Pineal Gland from the Cell Culture to Animal Models: A Review
Abstract
:1. Major Functions of Pineal Gland
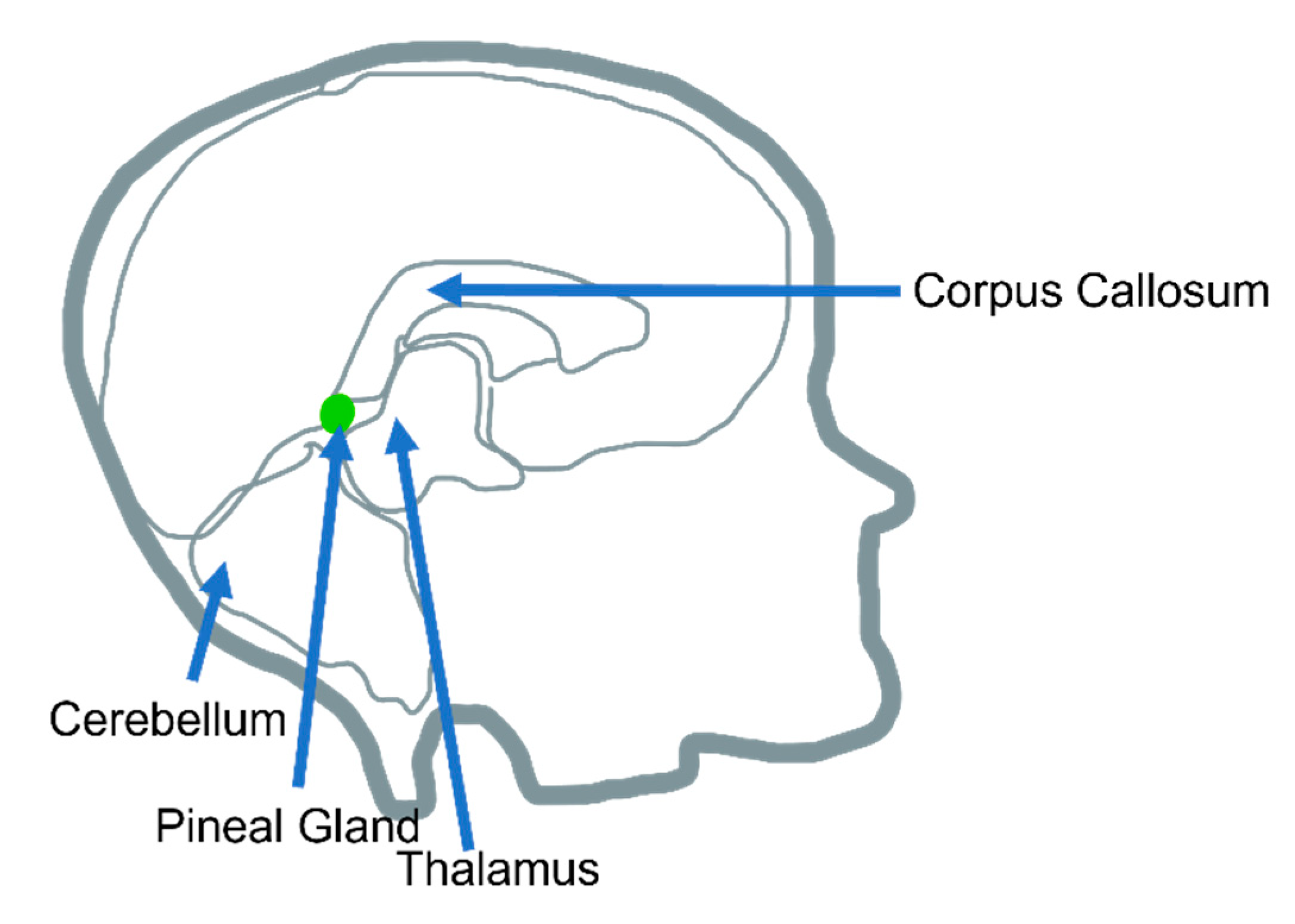
1.1. Pineal Gland Overall Morphology, Anatomy, and Function
1.2. Neuronal Interactions with the Hypothalamus
1.3. Pineal Gland and Its Correlation with Illness
1.4. Pineal Gland and It’s Relationship with the Central and Peripheral Nervous System Illness
2. In Vitro Models of Culturing Pineal Gland Cells
2.1. Two-Dimensional (2D) In Vitro Cell Culture
2.2. Three-Dimensional (3D) In Vitro Cell Culture
2.3. Alternative Culture Methods
3. In Vivo Animal Model for the Study of Pineal Gland
3.1. Non-Genetically Modified Animal Models
3.1.1. Swine
3.1.2. Rabbit
3.1.3. Rodents
3.2. Genetically Modified Animal Models for Pineal Gland Study
Common Animal Models Used for Specific Pineal Gland Diseases and/or Research
3.3. The Ideal Animal Model for Pineal Gland Study
4. Pineal-Gland-Derived Compounds Other Than Melatonin
5. Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Gheban, B.A.; Rosca, I.A.; Crisan, M. The morphological and functional characteristics of the pineal gland. Med. Pharm. Rep. 2019, 92, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.P.I.; Noctor, S.C.; Muñoz, E.M. Cellular Basis of Pineal Gland Development: Emerging Role of Microglia as Phenotype Regulator. PLoS ONE 2016, 11, e0167063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feingold, K.R.; Anawalt, B.; Boyce, A.; Chrousos, G.; de Herder, W.W.; Dhatariya, K.; Dungan, K.; Grossman, A.; Hershman, J.M.; Hofland, J.; et al. Pineal Gland; Endotext: Dartmouth, MA, USA, 2000. [Google Scholar]
- Axelrod, J. Comparative Biochemistry of the Pineal Gland. Am. Zool. 1970, 10, 259–267. [Google Scholar] [CrossRef]
- Aulinas, A. Physiology of the Pineal Gland and Melatonin. In Feingold KR; Anawalt, B., Boyce, A., Eds.; Endotext: Dartmouth, MA, USA, 2000. [Google Scholar]
- Doghramji, K. Melatonin and Its Receptors: A New Class of Sleep-Promoting Agents. J. Clin. Sleep Med. 2007, 3, S17–S23. [Google Scholar] [CrossRef] [Green Version]
- Borjigin, J.; Zhang, S.; Calinescu, A. Circadian regulation of pineal gland rhythmicity. Mol. Cell. Endocrinol. 2012, 349, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlow-Walden, L.R.; Reiter, R.J.; Abe, M.; Pablos, M.; Menendez-Pelaez, A.; Chen, L.-D.; Poeggeler, B. Melatonin stimulates brain glutathione peroxidase activity. Neurochem. Int. 1995, 26, 497–502. [Google Scholar] [CrossRef]
- Moore, R.Y.; Speh, J.C.; Leak, R.K. Suprachiasmatic nucleus organization. Cell Tissue Res. 2002, 309, 89–98. [Google Scholar] [CrossRef]
- Pablos, M.I.; Chuang, J.; Reiter, R.J.; Ortiz, G.G.; Daniels, W.M.; Sewerynek, E.; Melchiorri, D.; Poeggeler, B. Time Course of the Melatonin-Induced Increase in Glutathione Peroxidase Activity in Chick Tissues. Biol. Signals 1995, 4, 325–330. [Google Scholar] [CrossRef]
- Lee, J.G.; Woo, Y.S.; Park, S.W.; Seog, D.H.; Seo, M.K.; Bahk, W.M. The Neuroprotective Effects of Melatonin: Possible Role in the Pathophysiology of Neuropsychiatric Disease. Brain Sci. 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Ilahi, S.; Beriwal, N.; Ilahi, T.B. Physiology, Pineal Gland. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ackermann, K.; Stehle, J.H. Melatonin synthesis in the human pineal gland: Advantages, implications, and difficulties. Chrono Int. 2006, 23, 369–379. [Google Scholar] [CrossRef]
- Macchi, M.M.; Bruce, J.N. Human pineal physiology and functional significance of melatonin. Front. Neuroendocr. 2004, 25, 177–195. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J. CSF generation by pineal gland results in a robust melatonin circadian rhythm in the third ventricle as an unique light/dark signal. Med. Hypotheses 2016, 86, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Ritta, M.N. The role of prostaglandins in neuroendocrine junctions: Studies in the pineal gland and the hy-pothalamus. Neuroendocrinology 1983, 36, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Huebner, E.A.; Strittmatter, S.M. Axon regeneration in the peripheral and central nervous systems. Cell Biol. Axon 2009, 48, 339–351. [Google Scholar]
- Harvey, A.R.; Plant, G.W.; Tan, M.M. Schwann cells and the regrowth of axons in the mammalian CNS: A review of transplantation studies in the rat visual system. Clin. Exp. Pharmacol. Physiol. 1995, 22, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Benfey, M.; Aguayo, A. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature 1982, 296, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Jaisri, R.L.; Zigmond, R.E. Limited recovery of pineal function after regeneration of preganglionic sympathetic axons: Evidence for loss of ganglionic synaptic specificity. J. Neurosci. 2013, 33, 4867–4874. [Google Scholar]
- Dunn, J.C.; Eckhoff, M.D.; Nicholson, T.C.; Campbell, W.; Kenney, K.; Smith, J.; Landau, M.; Miller, M.; Souza, J.; Nesti, L.J. Combat-Sustained Peripheral Nerve Injuries in the United States Military. J. Hand Surg. 2021, 46, 148.e1–148.e8. [Google Scholar] [CrossRef]
- Jones, P.E.; Meyer, R.M.; Faillace, W.J.; Landau, M.E.; Smith, J.K.; McKay, P.L.; Nesti, L.J. Combat injury of the sciatic nerve—an institutional experience. Mil. Med. 2018, 183, e434–e441. [Google Scholar] [CrossRef] [Green Version]
- Kubiak, C.A.; Grochmal, J.; Kung, T.A.; Cederna, P.S.; Midha, R.; Kemp, S.W.P. Stem-cell-based therapies to enhance peripheral nerve regeneration. Muscle Nerve 2020, 61, 449–459. [Google Scholar] [CrossRef]
- Xie, Y.; Lou, D.; Zhang, D. Melatonin Alleviates Age-Associated Endothelial Injury of Atherosclerosis via Regulating Telomere Function. J. Inflamm. Res. 2021, 14, 6799–6812. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.T.; Atrakji, D.; Mehuaiden, D. The Effect of Melatonin on Thrombosis, Sepsis and Mortality Rate in COVID-19 Patients. Int. J. Infect. Dis. 2022, 114, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, H.L.; Gu, C.J.; Liu, Y.K.; Shao, J.; Zhu, R.; He, Y.Y.; Zhu, X.Y.; Li, M.Q. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2019, 43, 945–955. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.; Zhou, W.; Li, X.; Du, J.; Li, X.; Hao, H. Melatonin inhibits proliferation and viability and promotes apoptosis in colorectal cancer cells via upregulation of the microRNA-34a/449a cluster. Mol. Med. Rep. 2021, 23, 187. [Google Scholar] [CrossRef] [PubMed]
- Overberg, J.; Kalveram, L.; Keller, T.; Krude, H.; Kühnen, P.; Wiegand, S. Interactions between nocturnal melatonin secretion, metabolism, and sleeping behavior in adolescents with obesity. Int. J. Obes. 2022, 46, 1051–1058. [Google Scholar] [CrossRef]
- Pazo, J.H.; Gonzalez, M. Effects of central and peripheral inputs on single pineal cell activity in the rat. Neuroscience 1991, 43, 231–235. [Google Scholar] [CrossRef]
- Stazi, M.; Negro, S.; Megighian, A.; D’Este, G.; Solimena, M.; Jockers, R.; Lista, F.; Montecucco, C.; Rigoni, M. Melatonin promotes regeneration of injured motor axons via MT 1 receptors. J. Pineal Res. 2021, 70, e12695. [Google Scholar] [CrossRef]
- Jing, Y.; Bai, F.; Chen, H.; Dong, H. Melatonin prevents blood vessel loss and neurological impairment induced by spinal cord injury in rats. J. Spinal Cord Med. 2017, 40, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Araki, M. Developmental potency of cultured pineal cells: An approach to pineal developmental biology. Microsc. Res. Tech. 2001, 53, 33–42. [Google Scholar] [CrossRef]
- Khan, N.A.; Shacoori, V.; Havouis, R.; Querné, D.; Moulinoux, J.P.; Rault, B. Three Dimensional Culture of Pineal Cell Aggregates: A Model of Cell-Cell Co-operation. J. Neuroendocr. 1995, 7, 353–359. [Google Scholar] [CrossRef]
- Parfitt, A.; Weller, J.L.; Klein, D.C. Beta adrenergic-blockers decrease adrenergically stimulated N-acetyltransferase activity in pineal glands in organ culture. Neuropharmacology 1976, 15, 353–358. [Google Scholar] [CrossRef]
- Villela, D.; Atherino, V.F.; Lima, L.; Moutinho, A.A.; do Amaral, F.G.; Peres, R.; Martins de Lima, T.; Torrão, A.; Cipolla-Neto, J.; Scavone, C.; et al. Modulation of pineal melatonin synthesis by glutamate involves paracrine interactions between pinealocytes and astrocytes through NF-κB activation. BioMed. Res. Int. 2013, 2013, 618432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buda, M.; Klein, D.C. A suspension culture of pinealocytes: Regulation of N-acetyltransferase activity. Endocrinology 1978, 103, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.S.; Gago, A.G.; Naveda, F.A.; Calleja, J.G.; Zawadzka, A.; Czarnocki, Z.; Barrallo, J.; Menéndez, R.; Rodríguez-González, P.; Alonso, J. Evaluation of different internal standardization approaches for the quantification of melatonin in cell culture samples by multiple heart-cutting two dimensional liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2022, 1663, 462752. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery (Review). Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maritan, S.M.; Lian, E.Y.; Mulligan, L.M. An Efficient and Flexible Cell Aggregation Method for 3D Spheroid Production. J. Vis. Exp. 2017, 121, 55544. [Google Scholar] [CrossRef] [PubMed]
- Clinton, J.; McWilliams-Koeppen, P. Initiation, expansion, and cryopreservation of human primary tissue-derived normal and diseased organoids in embedded three-dimensional culture. Curr. Protoc. Cell Biol. 2019, 82, e66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khavinson, V.K.; Linkova, N.S.; Chalisova, N.I.; Dudkov, A.V.; Koncevaya, E.A. Effect of Short Peptides on Expression of Signaling Molecules in Organotypic Pineal Cell Culture. Bull. Exp. Biol. Med. 2011, 152, 138–141. [Google Scholar] [CrossRef]
- Walters, E.M.; Prather, R.S. Advancing swine models for human health and diseases. Mo. Med. 2013, 110, 212–215. [Google Scholar] [PubMed]
- Fabris, C.; Cozzi, B.; Hay-Schmidt, A.; Naver, B.; Møller, M. Demonstration of an orexinergic central innervation of the pineal gland of the pig. J. Comp. Neurol. 2004, 471, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Roblero, R.; González-Mariscal, G. Behavioral, neuroendocrine and physiological indicators of the circadian biology of male and female rabbits. Eur. J. Neurosci. 2020, 51, 429–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, J.; Zhou, Q.; Niu, J.; Wang, Y.; Yan, Q.; Wu, C.; Qian, J.; Yang, H.; Zou, J. Melatonin protects intervertebral disc from degeneration by improving cell survival and function via activation of the ERK1/2 signaling pathway. oxidative Med. Cell. Longev. 2019, 2019, 5120275. [Google Scholar] [CrossRef] [PubMed]
- Ozler, M.; Simsek, K.; Ozkan, C.; Akgul, E.O.; Topal, T.; Oter, S.; Korkmaz, A. Comparison of the effect of topical and systemic melatonin administration on delayed wound healing in rats that underwent pinealectomy. Scand. J. Clin. Lab. Investig. 2010, 70, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Turgut, M.; Uyanikgil, Y.; Baka, M.; Tunç, A.T.; Yavaşoğlu, A.; Yurtseven, M.E.; Kaplan, S. Pinealectomy exaggerates and melatonin treatment suppresses neuroma formation of transected sciatic nerve in rats: Gross morphological, histological and stereological analysis. J. Pineal Res. 2005, 38, 284–291. [Google Scholar] [CrossRef]
- Fernandes, P.A.C.M.; Cecon, E.; Markus, R.P.; Ferreira, Z.S. Effect of TNF-alpha on the melatonin synthetic pathway in the rat pineal gland: Basis for ‘feedback’ of the immune response on circadian timing. J. Pineal Res. 2006, 41, 344–350. [Google Scholar] [CrossRef]
- Tan, D.X.; Xu, B.; Zhou, X.; Reiter, R.J. Pineal Calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules 2018, 23, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.L.; Rui, C.; Li, Z.Z.; Li, S.S.; Fan, Y.J.; Qi, M.M. Melatonin mitigates traumatic brain injury-induced depression-like behaviors through HO-1/CREB signal in rats. Neurosci. Lett. 2022, 784, 136754. [Google Scholar] [CrossRef]
- Roy, J.; Wong, K.Y.; Aquili, L.; Uddin, M.S.; Heng, B.C.; Tipoe, G.L.; Wong, K.H.; Fung, M.L.; Lim, L.W. Role of melatonin in Alzheimer’s disease: From preclinical studies to novel melatonin-based therapies. Front. Neuroendocr. 2022, 65, 100986. [Google Scholar] [CrossRef]
- Liu, X.; Yao, S.; Bi, J.; Zheng, D.; Wang, P. Protective effects and regulatory mechanisms of melatonin in a neonatal mouse model of LPS-induced inflammation. Neurosci. Lett. 2022, 772, 136483. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K.; Bering, T.; Furukawa, T.; Rath, M.F. A modulatory role of the Rax homeobox gene in mature pineal gland function: Investigating the photoneuroendocrine circadian system of a Rax conditional knockout mouse. J. Neurochem. 2017, 143, 100–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewczuk, B.; Prusik, M.; Ziólkowska, N.; Dąbrowski, M.; Martniuk, K.; Hanuszewska, M.; Zielonka, L. Effects of streptozotocin-induced diabetes on the pineal gland in the domestic pig. Int. J. Mol. Sci. 2018, 19, 3077. [Google Scholar] [CrossRef] [Green Version]
- Egermann, M.; Gerhardt, C.; Barth, A.; Maestroni, G.J.; Schneider, E.; Alini, M. Pinealectomy affects bone mineral density and structure—An experimental study in sheep. BMC Musculoskelet. Disord. 2011, 12, 271. [Google Scholar] [CrossRef] [Green Version]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Künzle, H. Projections from the primary somatosensory cortex to basal ganglia and thalamus in the monkey. Exp. Brain Res. 1997, 30, 481–492. [Google Scholar] [CrossRef]
- Dean, J.; Liu, T.; Huff, S.; Sheler, B.; Barker, S.A.; Strassman, R.J.; Wang, M.M.; Borjigin, J. Biosynthesis and Extracellular Concentrations of N, N-dimethyltryptamine (DMT) in Mammalian Brain. Sci. Rep. 2019, 9, 9333. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peruri, A.; Morgan, A.; D’Souza, A.; Mellon, B.; Hung, C.W.; Kayal, G.; Shin, H.; Nguyen, K.; Zahed, M.; Yount, M.; et al. Pineal Gland from the Cell Culture to Animal Models: A Review. Life 2022, 12, 1057. https://doi.org/10.3390/life12071057
Peruri A, Morgan A, D’Souza A, Mellon B, Hung CW, Kayal G, Shin H, Nguyen K, Zahed M, Yount M, et al. Pineal Gland from the Cell Culture to Animal Models: A Review. Life. 2022; 12(7):1057. https://doi.org/10.3390/life12071057
Chicago/Turabian StylePeruri, Alekhya, Alexandra Morgan, Alida D’Souza, Bridget Mellon, Carey W. Hung, Gabriella Kayal, Haejung Shin, Kim Nguyen, Malek Zahed, Mason Yount, and et al. 2022. "Pineal Gland from the Cell Culture to Animal Models: A Review" Life 12, no. 7: 1057. https://doi.org/10.3390/life12071057





