Management of Combined Therapy (Ceritinib, A. cinnamomea, G. lucidum, and Photobiomodulation) in Advanced Non-Small-Cell Lung Cancer: A Case Report
Abstract
:1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamamoto, H.; Toyooka, S.; Mitsudomi, T. Impact of EGFR mutation analysis in non-small cell lung cancer. Lung Cancer 2009, 63, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.I.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Johne, W.; Paz-Ares, L.; Schmid-Bindert, G. KRAS-mutant non-small cell lung cancer: From biology to therapy. Lung Cancer 2018, 124, 53–64. [Google Scholar]
- Davies, K.D.; Le, A.T.; Theodoro, M.F.; Skokan, M.C.; Aisner, D.L.; Berge, E.M.; Terracciano, L.M.; Cappuzzo, F.; Incarbone, M.; Roncalli, M.; et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin. Cancer Res. 2012, 18, 4570–4579. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Torres, J.M.; Viteri, S.; Molina, M.A.; Rosell, R. BRAF mutant non-small cell lung cancer and treatment with BRAF inhibitors. Transl. Lung Cancer Res. 2013, 2, 244–250. [Google Scholar] [PubMed]
- Chuang, J.C.; Stehr, H.; Lianget, Y.; Das, M.; Huang, J.; Diehn, M.; Wakelee, H.A.; Neal, J.W. ERBB2-mutated metastatic non–small cell lung cancer: Response and resistance to targeted therapies. J. Thorac. Oncol. 2017, 12, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Stinchcombe, T.E. Current management of RET rearranged non-small cell lung cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920928634. [Google Scholar] [CrossRef]
- Pasquini, G.; Giaccone, G. C-MET inhibitors for advanced non-small cell lung cancer. Expert Opin. Investig. Drugs 2018, 27, 363–375. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Morgensztern, D.; Ng, S.H.; Gao, F.; Govindan, R. Trends in stage distribution for patients with non-small cell lung cancer: A national cancer database survey. J. Thorac. Oncol. 2010, 5, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Henk, H.J.; Ray, S. Treatment patterns and healthcare costs among patients with advanced non-small-cell lung cancer. Lung Cancer Manag. 2013, 2, 189–197. [Google Scholar] [CrossRef]
- Lu, T.; Yang, X.; Huang, Y.; Zhao, M.; Li, M.; Ma, K.; Yin, J.; Zhan, C.; Wang, Q. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res. 2019, 11, 943–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikhailov, V.A.; Skobelkin, O.K.; Denisov, I.N.; Frank, G.A.; Voltchenko, N.N. Investigations on the influence of low level diode laser irradiation on the growth of experimental tumours. Laser Ther. 1993, 5, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Skobelkin, O.K.; Michailov, V.A.; Zakharov, S.D. Preoperative activation of the immune system by low reactive level laser therapy (LLLT) in oncologic patients: A preliminary report. Laser Ther. 1991, 3, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Zecha, J.A.E.M.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Sonis, S.T.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.J.; et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 1: Mechanisms of action, dosimetric, and safety considerations. Support. Care Cancer 2016, 24, 2781–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zecha, J.A.E.M.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.J.; Genot, M.T.; et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 2: Proposed applications and treatments protocols. Support. Care Cancer 2016, 24, 2793–2805. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Chen, T.S.; Xing, D.; Wang, J.J.; Wu, Y.X. Measuring dynamics of caspase-3 activity in living cells using FRET technique during apoptosis induced by high fluence low-power laser irradiation. Lasers Surg. Med. 2005, 36, 2–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Zhang, M.; Zhang, L. Ganoderma sinense polysaccharide: An adjunctive drug used for cancer treatment. Prog. Mol. Biol. Transl. Sci. 2019, 163, 165–177. [Google Scholar]
- Sone, Y.; Okuda, R.; Wada, N.; Kishida, E.; Misaki, A. Structure and antitumor activities of the polysaccharides isolated from fruiting body and the growing culture of mycelium of Ganoderma lucidum. Agric. Biol. Chem. 1985, 49, 2641–2653. [Google Scholar]
- Mizuno, T.; Wang, G.; Zhang, J.; Kawagishi, H.; Nishitoba, T.; Li, J. Reishi, Ganoderma lucidum and Ganoderma tsugae: Bioactive substances and medicinal effects. Food Rev. Int. 1995, 11, 151–166. [Google Scholar] [CrossRef]
- Ooi, V.E.; Liu, F. Immunomodulation an anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.X.; Lin, Z.B.; Li, X.J.; Li, M.; Lu, J.; Duan, X.S.; Ge, Z.H.; Song, Y.X.; Xing, E.H.; Li, W.D. Promoting effects of Ganoderma lucidum polysaccharides on B16F10 cells to activate lymphocytes. Basic Clin. Pharmacol. Toxicol. 2011, 108, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Huang, T.S.; Hsu, M.L.; Chen, C.C.; Lin, W.S.; Lu, F.J.; Chang, W.H. Antitumor effects of the partially purified polysaccharides from Antrodia camphorata and the mechanism of its action. Toxicol. Appl. Pharmacol. 2004, 201, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Hu, C.T.; Weng, C.F. Antrodia Cinnamomea Prolongs Survival in a Patient with Small Cell Lung Cancer. Medicina 2019, 55, 640. [Google Scholar] [CrossRef] [Green Version]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef]
- Henriques, Á.C.G.; Ginani, F.; Oliveira, R.M.; Keesen, T.S.L.; Galvão Barboza, C.A.; Oliveira Rocha, H.A.; Castro, J.F.L.; Della Coletta, R.; Almeida Freitas, R. Low-level laser therapy promotes proliferation and invasion of oral squamous cell carcinoma cells. Laser Med. Sci. 2014, 29, 1385–1395. [Google Scholar] [CrossRef]
- Renno, A.C.M.; McDonnell, P.A.; Parizotto, N.A.; Laakso, E.L. The Effects of Laser Irradiation on Osteoblast and Osteosarcoma Cell Proliferation and Differentiation in Vitro. Photomed. Laser Surg. 2007, 25, 275–280. [Google Scholar] [CrossRef]
- Pinheiro, A.L.B.; Nascimento, S.C.; Vieira, A.L.B.; Brugnera, A., Jr.; Zanin, F.A.; Rolim, A.B.; Silva, P.S. Effects of Low-Level Laser Therapy on Malignant Cells: In Vitro Study. J. Clin. Laser Med. Surg. 2002, 20, 23–26. [Google Scholar] [CrossRef]
- Hode, L. Low-Level Laser Therapy May Have Cancer Fighting Role. Photomed. Laser Surg. 2006, 34, 221–222. [Google Scholar] [CrossRef]
- Mikhailov, V.A.; Denisov, I.N.; Frank, G.A.; Voltchenko, N.N. Results of treatment of patients with second- to third-stage breast cancer by combination of low-level laser therapy (LLLT) and surgery: Ten-year experience. In Laser Florence ‘99: A Window on the Laser Medicine World, Proceeding of the SPIE Laser Florence’ 99, Florence, Italy, 28–31 October 1999; Proceedings of SPIE; SPIE: Bellingham, WA, USA, 2000; Volume 4166, pp. 40–42. [Google Scholar]
- Friboulet, L.; Li, N.; Katayama, R.; Lee, C.C.; Gainor, J.F.; Crystal, A.S.; Michellys, P.Y.; Awad, M.M.; Yanagitani, N.; Kim, S.; et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014, 4, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Hseu, Y.C.; Chen, S.C.; Chen, H.C.; Liao, J.W.; Yang, H.L. Antrodia camphorata inhibits proliferation of human breast cancer cells in vitro and in vivo. Food Chem. Toxicol. 2008, 46, 2680–2688. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pan, C.L.; Yao, Y.C.; Chang, S.S.; Li, S.L.; Wu, T.F. Proteomic analysis of the effect of Antrodia camphorate extract on human lung cancer A549 cell. Proteomics 2006, 6, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Liu, F.C.; Chou, P.Y.; Chien, Y.C.; Chang, W.S.W.; Huang, G.J.; Wu, C.H.; Sheu, M.J. Ethanol extracts of fruiting bodies of Antrodia cinnamomea suppress CL1-5 human lung adenocarcinoma cells migration by inhibiting matrix metalloproteinase-2/9 through ERK, JNK, p38, and PI3K/Akt signaling pathways. Evid. Based Complement. Altern. Med. 2012, 2012, 378415. [Google Scholar]
- Huang, Y.L.; Chu, Y.L.; Ho, C.T.; Chung, J.G.; Lai, C.I.; Su, Y.C.; Kuo, Y.H.; Sheen, L.Y. Antcin K, an Active Triterpenoid from the Fruiting Bodies of Basswood-Cultivated Antrodia cinnamomea, Inhibits Metastasis via Suppression of Integrin-Mediated Adhesion, Migration, and Invasion in Human Hepatoma Cells. J. Agric. Food Chem. 2015, 63, 4561–4569. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.J.; Lee, S.S.; Lee, S.T.; Lin, W.W. Signaling mechanisms of enhanced neutrophil phagocytosis and chemotaxis by the polysaccharide purified from Ganoderma lucidum. Br. J. Pharmacol. 2003, 139, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Gou, X.; Xue, H.; Liu, K. Ganoderan (GDN) regulates the growth, motility and apoptosis of non-small cell lung cancer cells through ERK signaling pathway in vitro and in vivo. Oncol. Targets Ther. 2019, 12, 8821–8832. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhou, S.; Jiang, W.; Huang, M.; Dai, X. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol. Investig. 2003, 32, 201–215. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Z.P.; Yang, X.X.; Huang, M.; Gao, Y.; Tang, W.; Chan, S.Y.; Dai, X.; Ye, J.; Ho, P.C.; et al. Monitoring of immune responses to a herbal immuno-modulator in patients with advanced colorectal cancer. Int. Immunopharmacol. 2006, 6, 499–508. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, H.; Chan, E.; Tang, W.; Xu, A.; Yang, H.; Huang, M.; Lan, J.; Li, X.; Duan, W.; et al. Antitumor activity and underlying mechanisms of ganopoly, the refined polysaccharides extracted from Ganoderma lucidum, in mice. Immunol. Investig. 2005, 34, 171–198. [Google Scholar] [CrossRef]
- Haslett, P.A. Anticytokine approaches to the treatment of anorexia and cachexia. Semin. Oncol. 1998, 25, 53–57. [Google Scholar]
- Gelin, J.; Moldawer, L.L.; Lönnroth, C.; Sherry, B.; Chizzonite, R.; Lundholm, K. Role of Endogenous Tumor Necrosis Factor α and Interleukin 1 for Experimental Tumor Growth and the Development of Cancer Cachexia. Cancer Res. 1991, 51, 415–421. [Google Scholar] [PubMed]
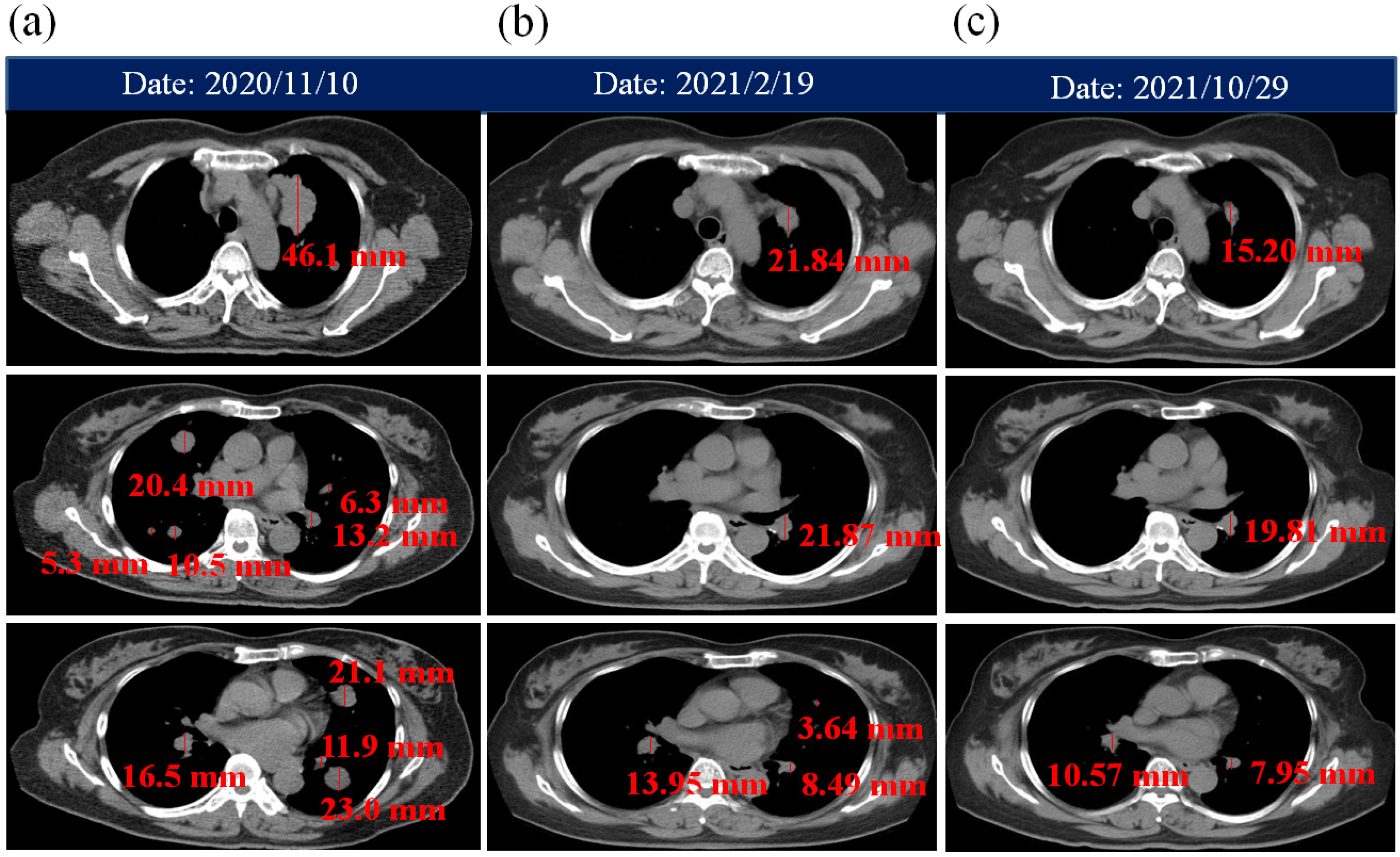


| Time | Image Modality | Clinical Findings | Combined Therapy |
|---|---|---|---|
| 10 November 2020 | Chest CT | Left upper lung cancer with lung-to-lung metastases. The size of left upper lung cancer tissue was larger than 5 cm. | ACGL and PBM therapy:
|
| 20 November 2020 | - | - | Increase ACGL dose: 8 capsules of ACGL per day. |
| 27 November 2020 | Brain CT | No definite metastatic lesion. | |
| 30 November 2020 | - | - | Increase ACGL dose: 10 capsules of ACGL per day |
| 18 December 2020 | - | Lung adenocarcinoma confirmed diagnosis (advanced NSCLC, Stage IVa) | Ceritinib, PBM and ACGL:
|
| 24 January 2021 | - |
| PBM therapy:
|
| 27 January 2021 | - | Severe dizziness (Side effect of Loperamide HCL). | PBM therapy: Severe dizziness stopped after 830 nm laser (30 mW, 20 min, 73.16 J/cm2) radiated on LU 11 and LU 07 acupoints. |
| 3 February 2021 | - | Red rashes on the back of the hand were significantly improved. | |
| 19 February 2021 | Chest CT Brain CT | For chest CT: Left upper lung cancer with bilateral lung metastases is smaller than prior image on 10 November 2020. For brain CT: No definite metastatic lesion. | |
| 16 April 2021 | - | Higher concentration was found in ASL, ALT, and BUN. | PBM therapy: The concentration of ASL, ALT, and BUN decreased after 830 nm laser radiated on BL 18 acupoint (30 mW, 10 min, 36.58 J/cm2) and KI 1 acupoint (30 mW, 30 min, 109.74 J/cm2) twice a day. |
| 12 May 2021 | Brain MRI | A tiny enhancing nodule in right frontal subcortical region and another in left cerebellum. | |
| 14 May 2021 | Chest CT | Left upper lung cancer is smaller than prior image on 19 February 2021. | |
| 3 August 2021 | Brain MRI | Stable tiny enhancing nodules in right frontal subcortical region and left cerebellum compared with prior image on 12 May 2021. | Increase ACGL dose: 12 capsules of ACGL per day. |
| 6 August 2021 | Chest CT | A small decreased of left upper lung cancer with bilateral lung metastases compared with prior image on 14 May 2021. | |
| 29 October 2021 | Chest CT | No significant interval changes as compared with prior CT image on 6 August 2021 | |
| 25 November 2021 | Brain MRI | Stable tiny enhancing nodules regions compared with prior image on 3 August 2021. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.-T.; Wu, J.-H. Management of Combined Therapy (Ceritinib, A. cinnamomea, G. lucidum, and Photobiomodulation) in Advanced Non-Small-Cell Lung Cancer: A Case Report. Life 2022, 12, 862. https://doi.org/10.3390/life12060862
Su C-T, Wu J-H. Management of Combined Therapy (Ceritinib, A. cinnamomea, G. lucidum, and Photobiomodulation) in Advanced Non-Small-Cell Lung Cancer: A Case Report. Life. 2022; 12(6):862. https://doi.org/10.3390/life12060862
Chicago/Turabian StyleSu, Chuan-Tsung, and Jih-Huah Wu. 2022. "Management of Combined Therapy (Ceritinib, A. cinnamomea, G. lucidum, and Photobiomodulation) in Advanced Non-Small-Cell Lung Cancer: A Case Report" Life 12, no. 6: 862. https://doi.org/10.3390/life12060862
APA StyleSu, C.-T., & Wu, J.-H. (2022). Management of Combined Therapy (Ceritinib, A. cinnamomea, G. lucidum, and Photobiomodulation) in Advanced Non-Small-Cell Lung Cancer: A Case Report. Life, 12(6), 862. https://doi.org/10.3390/life12060862







