Functionalised High-Performance Oxide Ceramics with Bone Morphogenic Protein 2 (BMP-2) Induced Ossification: An In Vivo Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.2. Surgical Procedure
2.3. Sample Preparation
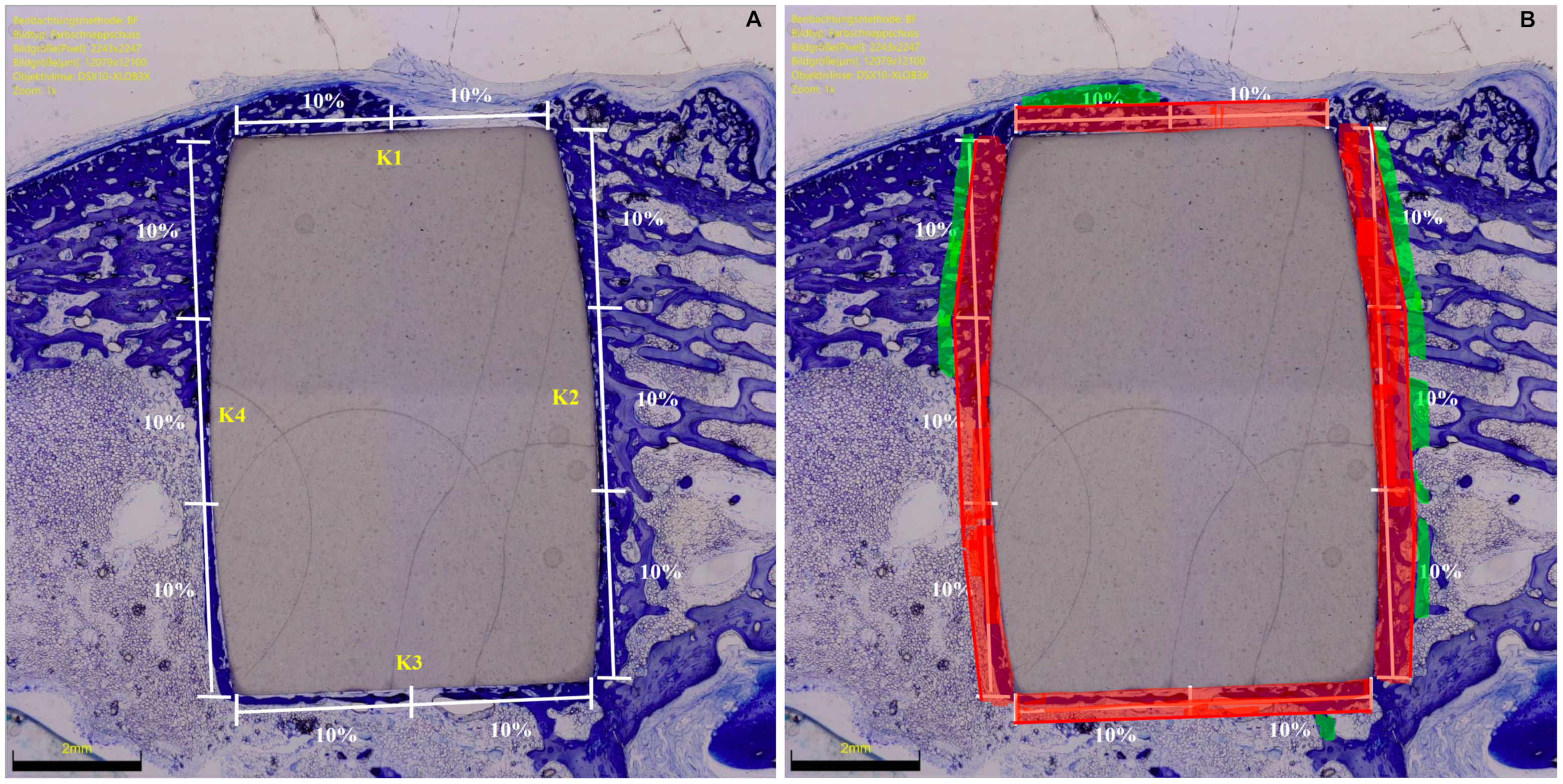
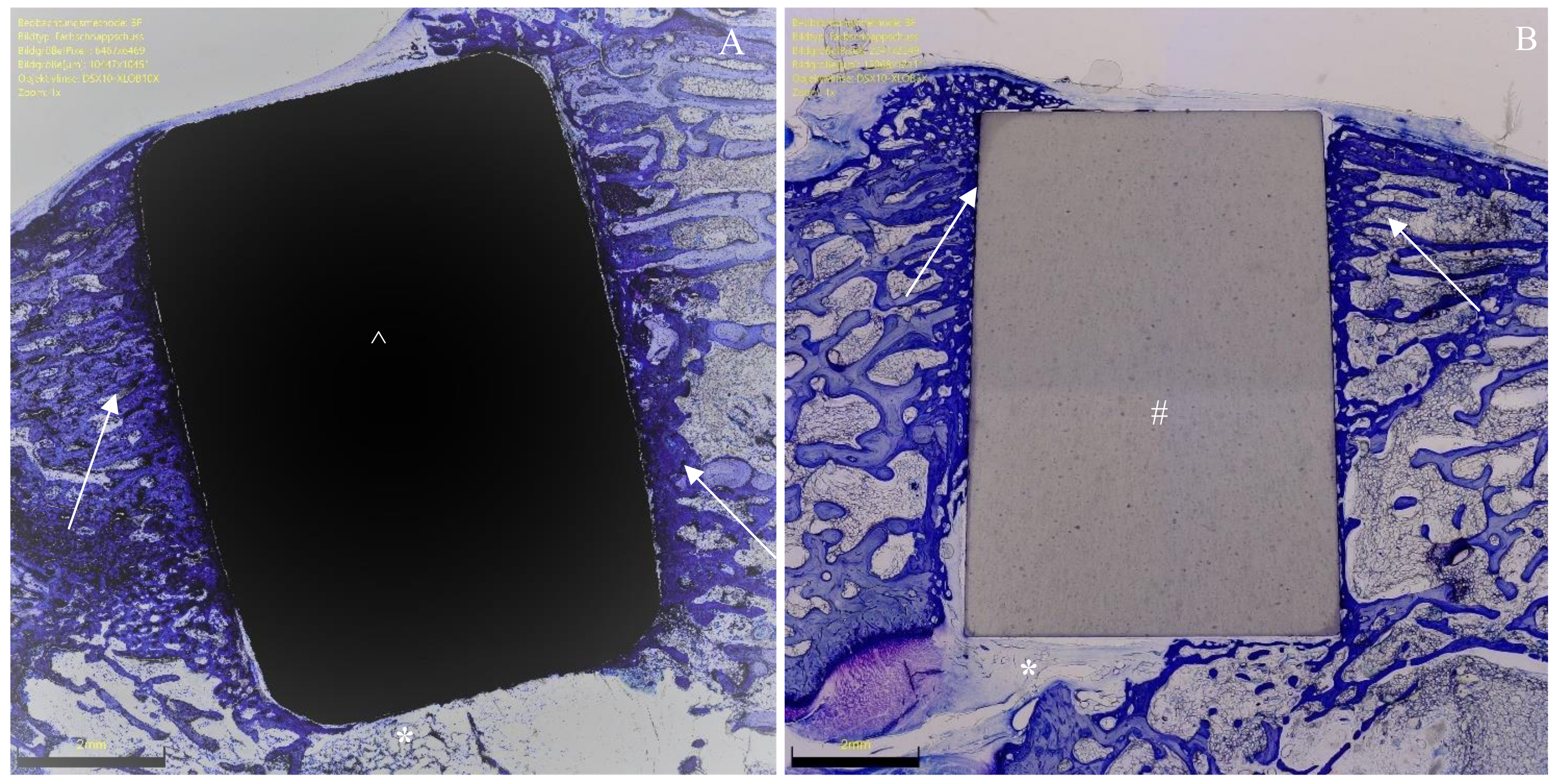
2.4. Histomorphometry
2.5. Outcomes of Interest
2.6. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Ossification from 6 to 12 Weeks of Follow-Up
3.3. Comparison of BMP-2-Functionalised HPOCs versus Titanium Implants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, O.Z.; Offermanns, V.; Sillassen, M.; Almtoft, K.P.; Andersen, I.H.; Sørensen, S.; Jeppesen, C.S.; Kraft, D.C.; Bøttiger, J.; Rasse, M.; et al. Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials 2013, 34, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Von Wilmowsky, C.; Moest, T.; Nkenke, E.; Stelzle, F.; Schlegel, K.A. Implants in bone: Part II. Research on implant osseointegration: Material testing, mechanical testing, imaging and histoanalytical methods. Oral Maxillofac. Surg. 2014, 18, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Yagüe, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Vladescu, A.; Cotrut, C.M.; Azem, F.A.; Bramowicz, M.; Pana, I.; Braic, V.; Birlik, I.; Kiss, A.; Braic, M.; Abdulgader, R.; et al. Sputtered Si and Mg doped hydroxyapatite for biomedical applications. Biomed. Mater. 2018, 13, 025011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, X.; Lin, Z.; Lin, H.; Zhang, Z.; Li, W.; Yang, X.; Cui, J. Ti-Based Biomedical Material Modified with TiOx/TiNx Duplex Bioactivity Film via Micro-Arc Oxidation and Nitrogen Ion Implantation. Nanomaterials 2017, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.J.; Lee, M.-H.; Bae, T.-S.; Lee, S.-J.; Gay, J.; Loch, C. Anodisation Increases Integration of Unloaded Titanium Implants in Sheep Mandible. BioMed Res. Int. 2015, 2015, 857969. [Google Scholar] [CrossRef]
- Zhang, L.; Haddouti, E.-M.; Welle, K.; Burger, C.; Kabir, K.; Schildberg, A.F. Local Cellular Responses to Metallic and Ceramic Nanoparticles from Orthopedic Joint Arthroplasty Implants. Int. J. Nanomed. 2020, 15, 6705–6720. [Google Scholar] [CrossRef]
- Trieb, K.; Glinz, J.; Reiter, M.; Kastner, J.; Senck, S. Non-Destructive Testing of Ceramic Knee Implants Using Micro-Computed Tomography. J. Arthroplast. 2019, 34, 2111–2117. [Google Scholar] [CrossRef]
- Li, C.; Ai, F.; Miao, X.; Liao, H.; Li, F.; Liu, M.; Yu, F.; Dong, L.; Li, T.; Wang, X. “The return of ceramic implants”: Rose stem inspired dual layered modification of ceramic scaffolds with improved mechanical and anti-infective properties. Mater. Sci. Eng. C 2018, 93, 873–879. [Google Scholar] [CrossRef]
- Teo, W.Z.W.; Schalock, P.C. Metal Hypersensitivity Reactions to Orthopedic Implants. Dermatol. Ther. 2017, 7, 53–64. [Google Scholar] [CrossRef]
- Kretzer, J.P.; Reinders, J.; Sonntag, R.; Hagmann, S.; Streit, M.; Jeager, S.; Moradi, B. Wear in total knee arthroplasty—Just a question of polyethylene?: Metal ion release in total knee arthroplasty. Int. Orthop. 2014, 38, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Solarino, G.; Piconi, C.; De Santis, V.; Piazzolla, A.; Moretti, B. Ceramic Total Knee Arthroplasty: Ready to Go? Joints 2017, 5, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Middleton, S.; Toms, A. Allergy in total knee arthroplasty: A review of the facts. Bone Jt. J. 2016, 98, 437–441. [Google Scholar] [CrossRef]
- Migliorini, F.; Schenker, H.; Maffulli, N.; Hildebrand, F.; Eschweiler, J. Histomorphometry of Ossification in Functionalised Ceramics with Tripeptide Arg-Gly-Asp (RGD): An In Vivo Study. Life 2022, 12, 761. [Google Scholar] [CrossRef]
- Böke, F.; Giner, I.; Keller, A.; Grundmeier, G.; Fischer, H. Plasma-Enhanced Chemical Vapor Deposition (PE-CVD) yields better Hydrolytical Stability of Biocompatible SiOx Thin Films on Implant Alumina Ceramics compared to Rapid Thermal Evaporation Physical Vapor Deposition (PVD). ACS Appl. Mater. Interfaces 2016, 8, 17805–17816. [Google Scholar] [CrossRef] [PubMed]
- Böke, F.; Labude, N.; Lauria, I.; Ernst, S.; Müller-Newen, G.; Neuss, S.; Fischer, H. Biological Activation of Bioinert Medical High-Performance Oxide Ceramics by Hydrolytically Stable Immobilization of c(RGDyK) and BMP-2. ACS Appl. Mater. Interfaces 2018, 10, 38669–38680. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zhang, W.; Shi, B.; Cheng, X.; Zhang, Y. Enhanced bone regeneration around dental implant with bone morphogenetic protein 2 gene and vascular endothelial growth factor protein delivery. Clin. Oral Implant. Res. 2012, 23, 467–473. [Google Scholar] [CrossRef]
- Balasundaram, G.; Yao, C.; Webster, T.J. TiO2 nanotubes functionalized with regions of bone morphogenetic protein-2 increases osteoblast adhesion. J. Biomed. Mater. Res. Part A 2008, 84, 447–453. [Google Scholar] [CrossRef]
- Benoit, D.S.; Collins, S.D.; Anseth, K.S. Multifunctional Hydrogels that Promote Osteogenic Human Mesenchymal Stem Cell Differentiation Through Stimulation and Sequestering of Bone Morphogenic Protein 2. Adv. Funct. Mater. 2007, 17, 2085–2093. [Google Scholar] [CrossRef]
- Vasudev, M.C.; Anderson, K.D.; Bunning, T.J.; Tsukruk, V.V.; Naik, R.R. Exploration of Plasma-Enhanced Chemical Vapor Deposition as a Method for Thin-Film Fabrication with Biological Applications. ACS Appl. Mater. Interfaces 2013, 5, 3983–3994. [Google Scholar] [CrossRef]
- Li, Y.; Ren, J.; Wang, B.; Lu, W.; Wang, H.; Hou, W. Development of biobased multilayer films with improved compatibility between polylactic acid-chitosan as a function of transition coating of SiOx. Int. J. Biol. Macromol. 2020, 165, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Sung, G.Y.; Park, M. Efficient Portable Urea Biosensor Based on Urease Immobilized Membrane for Monitoring of Physiological Fluids. Biomedicines 2020, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.J.; Ren, Q.H.; Bi, L. miR-135b-5p regulates human mesenchymal stem cell osteogenic differentiation by facilitating the Hippo signaling pathway. Int. J. Clin. Exp. Pathol. 2017, 10, 7767–7775. [Google Scholar] [PubMed]
- Walker, J.M. (Ed.) The bicinchoninic acid (BCA) assay for protein quantitation. In Basic Protein and Peptide Protocols, 1st ed.; Humana Press: Totowa, NJ, USA, 1994; pp. 5–8. [Google Scholar]
- Tufekci, P.; Tavakoli, A.; Dlaska, C.; Neumann, M.; Shanker, M.; Saifzadeh, S.; Steck, R.; Schuetz, M.; Epari, D. Early mechanical stimulation only permits timely bone healing in sheep. J. Orthop. Res. 2018, 36, 1790–1796. [Google Scholar] [CrossRef]
- Hunziker, E.; Enggist, L.; Küffer, A.; Buser, D.; Liu, Y. Osseointegration: The slow delivery of BMP-2 enhances osteoinductivity. Bone 2012, 51, 98–106. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Suzuki, S.; Kobayashi, H.; Ogawa, T. Implant Stability Change and Osseointegration Speed of Immediately Loaded Photofunctionalized Implants. Implant Dent. 2013, 22, 481–490. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Bagno, A.; Di Bello, C. Surface treatments and roughness properties of Ti-based biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 935–949. [Google Scholar] [CrossRef]
- Cordova, L.A.; Stresing, V.; Gobin, B.; Rosset, P.; Passuti, N.; Gouin, F.; Trichet, V.; Layrolle, P.; Heymann, D. Orthopaedic implant failure: Aseptic implant loosening–the contribution and future challenges of mouse models in translational research. Clin. Sci. 2014, 127, 277–293. [Google Scholar] [CrossRef]
- Tang, K.; Deng, Z.; Wang, T.; Nie, M. Aseptic Loosening after total hip arthroplasty secondary to the disappearing coating? Asian J. Surg. 2022, 45, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium Enhances Osteogenic Differentiation of Mesenchymal Stem Cells and In Vivo Bone Formation by Activating Wnt/Catenin Signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M. Is the calcium receptor a molecular target for the actions of strontium on bone? Osteoporos. Int. 2003, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Gomes, N.; Romero-Gavilán, F.; García-Arnáez, I.; Martínez-Ramos, C.; Sánchez-Pérez, A.M.; Azkargorta, M.; Elortza, F.; de Llano, J.J.M.; Gurruchaga, M.; Goñi, I.; et al. Osseointegration mechanisms: A proteomic approach. JBIC J. Biol. Inorg. Chem. 2018, 23, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Papale, F.; Bollino, F. Coatings of titanium substrates with xCaO(1 − x)SiO2 sol–gel materials: Characterization, bioactivity and biocompatibility evaluation. Mater. Sci. Eng. C 2016, 58, 846–851. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Rong, M.; Lu, H.; Wan, L.; Zhang, X.; Lin, X.; Li, S.; Zhou, L.; Lv, Y.; Su, Y. Comparison of early osseointegration between laser-treated/acid-etched and sandblasted/acid-etched titanium implant surfaces. J. Mater. Sci. Mater. Med. 2018, 29, 43. [Google Scholar] [CrossRef]
- Levine, R.A.; Sendi, P.; Bornstein, M.M. Immediate restoration of nonsubmerged titanium implants with a sandblasted and acid-etched surface: Five-year results of a prospective case series study using clinical and radiographic data. Int. J. Periodont. Restor. Dent. 2012, 32, 39–47. [Google Scholar]
- Shimer, A.L.; Öner, F.C.; Vaccaro, A.R. Spinal reconstruction and bone morphogenetic proteins: Open questions. Injury 2009, 40, S32–S38. [Google Scholar] [CrossRef]
- Lan, J.; Wang, Z.; Shi, B.; Xia, H.; Cheng, X. The influence of recombinant human BMP-2 on bone–implant osseointegration: Biomechanical testing and histomorphometric analysis. Int. J. Oral Maxillofac. Surg. 2007, 36, 345–349. [Google Scholar] [CrossRef]
- Song, Y.; Wan, L.; Zhang, S.; Du, Y. Biological Response to Recombinant Human Bone Morphogenetic Protein-2 on Bone-Implant Osseointegration in Ovariectomized Experimental Design. J. Craniofacial Surg. 2019, 30, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Chen, Y.-S.; Ko, C.-L.; Lin, Y.; Kuo, T.-H.; Kuo, H.-N. Interaction of progenitor bone cells with different surface modifications of titanium implant. Mater. Sci. Eng. C 2014, 37, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, Y.; Ma, A.; Li, C. Surface Immobilization of TiO2 Nanotubes with Bone Morphogenetic Protein-2 Synergistically Enhances Initial Preosteoblast Adhesion and Osseointegration. BioMed. Res. Int. 2019, 2019, 5697250. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, Y.; Wu, C.; Dunstan, C.R.; Hewson, B.; Eindorf, T.; Anderson, G.I.; Zreiqat, H. Sphene ceramics for orthopedic coating applications: An in vitro and in vivo study. Acta Biomater. 2009, 5, 3192–3204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, G.; Liu, Y.; Zhao, X.; Zou, D.; Zhu, C.; Jin, Y.; Huang, Q.; Sun, J.; Liu, X.; et al. The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration. Biomaterials 2013, 34, 3184–3195. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.D.; Lotfibakhshaiesh, N.; O’Donnell, M.; Walboomers, X.F.; Horwood, N.; Jansen, J.A.; Amis, A.A.; Cobb, J.P.; Stevens, M.M. Enhanced Osseous Implant Fixation with Strontium-Substituted Bioactive Glass Coating. Tissue Eng. Part A 2014, 20, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Van Oirschot, B.A.J.A.; Alghamdi, H.; Närhi, T.O.; Anil, S.; Aldosari, A.A.F.; Beucken, J.v.D.; Jansen, J.A. In vivoevaluation of bioactive glass-based coatings on dental implants in a dog implantation model. Clin. Oral Implant. Res. 2014, 25, 21–28. [Google Scholar] [CrossRef]


| Endpoint | 6 Weeks | 12 Weeks | MD | SE | 95% CI | t Value | p | |
|---|---|---|---|---|---|---|---|---|
| Lateral | BIC (%) | 2.5 ± 2.0 | 4.4 ± 3.3 | 1.9 | 0.67 | 0.56 to 3.24 | 2.83 | 0.006 |
| OIC (%) | 2.3 ± 2.3 | 2.4 ± 1.7 | 0.1 | 0.50 | −0.90 to 1.10 | 0.20 | 0.8 | |
| Distal | BIC (%) | 12.0 ± 6.4 | 15.6 ± 6.4 | 3.6 | 1.58 | 0.45 to 6.75 | 2.29 | 0.03 |
| OIC (%) | 6.6 ± 4.7 | 6.0 ± 6.9 | −0.6 | 1.45 | −3.50 to 2.30 | −0.41 | 0.7 | |
| Medial | BIC (%) | 5.3 ± 3.5 | 6.1 ± 2.4 | 0.8 | 0.74 | −0.68 to 2.28 | 1.08 | 0.3 |
| OIC (%) | 2.6 ± 3.5 | 2.8 ± 3.6 | −1.8 | 0.87 | −3.55 to −0.05 | −2.06 | 0.04 | |
| Proximal | BIC (%) | 9.8 ± 7.0 | 13.8 ± 6.1 | 4.0 | 1.62 | 0.77 to 7.23 | 2.48 | 0.02 |
| OIC (%) | 5.0 ± 0.0 | 5.0 ± 0.1 | 0.0 | 0.02 | −0.04 to 0.04 | 0.00 | 0.99 | |
| Overall | BIC (%) | 29.6 ± 5.1 | 39.9 ± 4.4 | 10.3 | 1.17 | 7.96 to 12.64 | 8.78 | <0.0001 |
| OIC (%) | 16.5 ± 1.2 | 16.2 ± 3.4 | −0.3 | 0.63 | −1.55 to 0.95 | 0.48 | 0.6 | |
| End Point | 6 Weeks | 12 Weeks | |||
|---|---|---|---|---|---|
| MD | p | MD | p | ||
| Lateral | BIC (%) | −0.6 | 0.4 | 0.6 | 0.4 |
| OIC (%) | 2.3 | 0.007 | 2.0 | 0.005 | |
| Distal | BIC (%) | 0.5 | 0.4 | 1.9 | 0.3 |
| OIC (%) | 5.1 | 0.01 | 1.0 | 0.4 | |
| Medial | BIC (%) | −0.1 | 0.5 | −3.1 | 0.009 |
| OIC (%) | 2.9 | 0.02 | 1.3 | 0.2 | |
| Proximal | BIC (%) | −1.1 | 0.4 | 2.1 | 0.2 |
| OIC (%) | 0.0 | 1.0 | 0.7 | 0.1 | |
| Overall | BIC (%) | 0.0 | 0.4 | 0.1 | 0.2 |
| OIC (%) | 10.2 | 0.002 | 5.1 | 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliorini, F.; Eschweiler, J.; Maffulli, N.; Hildebrand, F.; Schenker, H. Functionalised High-Performance Oxide Ceramics with Bone Morphogenic Protein 2 (BMP-2) Induced Ossification: An In Vivo Study. Life 2022, 12, 866. https://doi.org/10.3390/life12060866
Migliorini F, Eschweiler J, Maffulli N, Hildebrand F, Schenker H. Functionalised High-Performance Oxide Ceramics with Bone Morphogenic Protein 2 (BMP-2) Induced Ossification: An In Vivo Study. Life. 2022; 12(6):866. https://doi.org/10.3390/life12060866
Chicago/Turabian StyleMigliorini, Filippo, Jörg Eschweiler, Nicola Maffulli, Frank Hildebrand, and Hanno Schenker. 2022. "Functionalised High-Performance Oxide Ceramics with Bone Morphogenic Protein 2 (BMP-2) Induced Ossification: An In Vivo Study" Life 12, no. 6: 866. https://doi.org/10.3390/life12060866







