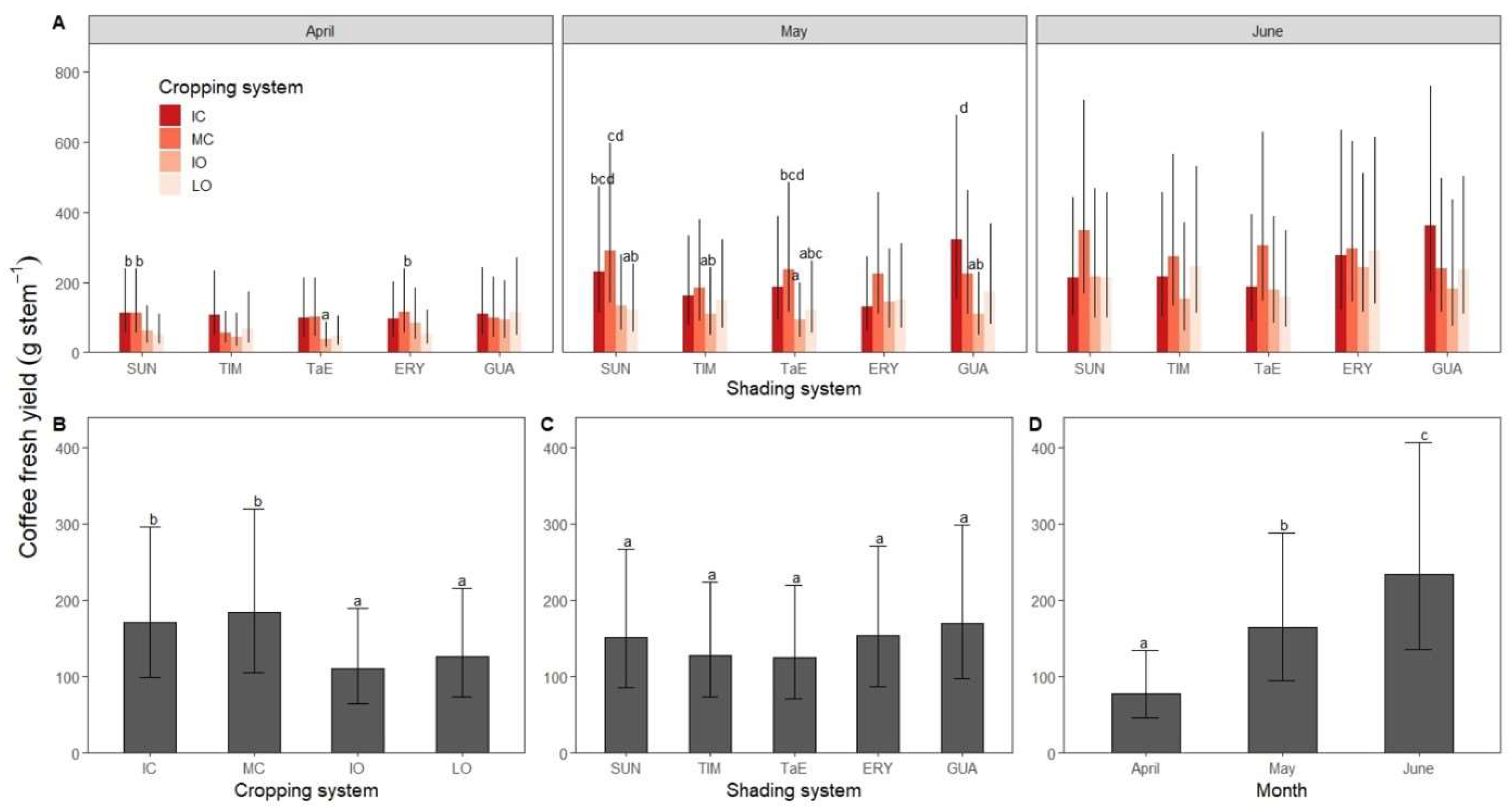
3.2. Coffee Fresh Yield
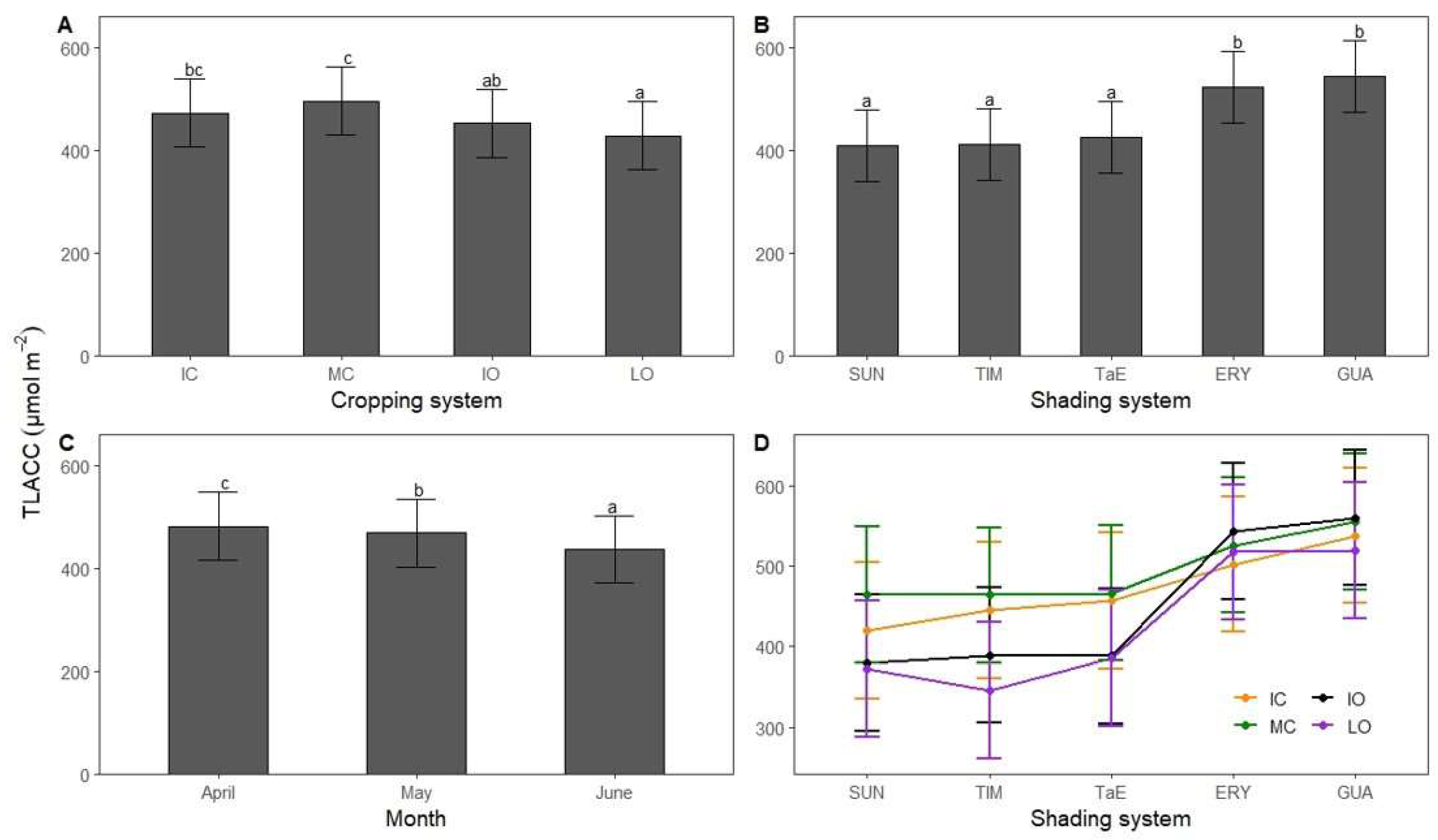
3.3. Total Leaf Absolute Chlorophyll Concentration
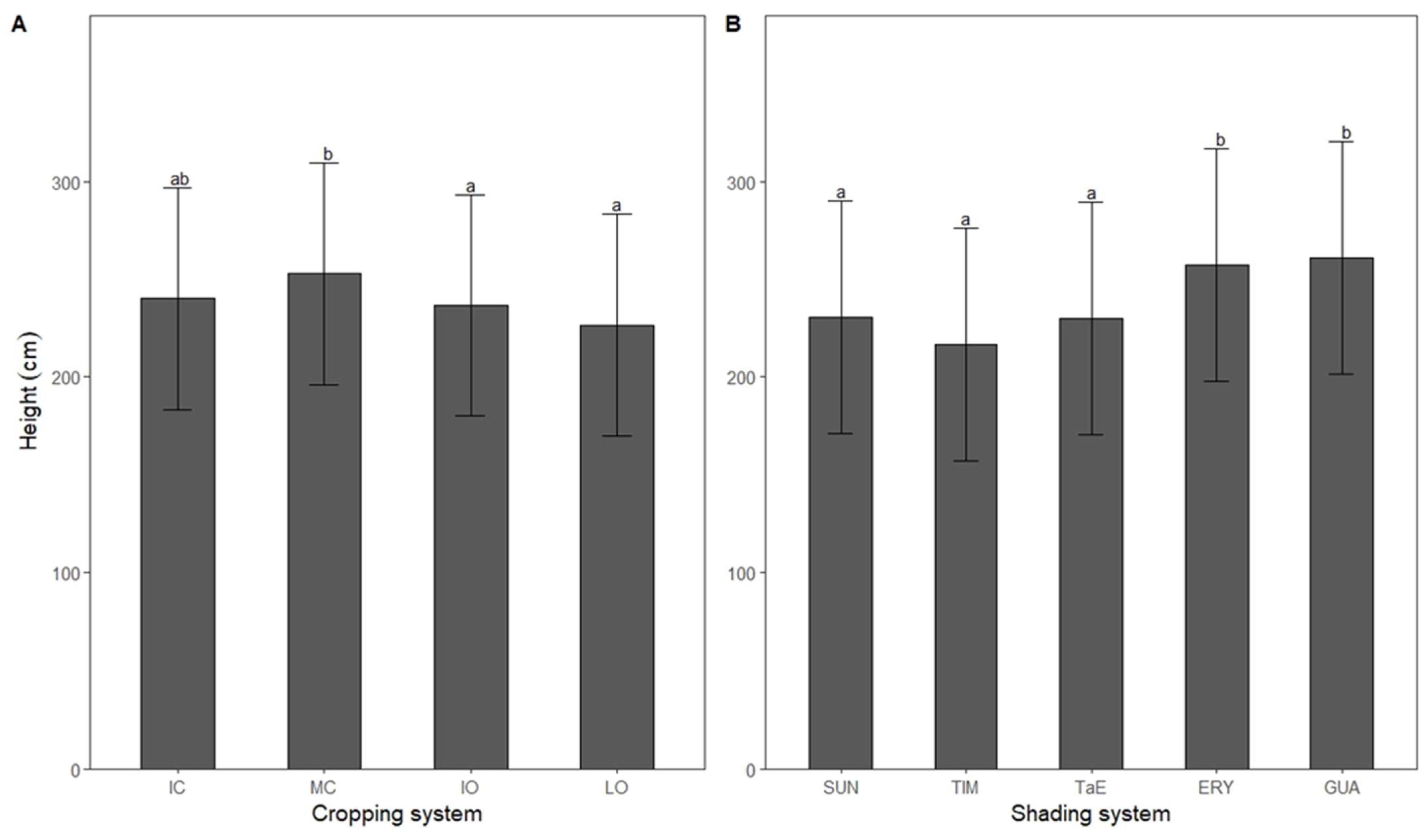
3.4. Height
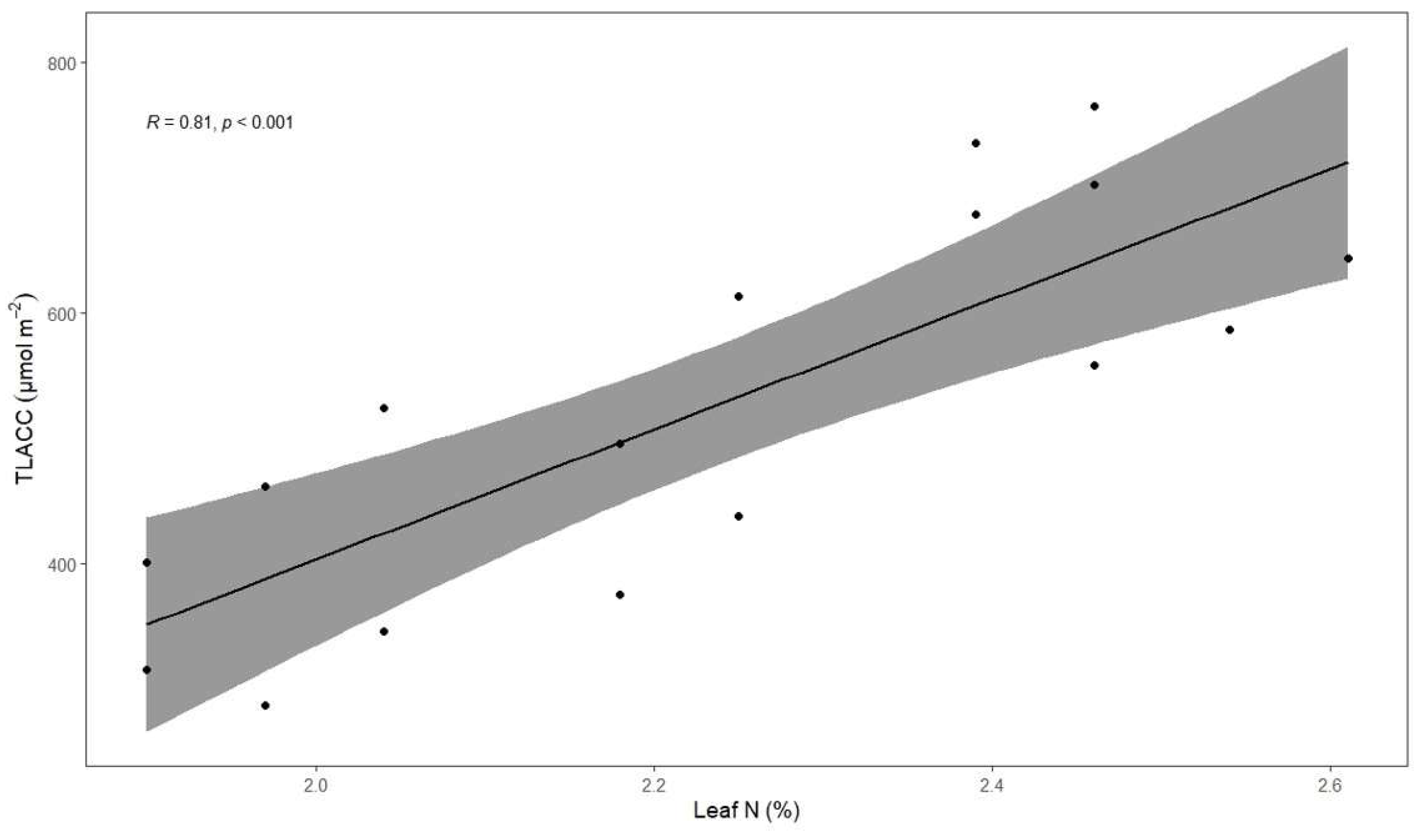
3.5. Associations between Total Leaf Absolute Chlorophyll Concentration, Coffee Fresh Yield, Height and Leaf Total N Concentration
4. Discussion
4.1. Coffee Fresh Yield
4.2. Total Leaf Absolute Chlorophyll Concentration
4.3. Height
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. PSD Data Sets: Coffee. Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/downloads (accessed on 5 October 2020).
- Waller, J.M.; Bigger, M.; Hillocks, R.J. Coffee Pests, Diseases and Their Management; CABI Pub: Wallingford, UK; Cambridge, MA, USA, 2007; ISBN 1845931297. [Google Scholar]
- Fridell, G. Coffee; Polity: Cambridge, UK, 2014; ISBN 9780745670768. [Google Scholar]
- Craparo, A.; van Asten, P.; Läderach, P.; Jassogne, L.; Grab, S.W. Coffea arabica yields decline in Tanzania due to climate change: Global implications. Agric. For. Meteorol. 2015, 207, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Torres Castillo, N.E.; Melchor-Martínez, E.M.; Ochoa Sierra, J.S.; Ramirez-Mendoza, R.A.; Parra-Saldívar, R.; Iqbal, H.M.N. Impact of climate change and early development of coffee rust—An overview of control strategies to preserve organic cultivars in Mexico. Sci. Total Environ. 2020, 738, 140225. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, M.; Rajavel, M.; Surendran, U.; Gopinath, G.; Ramamoorthy, K. Impact of climate variability on coffee yield in India—with a micro-level case study using long-term coffee yield data of humid tropical Kerala. Clim. Chang. 2017, 145, 335–349. [Google Scholar] [CrossRef]
- Pham, Y.; Reardon-Smith, K.; Mushtaq, S.; Cockfield, G. The impact of climate change and variability on coffee production: A systematic review. Clim. Chang. 2019, 156, 609–630. [Google Scholar] [CrossRef]
- Lambot, C.; Herrera, J.C.; Bertrand, B.; Sadeghian, S.; Benavides, P.; Gaitán, A. Chapter 2—Cultivating Coffee Quality—Terroir and Agro-Ecosystem. In The Craft and Science of Coffee; Folmer, B., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 17–49. ISBN 978-0-12-803520-7. [Google Scholar]
- Gomes, L.C.; Bianchi, F.; Cardoso, I.M.; Fernandes, R.; Filho, E.F.; Schulte, R. Agroforestry systems can mitigate the impacts of climate change on coffee production: A spatially explicit assessment in Brazil. Agric. Ecosyst. Environ. 2020, 294, 106858. [Google Scholar] [CrossRef]
- Moreira, C.C.; Celestino, D.; Guerra Sobrinho, T.; Cardoso, I.M.; Elliot, S.L. Agroforestry coffee soils increase the insect-suppressive potential offered by entomopathogenic fungi over full-sun soils: A case proposing a “bait survival technique”. Ecol. Evol. 2019, 9, 10777–10787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, H.R.; Grandinete, Y.C.; Manning, P.; Harper, K.A.; Cutler, G.C.; Tyedmers, P.; Righi, C.A.; Ribeiro, M.C. Forest cover enhances natural enemy diversity and biological control services in Brazilian sun coffee plantations. Agron. Sustain. Dev. 2019, 39, 50. [Google Scholar] [CrossRef] [Green Version]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES secretariat: Bonn, Germany, 2019. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAOSTAT: Crops. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 22 July 2020).
- Nieto, C.; Caicedo, C.E.V. Análisis Reflexivo Sobre el Desarrollo Agropecuario Sostenible en la Amazonia Ecuatoriana. Publicación Miscelánea, 405. INIAP: Joya de los Sachas, Ecuador, 118 p. Available online: http://repositorio.iniap.gob.ec/handle/41000/3791 (accessed on 22 July 2020).
- Alves, V.; Goulart, F.F.; Jacobson, T.K.B.; Filho, R.J.D.M.; Ribas, C.E.D.C. Shade’s Benefit: Coffee Production under Shade and Full Sun. JAS 2016, 8, 11. [Google Scholar] [CrossRef]
- Rigal, C.; Xu, J.; Hu, G.; Qiu, M.; Vaast, P. Coffee production during the transition period from monoculture to agroforestry systems in near optimal growing conditions, in Yunnan Province. Agric. Syst. 2020, 177, 102696. [Google Scholar] [CrossRef]
- Jha, S.; Bacon, C.M.; Philpott, S.M.; Ernesto Méndez, V.; Läderach, P.; Rice, R.A. Shade Coffee: Update on a Disappearing Refuge for Biodiversity. Bioscience 2014, 64, 416–428. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.B. The role of agroforestry in reducing water loss through soil evaporation and crop transpiration in coffee agroecosystems. Agric. For. Meteorol. 2010, 150, 510–518. [Google Scholar] [CrossRef]
- The Intergovernmental Panel on Climate Change. An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. Available online: https://www.ipcc.ch/srccl/ (accessed on 23 July 2020).
- Jaramillo, J.; Setamou, M.; Muchugu, E.; Chabi-Olaye, A.; Jaramillo, A.; Mukabana, J.; Maina, J.; Gathara, S.; Borgemeister, C. Climate change or urbanization? Impacts on a traditional coffee production system in East Africa over the last 80 years. PLoS ONE 2013, 8, e51815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenbergerová, L.; Cienciala, E.; Kučera, A.; Guy, L.; Habrová, H. Carbon stock in agroforestry coffee plantations with different shade trees in Villa Rica, Peru. Agrofor. Syst. 2016, 90, 433–445. [Google Scholar] [CrossRef]
- Buchanan, S.; Isaac, M.E.; van den Meersche, K.; Martin, A.R. Functional traits of coffee along a shade and fertility gradient in coffee agroforestry systems. Agrofor. Syst. 2019, 93, 1261–1273. [Google Scholar] [CrossRef]
- Sauvadet, M.; van den Meersche, K.; Allinne, C.; Gay, F.; de Melo Virginio Filho, E.; Chauvat, M.; Becquer, T.; Tixier, P.; Harmand, J.-M. Shade trees have higher impact on soil nutrient availability and food web in organic than conventional coffee agroforestry. Sci. Total Environ. 2019, 649, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Villers, L.E.; Geris, J.; Alvarado-Barrientos, M.S.; Holwerda, F.; Dawson, T. Coffee and shade trees show complementary use of soil water in a traditional agroforestry ecosystem. Hydrol. Earth Syst. Sci. 2020, 24, 1649–1668. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Alvarez, E.A.; Almazán-Núñez, R.C.; González-García, F.; Brito-Millán, M.; Méndez-Bahena, A.; García-Ibáñez, S. Shade coffee plantations maintain woody plant diversity and structure in a cloud forest landscape of southern Mexico. J. For. Res. 2020, 32, 637–648. [Google Scholar] [CrossRef]
- Durand-Bessart, C.; Tixier, P.; Quinteros, A.; Andreotti, F.; Rapidel, B.; Tauvel, C.; Allinne, C. Analysis of interactions amongst shade trees, coffee foliar diseases and coffee yield in multistrata agroforestry systems. Crop Prot. 2020, 133, 105137. [Google Scholar] [CrossRef]
- Jezeer, R.E.; Santos, M.J.; Verweij, P.A.; Boot, R.G.; Clough, Y. Benefits for multiple ecosystem services in Peruvian coffee agroforestry systems without reducing yield. Ecosyst. Serv. 2019, 40, 101033. [Google Scholar] [CrossRef]
- Avelino, J.; Vílchez, S.; Segura-Escobar, M.B.; Brenes-Loaiza, M.A.; Virginio Filho, E.d.M.; Casanoves, F. Shade tree Chloroleucon eurycyclum promotes coffee leaf rust by reducing uredospore wash-off by rain. Crop Prot. 2020, 129, 105038. [Google Scholar] [CrossRef]
- Haggar, J.; Barrios, M.; Bolaños, M.; Merlo, M.; Moraga, P.; Munguia, R.; Ponce, A.; Romero, S.; Soto, G.; Staver, C.; et al. Coffee agroecosystem performance under full sun, shade, conventional and organic management regimes in Central America. Agrofor. Syst. 2011, 82, 285–301. [Google Scholar] [CrossRef]
- Bosselmann, A.S.; Dons, K.; Oberthur, T.; Olsen, C.S.; Ræbild, A.; Usma, H. The influence of shade trees on coffee quality in small holder coffee agroforestry systems in Southern Colombia. Agric. Ecosyst. Environ. 2009, 129, 253–260. [Google Scholar] [CrossRef]
- Vaast, P.; Bertrand, B.; Perriot, J.-J.; Guyot, B.; Génard, M. Fruit thinning and shade improve bean characteristics and beverage quality of coffee (Coffea arabica L.) under optimal conditions. J. Sci. Food Agric. 2006, 86, 197–204. [Google Scholar] [CrossRef]
- Sarmiento-Soler, A.; Vaast, P.; Hoffmann, M.P.; Jassogne, L.; van Asten, P.; Graefe, S.; Rötter, R.P. Effect of cropping system, shade cover and altitudinal gradient on coffee yield components at Mt. Elgon, Uganda. Agric. Ecosyst. Environ. 2020, 295, 106887. [Google Scholar] [CrossRef]
- Siles, P.; Harmand, J.-M.; Vaast, P. Effects of Inga densiflora on the microclimate of coffee (Coffea arabica L.) and overall biomass under optimal growing conditions in Costa Rica. Agrofor. Syst. 2010, 78, 269–286. [Google Scholar] [CrossRef]
- Piato, K.; Subía, C.; Pico, J.; Calderón, D.; Norgrove, L.; Lefort, F. Organic Farming Practices and Shade Trees Reduce Pest Infestations in Robusta Coffee Systems in Amazonia. Life 2021, 11, 413. [Google Scholar] [CrossRef]
- Boreux, V.; Vaast, P.; Madappa, L.P.; Cheppudira, K.G.; Garcia, C.; Ghazoul, J. Agroforestry coffee production increased by native shade trees, irrigation, and liming. Agron. Sustain. Dev. 2016, 36, 42. [Google Scholar] [CrossRef] [Green Version]
- Moreira, S.L.; Pires, C.V.; Marcatti, G.E.; Santos, R.H.; Imbuzeiro, H.M.; Fernandes, R.B. Intercropping of coffee with the palm tree, macauba, can mitigate climate change effects. Agric. For. Meteorol. 2018, 256, 379–390. [Google Scholar] [CrossRef]
- Staver, C.; Guharay, F.; Monterroso, D.; Muschler, R.G. Designing pest-suppressive multistrata perennial crop systems: Shade-grown coffee in Central America. Agrofor. Syst. 2001, 53, 151–170. [Google Scholar] [CrossRef]
- Piato, K.; Lefort, F.; Subía, C.; Caicedo, C.; Calderón, D.; Pico, J.; Norgrove, L. Effects of shade trees on robusta coffee growth, yield and quality. A meta-analysis. Agron. Sustain. Dev. 2020, 40, 1–13. [Google Scholar] [CrossRef]
- Cannell, M.G.R. Physiology of the Coffee Crop. In Coffee: Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Willson, K.C., Clifford, M.N., Willson, K.C., Eds.; Springer: Boston, MA, USA, 1985; pp. 108–134. ISBN 978-1-4615-6657-1. [Google Scholar]
- Wintgens, J.N. The Coffee Plant. In Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders and Researchers; Wintgens, J.N., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 1–24. ISBN 9783527619627. [Google Scholar]
- Prado, S.G.; Collazo, J.A.; Irwin, R.E. Resurgence of specialized shade coffee cultivation: Effects on pollination services and quality of coffee production. Agric. Ecosyst. Environ. 2018, 265, 567–575. [Google Scholar] [CrossRef]
- Damatta, F.M. Ecophysiological constraints on the production of shaded and unshaded coffee: A review. Field Crops Res. 2004, 86, 99–114. [Google Scholar] [CrossRef]
- Araujo, A.V.; Partelli, F.L.; Oliosi, G.; Pezzopane, J.R.M. Microclimate, development and productivity of robusta coffee shaded by rubber trees and at full sun. Rev. Ciênc. Agron. 2016, 47, 700–709. [Google Scholar] [CrossRef] [Green Version]
- Partelli, F.L.; Araujo, A.V.; Vieira, H.D.; Machado Dias, J.R.; de Menezes, T.; Fernando, L.; Ramalho, J.C. Microclimate and development of ‘Conilon’ coffee intercropped with rubber trees. Pesqui. Agropecuária Bras. 2014, 49, 872–881. [Google Scholar] [CrossRef] [Green Version]
- Reis, A.R.; Favarin, J.L.; Malavolta, E.; Júnior, J.L.; Moraes, M.F. Photosynthesis, Chlorophylls, and SPAD Readings in Coffee Leaves in Relation to Nitrogen Supply. Commun. Soil Sci. Plant Anal. 2009, 40, 1512–1528. [Google Scholar] [CrossRef]
- Cheng, B.; Smyth, H.E.; Furtado, A.; Henry, R.J. Slower development of lower canopy beans produces better coffee. J. Exp. Bot. 2020, 71, 4201–4214. [Google Scholar] [CrossRef] [PubMed]
- Pompelli, M.F.; Martins, S.C.V.; Antunes, W.C.; Chaves, A.R.M.; Damatta, F.M. Photosynthesis and photoprotection in coffee leaves is affected by nitrogen and light availabilities in winter conditions. J. Plant Physiol. 2010, 167, 1052–1060. [Google Scholar] [CrossRef]
- Gutierrez, M.V.; Meinzer, F.C. Carbon Isotope Discrimination and Photosynthetic Gas Exchange in Coffee Hedgerows during Canopy Development. Funct. Plant Biol. 1994, 21, 207–219. [Google Scholar] [CrossRef]
- Damatta, F.M.; Loos, R.A.; Silva, E.A.; Loureiro, M.E. Limitations to photosynthesis in Coffea canephora as a result of nitrogen and water availability. J. Plant Physiol. 2002, 159, 975–981. [Google Scholar] [CrossRef]
- Franck, N.; Vaast, P. Limitation of coffee leaf photosynthesis by stomatal conductance and light availability under different shade levels. Trees 2009, 23, 761–769. [Google Scholar] [CrossRef]
- Fahl, J.I.; Carelli, M.L.C.; Vega, J.; Magalhães, A.C. Nitrogen and irradiance levels affecting net photosynthesis and growth of young coffee plants (Coffea arabica L.). J. Hortic. Sci. 1994, 69, 161–169. [Google Scholar] [CrossRef]
- Ponce Vaca, L.A.; Acuna Velazquez, I.R.; Proano Ponce, W.P.; Oreliana Suarez, K.D. The coffee agroforestry system. Its importance for the agro-alimentary and nutritional security in Ecuador. Rev. Cuba. De Cienc. For. 2018, 6, 116–129. [Google Scholar]
- Davis, A.; Govaerts, R.; Bridson, D.; Stoffelen, P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef] [Green Version]
- Nesper, M.; Kueffer, C.; Krishnan, S.; Kushalappa, C.G.; Ghazoul, J. Shade tree diversity enhances coffee production and quality in agroforestry systems in the Western Ghats. Agric. Ecosyst. Environ. 2017, 247, 172–181. [Google Scholar] [CrossRef]
- Tumwebaze, S.B.; Byakagaba, P. Soil organic carbon stocks under coffee agroforestry systems and coffee monoculture in Uganda. Agric. Ecosyst. Environ. 2016, 216, 188–193. [Google Scholar] [CrossRef]
- Ferwerda, F.P. Coffee Breeding in Java. Econ. Bot. 1948, 2, 258–272. [Google Scholar] [CrossRef]
- Mariño, Y.A.; Vega, V.J.; García, J.M.; Rodrigues, V.; García, N.M.; Bayman, P. The Coffee Berry Borer (Coleoptera: Curculionidae) in Puerto Rico: Distribution, infestation, and population per fruit. J. Insect. Sci. 2017, 17, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Damatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Ecophysiology of coffee growth and production. Braz. J. Plant Physiol. 2007, 19, 485–510. [Google Scholar] [CrossRef] [Green Version]
- Beer, J.; Muschler, R.; Kass, D.; Somarriba, E. Shade management in coffee and cacao plantations. Agrofor. Syst. 1997, 38, 139–164. [Google Scholar] [CrossRef]
- Cerda, R.; Allinne, C.; Gary, C.; Tixier, P.; Harvey, C.A.; Krolczyk, L.; Mathiot, C.; Clément, E.; Aubertot, J.-N.; Avelino, J. Effects of shade, altitude and management on multiple ecosystem services in coffee agroecosystems. Eur. J. Agron. 2017, 82, 308–319. [Google Scholar] [CrossRef]
- Ehrenbergerova, L.; Senfeldr, M.; Habrova, H. Impact of tree shading on the microclimate of a coffee plantation: A case study from the Peruvian Amazon. Bois Et For. Des Trop. 2017, 334, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Glover, N.; Beer, J. Nutrient cycling in two traditional Central American agroforestry systems. Agrofor. Syst. 1986, 4, 77–87. [Google Scholar] [CrossRef]
- Haggar, J.P.; Tanner, E.; Beer, J.W.; Kass, D. Nitrogen dynamics of tropical agroforestry and annual cropping systems. Soil Biol. Biochem. 1993, 25, 1363–1378. [Google Scholar] [CrossRef]
- Batterman, S.A.; Hedin, L.O.; van Breugel, M.; Ransijn, J.; Craven, D.J.; Hall, J.S. Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature 2013, 502, 224–227. [Google Scholar] [CrossRef]
- Sierra, J.; Nygren, P. Transfer of N fixed by a legume tree to the associated grass in a tropical silvopastoral system. Soil Biol. Biochem. 2006, 38, 1893–1903. [Google Scholar] [CrossRef]
- Vargas Tierras, Y.B.; Prado-Beltrán, J.K.; Nicolalde-Cruz, J.R.; Casanoves, F.; Virginio-Filho, E.d.M.; Viera Arroyo, W.F. Caracterización y rol de los frutales amazónicos en fincas familiares en las provincias de Sucumbíos y Orellana (Ecuador). CTA 2018, 19, 485–499. [Google Scholar] [CrossRef] [Green Version]
- Barrance, A.; Beer, J.; Boshier, D.H.; Chamberlain, J.; Cordero, J.; Detlefsen, G.; Finegan, B.; Galloway, G.; Gómez, M.; Gordon, J.; et al. Inga edulis Mart. In Árboles de Centroamérica: Un Manual Para Extensionistas; Cordero, J., Ed.; CATIE: Turrialba, Costa Rica, 2003; pp. 605–606. ISBN 0850741610. [Google Scholar]
- Kass, D.L. Erythrina species: Pantropical multipurpose tree legumes. In Forage Tree Legumes in Tropical Agriculture; Gutteridge, R.C., Shelton, H.M., Eds.; CAB International: Wallingford, UK, 1994; pp. 84–96. ISBN 0851988687. [Google Scholar]
- Riesmeier, M.; Mattonai, M.; Wong, S.S.; Veall, M.-A.; Betts, J.; Johnston, M.; Ribechini, E.; Devièse, T. Molecular profiling of Peru Balsam reveals active ingredients responsible for its pharmaceutical properties. Nat. Prod. Res. 2020, 35, 5311–5316. [Google Scholar] [CrossRef]
- Schnabel, F.; Filho, E.D.M.V.; Xu, S.; Fisk, I.D.; Roupsard, O.; Haggar, J. Shade trees: A determinant to the relative success of organic versus conventional coffee production. Agrofor. Syst. 2017, 92, 1535–1549. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture (USDA); Natural Resources Conservation Service (NRCS): Washington, DC, USA, 2014; ISBN 9780359573240.
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Holdridge, L.R.; Tosi, J.A. Life Zone Ecology; Tropical Science Center: San Jose, CA, USA, 1967. [Google Scholar]
- INAMHI. Mapa de Temperatura Media Multianual: Serie 1965–1999; Instituto Nacional de Meteorología e Hidrología (INAMHI): Quito, Ecuador, 2000. [Google Scholar]
- INAMHI. Mapa de Precipitación Media Multianual: Serie 1965–1999; Instituto Nacional de Meteorología e Hidrología (INAMHI): Quito, Ecuador, 2000. [Google Scholar]
- INAMHI. Meteorological Data; EECA: La Joya de los Sachas, Ecuador, 2020. [Google Scholar]
- Bote, A.D.; Ayalew, B.; Ocho, F.L.; Anten, N.P.; Vos, J. Analysis of coffee (Coffea arabica L.) performance in relation to radiation levels and rates of nitrogen supply I. Vegetative growth, production and distribution of biomass and radiation use efficiency. Eur. J. Agron. 2018, 92, 115–122. [Google Scholar] [CrossRef]
- Morais, H.; Marur, C.J.; Caramori, P.H.; Ribeiro, A.M.d.A.; Gomes, J.C. Características fisiológicas e de crescimento de cafeeiro sombreado com guandu e cultivado a pleno sol. Pesqui. Agropecuária Bras. 2003, 38, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Netto, A.T.; Campostrini, E.; Oliveira, J.G.d.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Salinas, J.G.; García, R. Métodos Químicos Para el Análisis de Suelos Ácidos y Plantas Forrajeras; Centro Internacional de Agricultura Tropical, Programa de Pastos Tropicales: Cali, Colombia, 1985; ISBN 8489206503. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008; ISBN 978-0-387-75968-5. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Russell, V.L. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.G.; Carl, P.; Boudt, K.; Bennett, R.; Ulrich, J.; Zivot, E.; Cornilly, D.; Hung, E.; Lestel, M.; Balkissoon, K.; et al. Performance Analytics: Econometric Tools for Performance and Risk Analysis, R Package Version 2.0.4; 2020. Available online: https://cran.r-project.org/web/packages/PerformanceAnalytics/PerformanceAnalytics.pdf (accessed on 17 September 2021).
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots, R Pacckage. Version 0.4.0.; 2020. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 17 September 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- De Oliveira Aparecido, L.E.; Rolim, G.d.S.; Da Moraes, J.R.S.C.d.; Valeriano, T.T.B.; Lense, G.H.E. Maturation periods for Coffea arabica cultivars and their implications for yield and quality in Brazil. J. Sci. Food Agric. 2018, 98, 3880–3891. [Google Scholar] [CrossRef] [PubMed]
- Bote, A.D.; Struik, P.C. Effects of shade on growth, production and quality of coffee (Coffea arabica) in Ethiopia. J. Hortic. For. 2011, 3, 336–341. [Google Scholar]
- Sarmiento-Soler, A.; Rötter, R.P.; Hoffmann, M.P.; Jassogne, L.; van Asten, P.; Graefe, S.; Vaast, P. Disentangling effects of altitude and shade cover on coffee fruit dynamics and vegetative growth in smallholder coffee systems. Agric. Ecosyst. Environ. 2022, 326, 107786. [Google Scholar] [CrossRef]
- Merle, I.; Villarreyna-Acuña, R.; Ribeyre, F.; Roupsard, O.; Cilas, C.; Avelino, J. Microclimate estimation under different coffee-based agroforestry systems using full-sun weather data and shade tree characteristics. Eur. J. Agron. 2022, 132, 126396. [Google Scholar] [CrossRef]
- Ehrenbergerová, L.; Klimková, M.; Cano, Y.G.; Habrová, H.; Lvončík, S.; Volařík, D.; Khum, W.; Němec, P.; Kim, S.; Jelínek, P.; et al. Does Shade Impact Coffee Yield, Tree Trunk, and Soil Moisture on Coffea canephora Plantations in Mondulkiri, Cambodia? Sustainability 2021, 13, 13823. [Google Scholar] [CrossRef]
- Chaves, A.R.; Martins, S.C.; Batista, K.D.; Celin, E.F.; Damatta, F.M. Varying leaf-to-fruit ratios affect branch growth and dieback, with little to no effect on photosynthesis, carbohydrate or mineral pools, in different canopy positions of field-grown coffee trees. Environ. Exp. Bot. 2012, 77, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Reis, A.R.; Furlani Junior, E.; Buzetti, S.; Andreotti, M. Diagnóstico da exigência do cafeeiro em nitrogênio pela utilização do medidor portátil de clorofila. Bragantia 2006, 65, 163–171. [Google Scholar] [CrossRef]
- Rodríguez-López, N.F.; Cavatte, P.C.; Silva, P.E.M.; Martins, S.C.V.; Morais, L.E.; Medina, E.F.; Damatta, F.M. Physiological and biochemical abilities of robusta coffee leaves for acclimation to cope with temporal changes in light availability. Physiol. Plant. 2013, 149, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.C.; Pons, T.L.; Groeneveld, H.W.; Azinheira, H.G.; Nunes, M.A. Photosynthetic acclimation to high light conditions in mature leaves of Coffea arabica L.: Role of xanthophylls, quenching mechanisms and nitrogen nutrition. Funct. Plant Biol. 2000, 27, 43–51. [Google Scholar] [CrossRef]
- Reis, A.R.; Favarin, J.L.; Gratão, P.L.; Capaldi, F.R.; Azevedo, R.A. Antioxidant metabolism in coffee (Coffea arabica L.) plants in response to nitrogen supply. Theor. Exp. Plant Physiol. 2015, 27, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Dossa, E.L.; Fernandes, E.C.M.; Reid, W.S.; Ezui, K. Above- and belowground biomass, nutrient and carbon stocks contrasting an open-grown and a shaded coffee plantation. Agrofor. Syst. 2008, 72, 103–115. [Google Scholar] [CrossRef]
- Anim-Kwapong, G.J.; Anim-Kwapong, E.; Amoah, F.M. Nutrient status and rooting of Robusta coffee (Coffea canephora Pierre ex Froehner) cuttings from stock plants under Gliricidia sepium. Trop. Agr. 1999, 76, 263–268. [Google Scholar]
- Ordoñez, J.C.; van Bodegom, P.M.; Witte, J.-P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Mamani-Pati, F.; Clay, D.E.; Clay, S.A.; Smeltekop, H.; Yujra-Callata, M.A. The Influence of Strata on the Nutrient Recycling within a Tropical Certified Organic Coffee Production System. ISRN Agron. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Celentano, D.; Zahawi, R.A.; Finegan, B.; Ostertag, R.; Cole, R.J.; Holl, K.D. Litterfall Dynamics Under Different Tropical Forest Restoration Strategies in Costa Rica. Biotropica 2011, 43, 279–287. [Google Scholar] [CrossRef]
- Duarte, E.M.G.; Cardoso, I.M.; Stijnen, T.; Mendonça, M.A.F.C.; Coelho, M.S.; Cantarutti, R.B.; Kuyper, T.W.; Villani, E.M.A.; Mendonça, E.S. Decomposition and nutrient release in leaves of Atlantic Rainforest tree species used in agroforestry systems. Agrofor. Syst. 2013, 87, 835–847. [Google Scholar] [CrossRef]
- Palm, C.A.; Sanchez, P.A. Decomposition and Nutrient Release Patterns of the Leaves of Three Tropical Legumes. Biotropica 1990, 22, 330–338. [Google Scholar] [CrossRef]
- Amaral, J.T.; Da Matta, F.; Rena, A. Effects of fruiting on the growth of Arabica coffee trees as related to carbohydrate and nitrogen status and to nitrate reductase activity. Rev. Bras. Fisiol. Veg. 2001, 13, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Partelli, F.L.; Oliveira, M.G.; Covre, A.M.; Vieira, H.D.; Machado Dias, J.R.; Braun, H. Nutritional standards and nutritional diagnosis of the Conilon coffee plants in phenological growth stages. J. Plant Nutr. 2018, 41, 2536–2546. [Google Scholar] [CrossRef]
- Neto, A.P.; Favarin, J.L.; de Almeida, R.E.M.; dos Santos Dias, C.T.; Tezotto, T.; Alves, A.L.G.; Moraes, M.F. Changes of Nutritional Status during a Phenological Cycle of Coffee under High Nitrogen Supply by Fertigation. Commun. Soil Sci. Plant Anal. 2011, 42, 2414–2425. [Google Scholar] [CrossRef]
- Venancio, L.P.; Do Amaral, J.F.T.; Cavatte, P.C.; Vargas, C.T.; Dos Reis, E.F.; Dias, J.R. Vegetative growth and yield of robusta coffee genotypes cultivated under different shading levels. Biosci. J. 2019, 35, 1490–1503. [Google Scholar] [CrossRef] [Green Version]
- Charbonnier, F.; Roupsard, O.; Le Maire, G.; Guillemot, J.; Casanoves, F.; Lacointe, A.; Vaast, P.; Allinne, C.; Audebert, L.; Cambou, A.; et al. Increased light-use efficiency sustains net primary productivity of shaded coffee plants in agroforestry system. Plant Cell Environ. 2017, 40, 1592–1608. [Google Scholar] [CrossRef]
- Casal, J.J. Shade avoidance. Arab. Book 2012, 10, e0157. [Google Scholar] [CrossRef] [Green Version]
- Van Long, N.; Ngoc, N.Q.; Dung, N.N.; Kristiansen, P.; Yunusa, I.; Fyfe, C. The Effects of Shade Tree Types on Light Variation and Robusta Coffee Production in Vietnam. Engineering 2015, 7, 742–753. [Google Scholar] [CrossRef]
- Aristizábal, L.F.; Johnson, M.; Shriner, S.; Hollingsworth, R.; Manoukis, N.C.; Myers, R.; Bayman, P.; Arthurs, S.P. Integrated Pest Management of Coffee Berry Borer in Hawaii and Puerto Rico: Current Status and Prospects. Insects 2017, 8, 123. [Google Scholar] [CrossRef] [Green Version]



| Total Precipitation (mm) | Mean Daily Temperature (°C) | Relative Humidity (%) | ||||
|---|---|---|---|---|---|---|
| Month | Max | Min | 7 a.m. | 1 p.m. | 7 p.m. | |
| April | 86 | 33 | 19 | 97.5 | 92 | 96 |
| May | 50 | 32.5 | 20.5 | 97 | 90 | 96 |
| June | 238.5 | 31 | 21.5 | 97 | 85 | 92 |
| Shading System | |||||
|---|---|---|---|---|---|
| SUN | TIM | ERY | GUA | TaE | |
| Shade Tree Species | - | Myroxylon balsamum | Erythrina spp. | Inga edulis | M. balsamum; E. spp. |
| # of shade tree species | 0 | 1 | 1 | 1 | 2 |
| Shade tree density (ha−1) | 0 | 83 | 333 | 83 | 83 |
| Shade amount (%) | 0 | 7 | 25 | 24 | 9 |
| # of shade tree pruning (year−1) | 0 | 0 | 1–2 | 1 | 1–2 |
| Pruning type | - | - | ½ pollarding at 2 m; ½ crown reduction (50%) | Crown thinning | Only E. spp. crown reduction (50%) |
| Response Variable | Factor | Sum of sq. | Mean sq. | Num. d.f. | Den. D.f. | F-Value | p-Value | |
|---|---|---|---|---|---|---|---|---|
| CFY (g) | M | 166.6 | 83.3 | 2 | 869.6 | 277.2 | <0.001 | *** |
| CS | 11.1 | 3.7 | 3 | 37.9 | 12.3 | <0.001 | *** | |
| SS | 3.3 | 0.8 | 4 | 38.2 | 2.8 | 0.042 | * | |
| M:CS | 5.6 | 1.0 | 6 | 857 | 3.2 | 0.003 | ** | |
| M:SS | 3.9 | 0.5 | 8 | 864.9 | 1.6 | 0.113 | ||
| CS:SS | 4.5 | 0.4 | 12 | 37.1 | 1.3 | 0.288 | ||
| M:CS:SS | 12.9 | 0.5 | 24 | 846.9 | 1.8 | 0.011 | * | |
| Height (cm) | CS | 19,199 | 6399.7 | 3 | 38.2 | 7.4 | <0.001 | *** |
| SS | 63,632 | 15,908.1 | 4 | 38.1 | 18.4 | <0.001 | *** | |
| CS:SS | 19,941 | 1661.8 | 12 | 38 | 1.9 | 0.062 | ||
| TLACC (μmol m−2) | M | 1,167,195 | 58,3598 | 2 | 3133.2 | 75.8 | <0.001 | *** |
| CS | 391,475 | 130,492 | 3 | 38.2 | 16.9 | <0.001 | *** | |
| SS | 2,178,395 | 544,599 | 4 | 38.17 | 70.7 | <0.001 | *** | |
| M:CS | 59,060 | 9843 | 6 | 3133.2 | 1.3 | 0.263 | ||
| M:SS | 102,495 | 12,812 | 8 | 3133.2 | 1.7 | 0.102 | ||
| CS:SS | 300,364 | 25,030 | 12 | 38.03 | 3.3 | 0.003 | ** | |
| M:CS:SS | 126,354 | 5265 | 24 | 3133.2 | 0.7 | 0.872 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piato, K.; Subía, C.; Lefort, F.; Pico, J.; Calderón, D.; Norgrove, L. No Reduction in Yield of Young Robusta Coffee When Grown under Shade Trees in Ecuadorian Amazonia. Life 2022, 12, 807. https://doi.org/10.3390/life12060807
Piato K, Subía C, Lefort F, Pico J, Calderón D, Norgrove L. No Reduction in Yield of Young Robusta Coffee When Grown under Shade Trees in Ecuadorian Amazonia. Life. 2022; 12(6):807. https://doi.org/10.3390/life12060807
Chicago/Turabian StylePiato, Kevin, Cristian Subía, François Lefort, Jimmy Pico, Darío Calderón, and Lindsey Norgrove. 2022. "No Reduction in Yield of Young Robusta Coffee When Grown under Shade Trees in Ecuadorian Amazonia" Life 12, no. 6: 807. https://doi.org/10.3390/life12060807
APA StylePiato, K., Subía, C., Lefort, F., Pico, J., Calderón, D., & Norgrove, L. (2022). No Reduction in Yield of Young Robusta Coffee When Grown under Shade Trees in Ecuadorian Amazonia. Life, 12(6), 807. https://doi.org/10.3390/life12060807






