Effectual Endeavors of Silk Protein Sericin against Isoproterenol Induced Cardiac Toxicity and Hypertrophy in Wistar Rats
Abstract
:1. Introduction
2. Material and Method
2.1. Chemicals
2.2. Animals
2.3. Ethical Approval
2.4. Treatment Protocol
2.4.1. Dose Determination and Its Administration
2.4.2. Processing of Tissue and Blood Samples
Serum Preparation
Plasma Preparation
Tissue Homogenate Preparation
2.5. Parameter Estimated
2.5.1. Grading of Heart
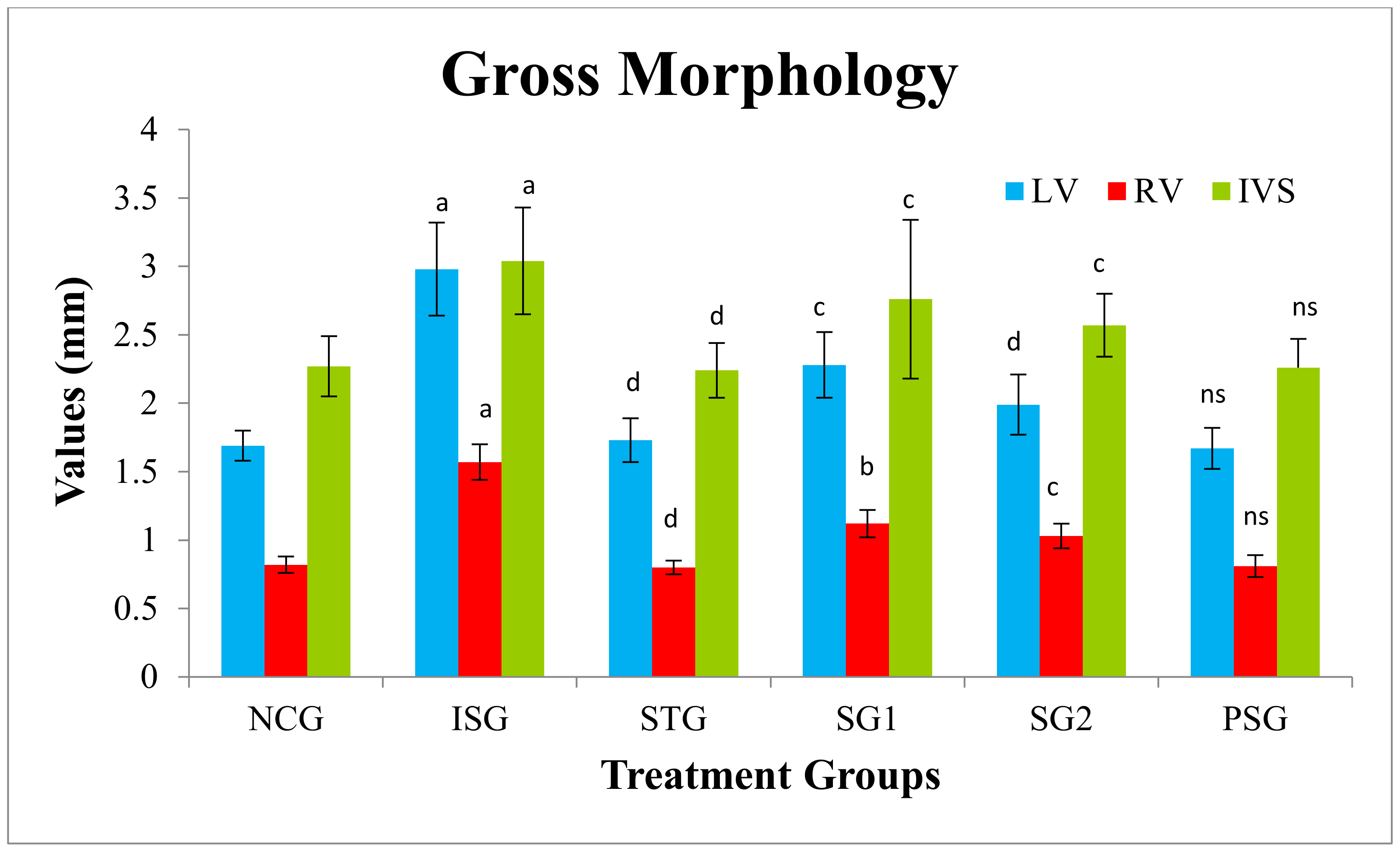
2.5.2. Heart:Body Weight Ratio
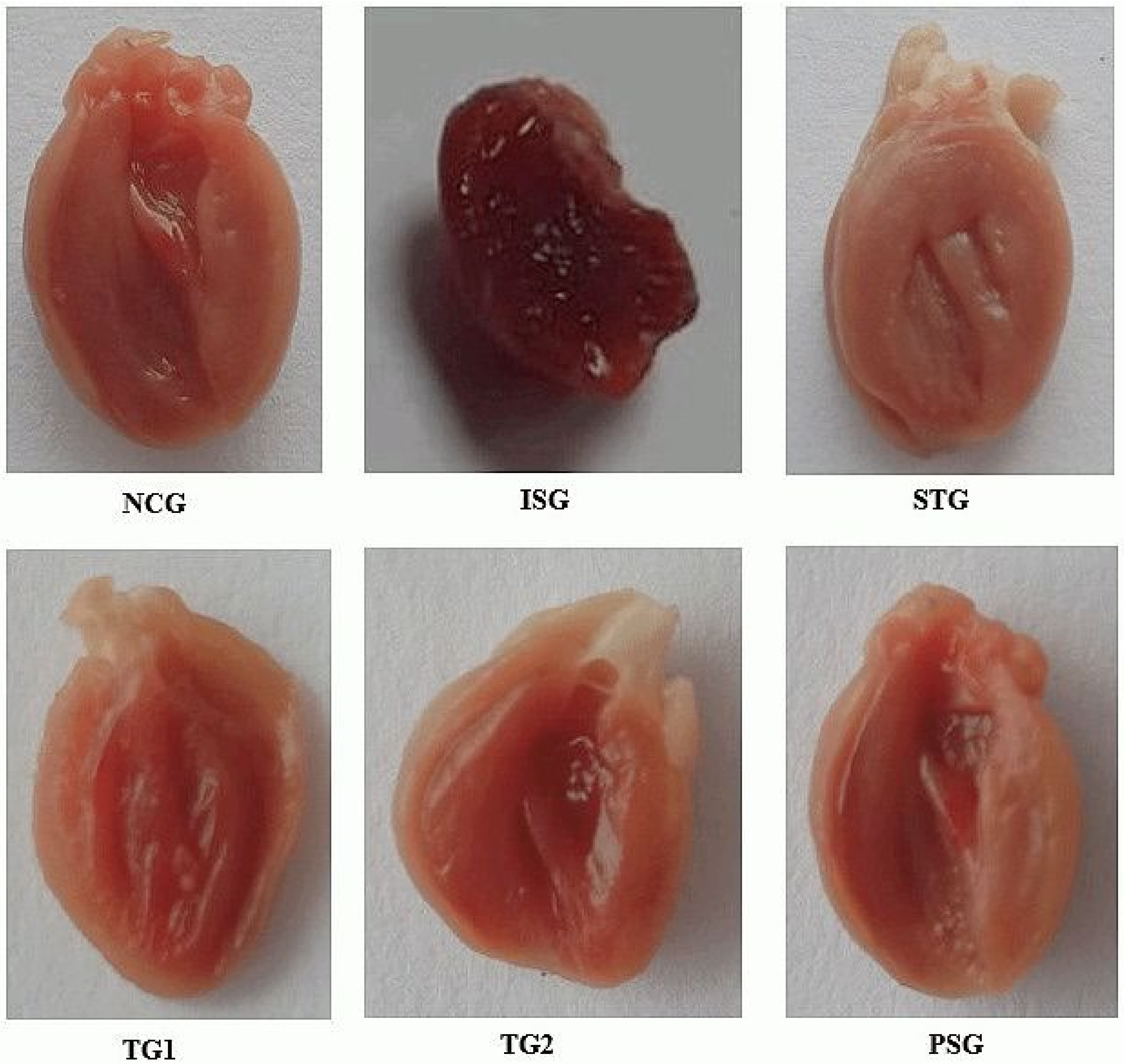
2.5.3. Gross Morphology
2.5.4. Cardiac Markers
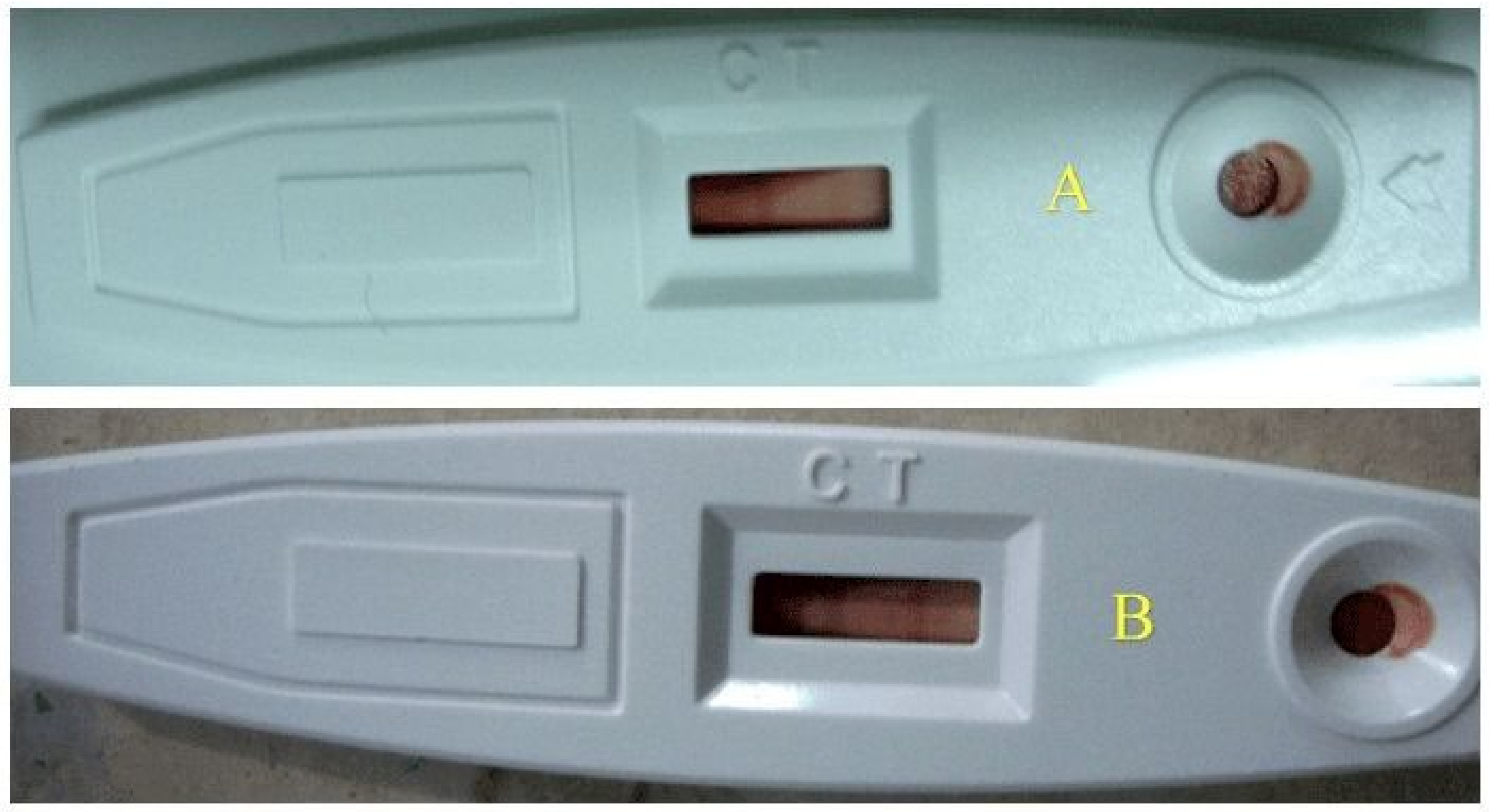
2.5.5. Troponin
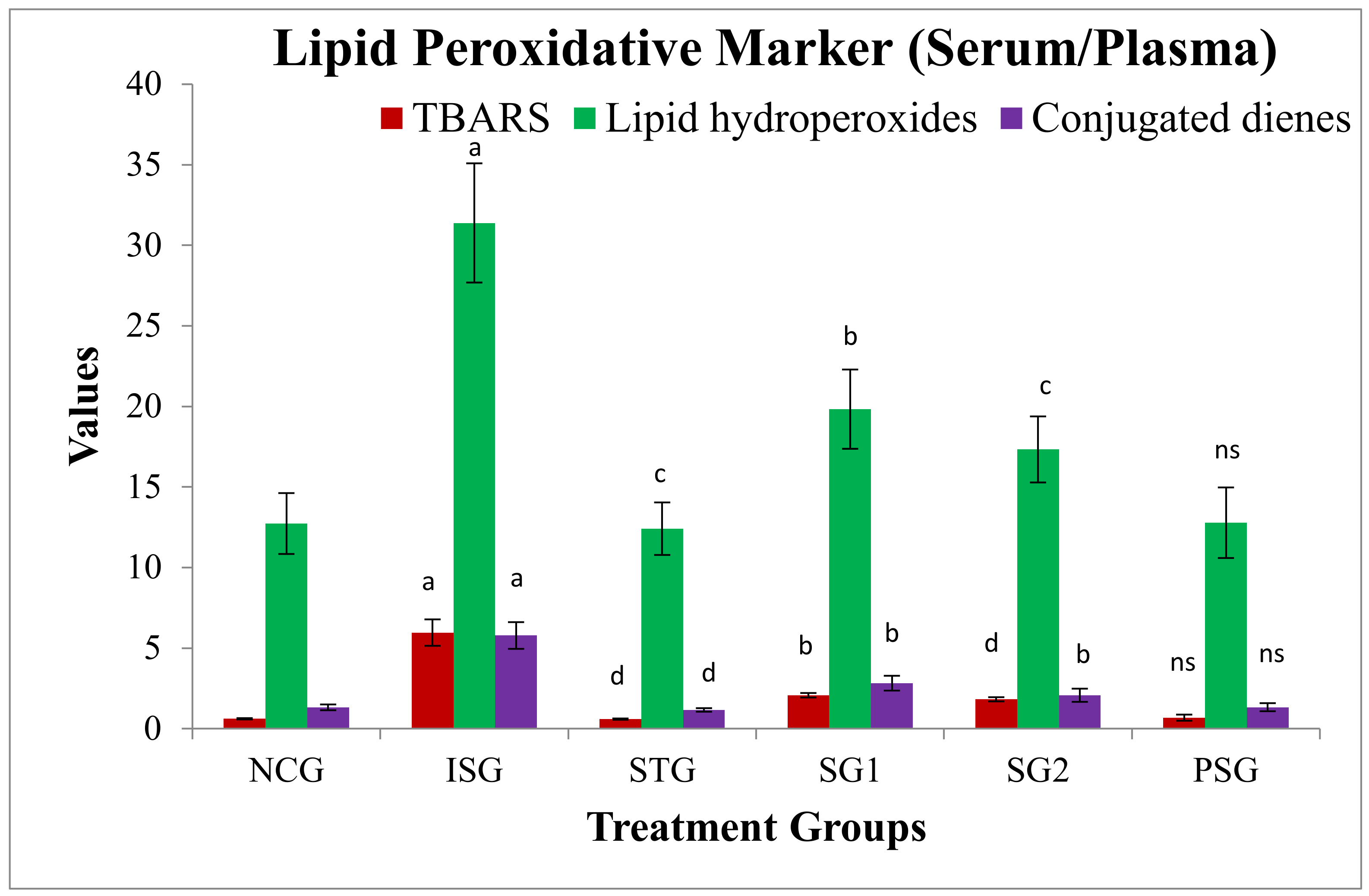
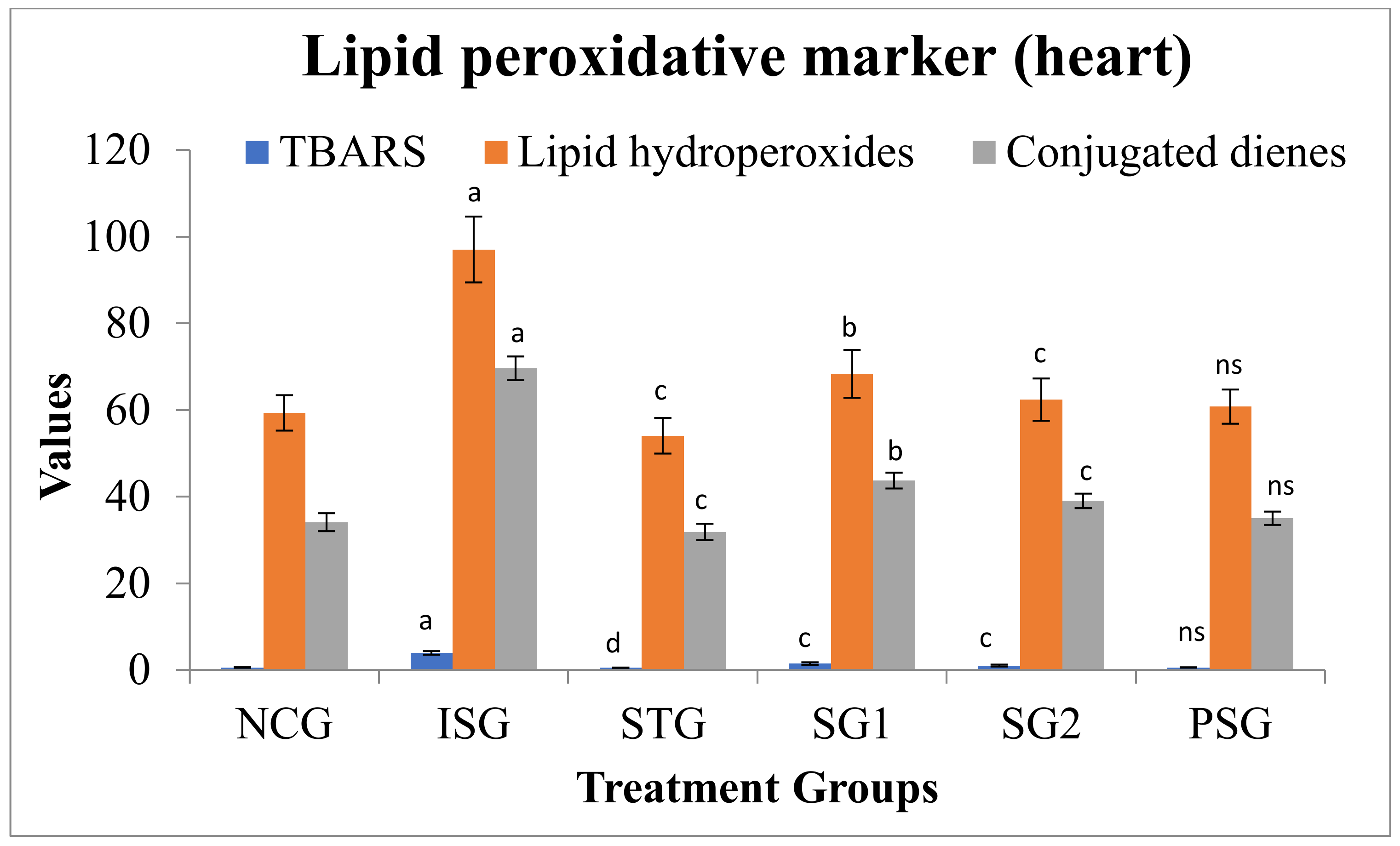
2.5.6. Oxidative Stress Parameter
2.5.7. Antioxidant Parameters
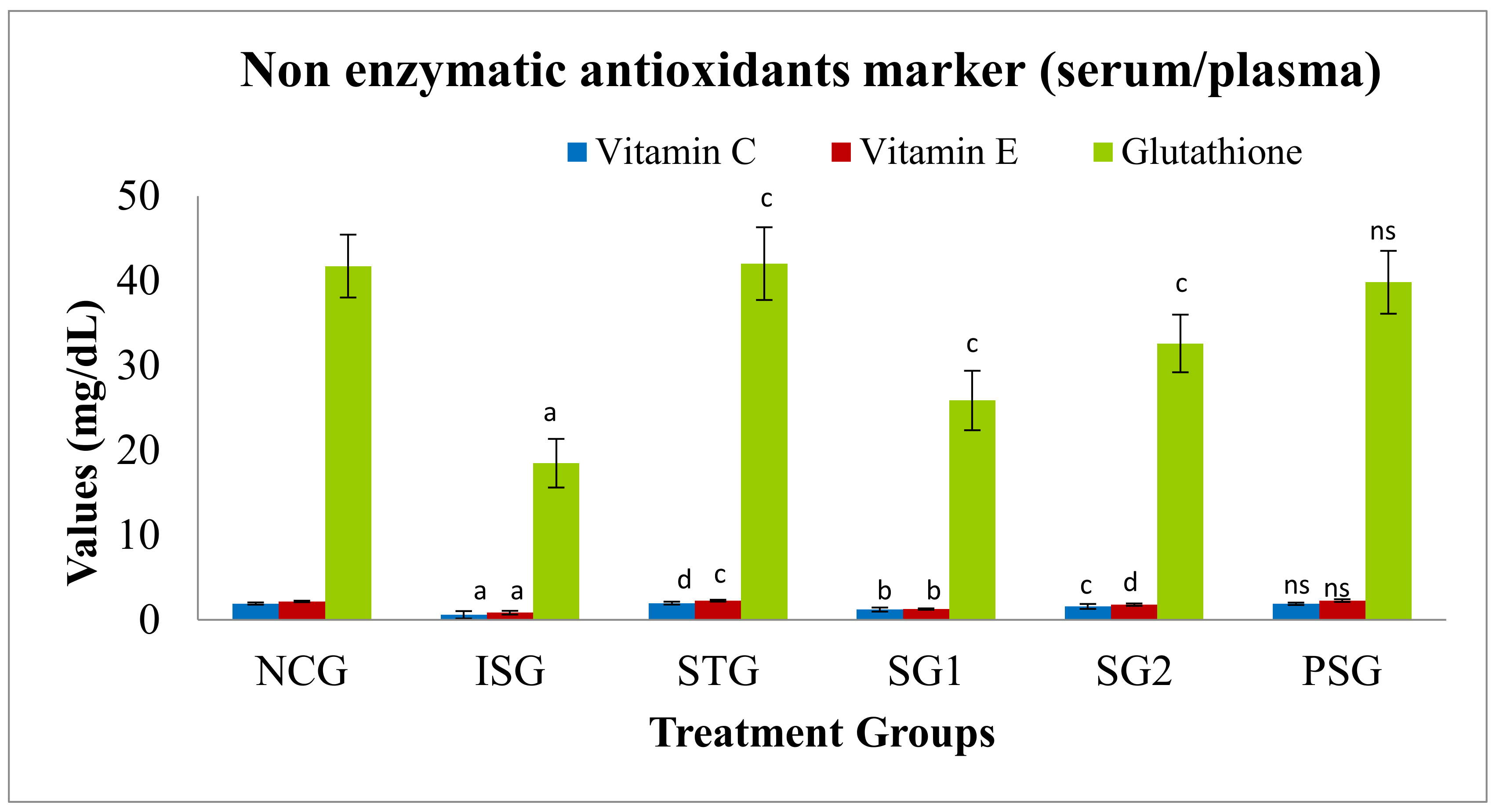
Non-Enzymatic Antioxidants Marker (Serum/Plasma)
Non-Enzymatic Antioxidants Marker (Heart)
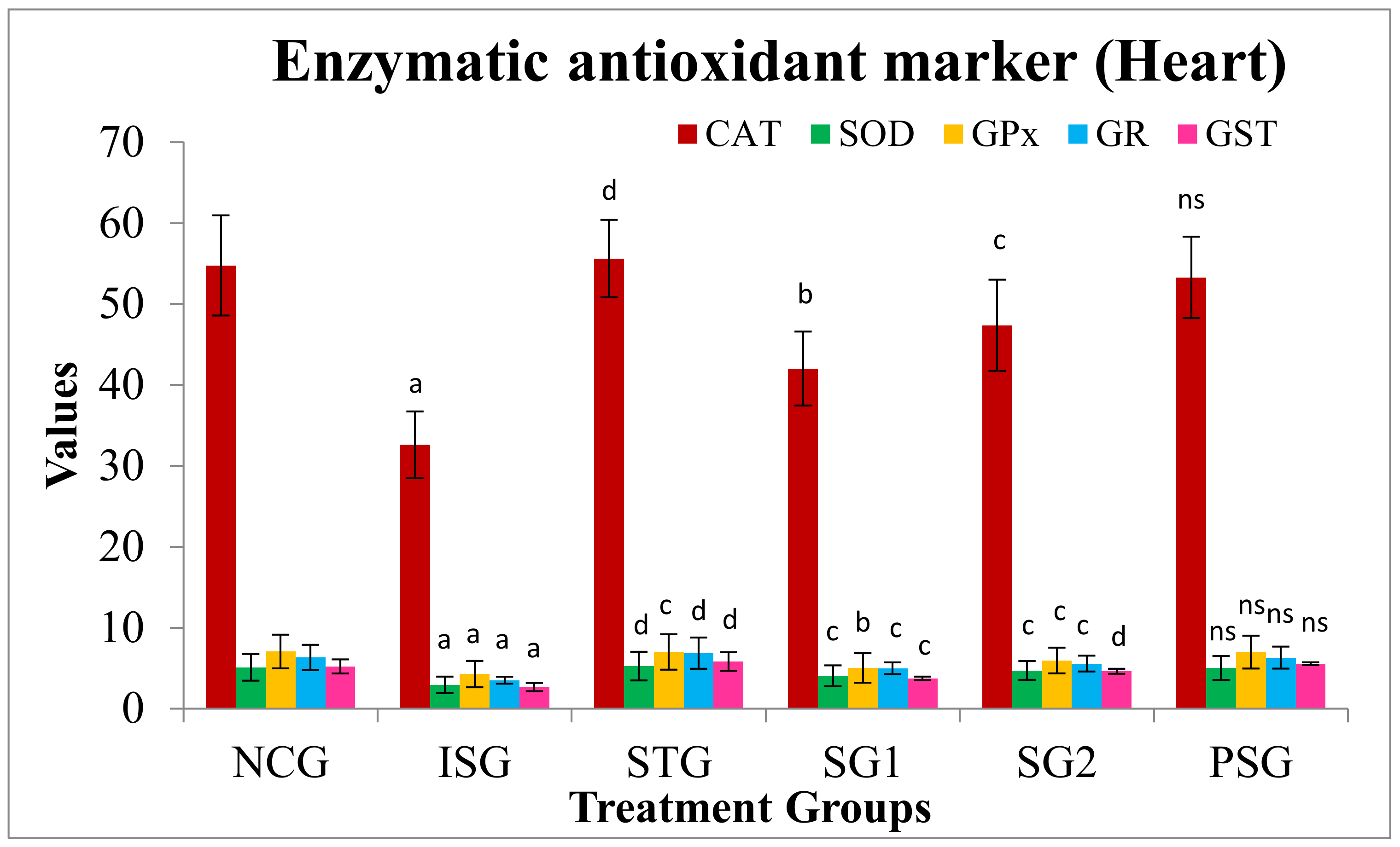
2.5.8. Enzymatic Antioxidant Marker (Heart)
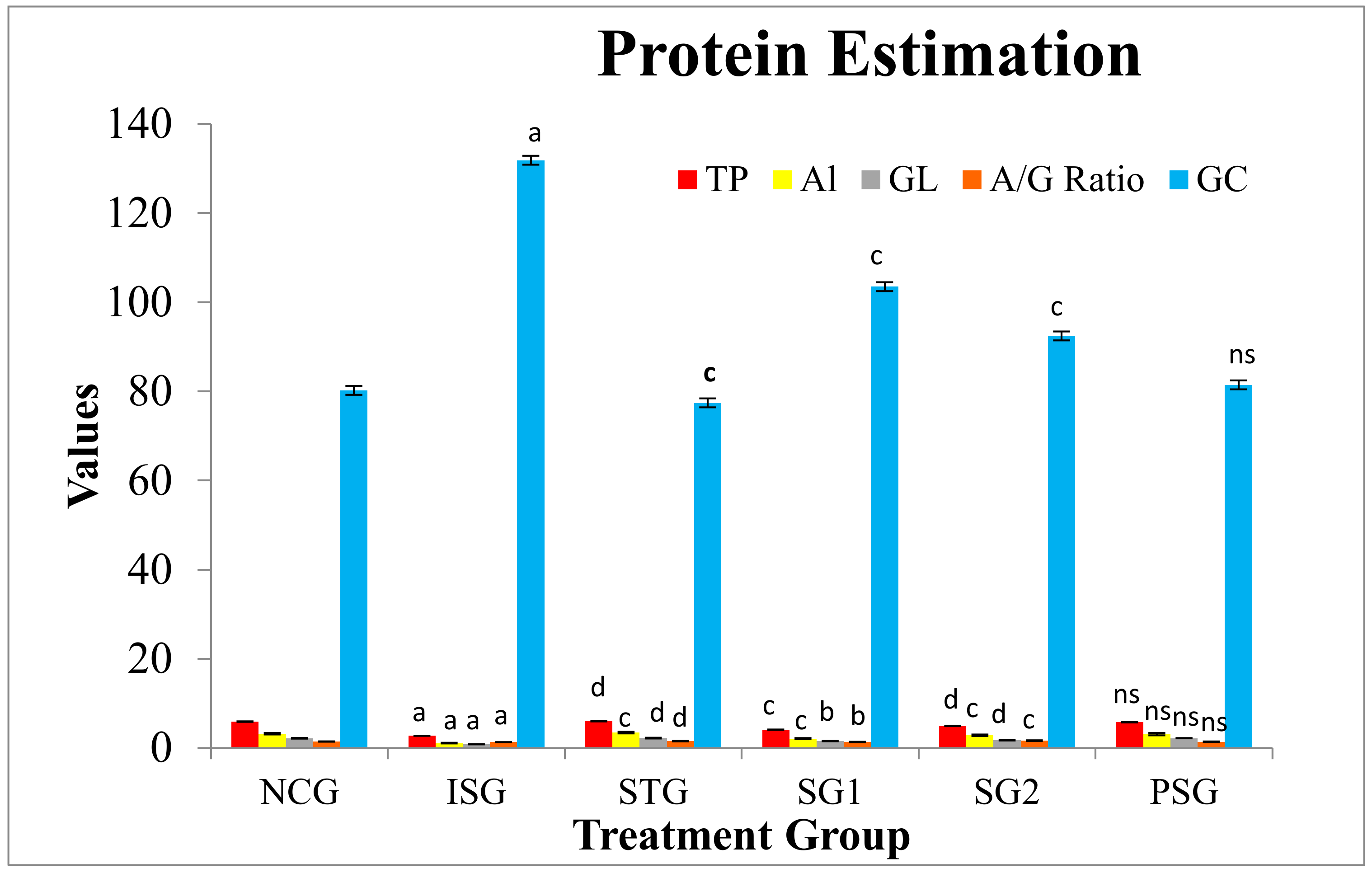
2.5.9. Protein Estimation
2.5.10. Estimation of Glucose
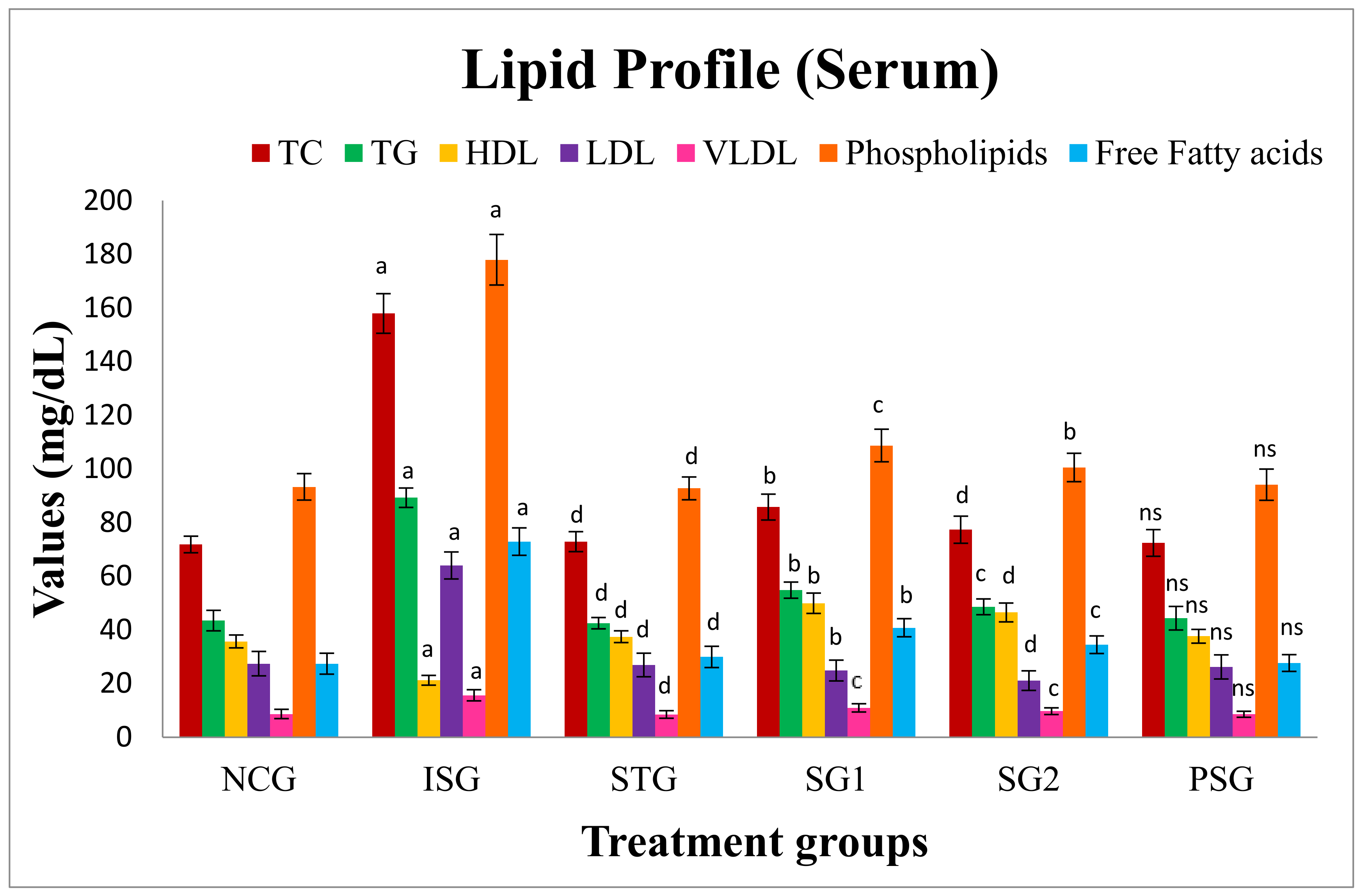
2.5.11. Lipid Profile
Estimation of Serum Lipids
Estimation of Tissue Lipids
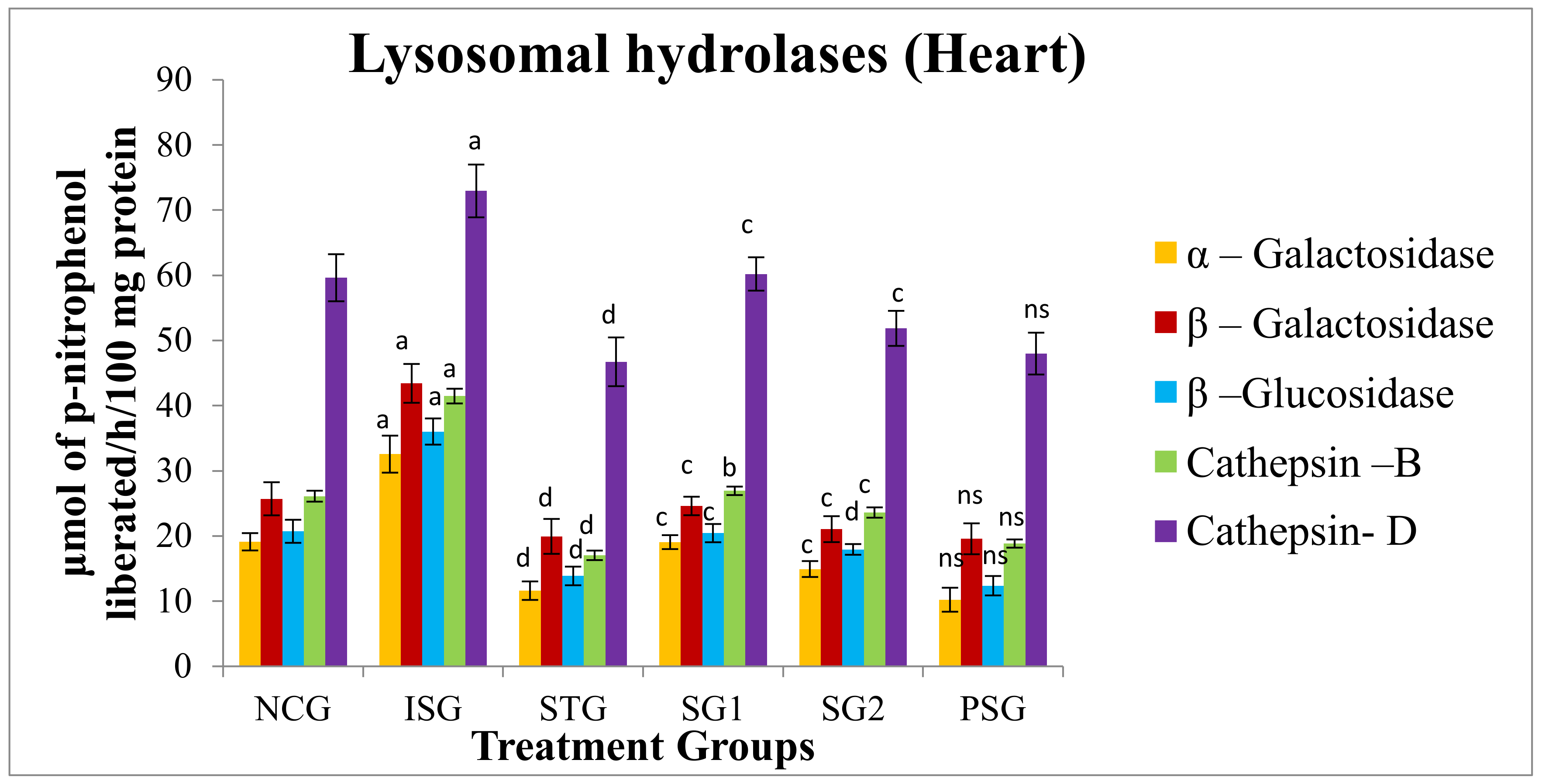
2.5.12. Lysosomal hydrolases
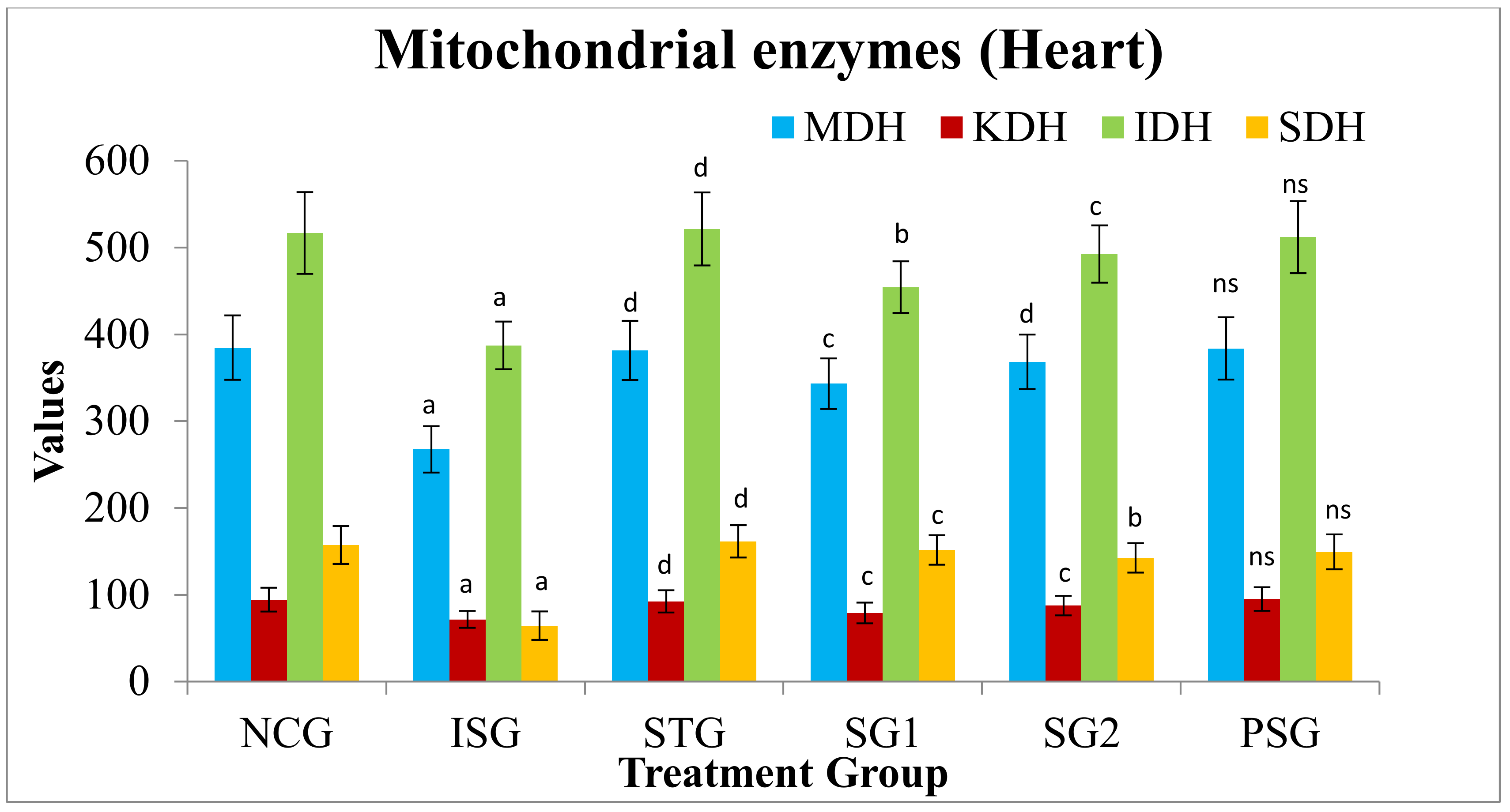
2.5.13. Heart Mitochondrial Enzymes
2.5.14. Apoptotic Marker
α- and β-Myosin Heavy Chain (α, β-MHC) Expression
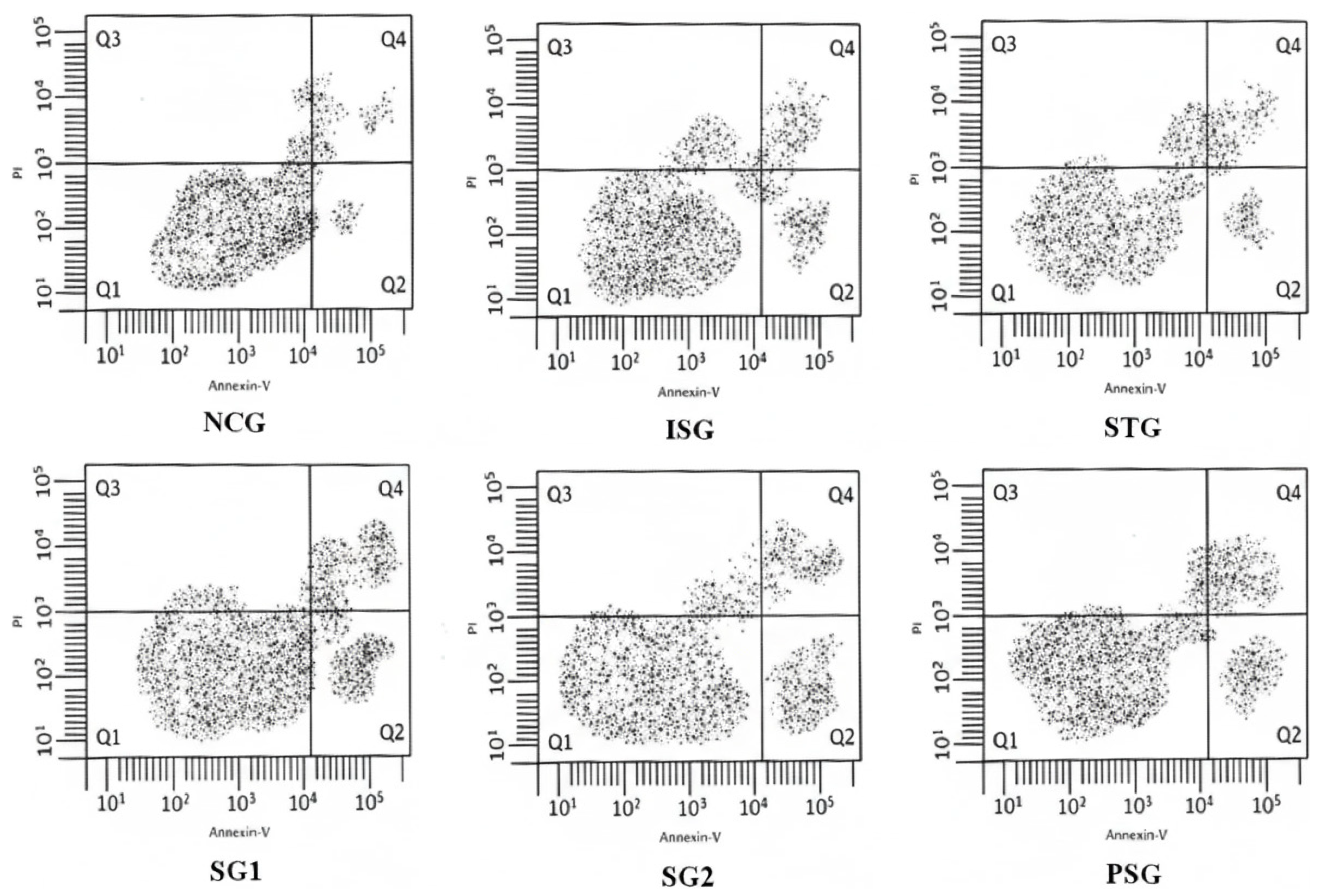
Flow Cytometry for the Measurement of Myocardial Apoptosis
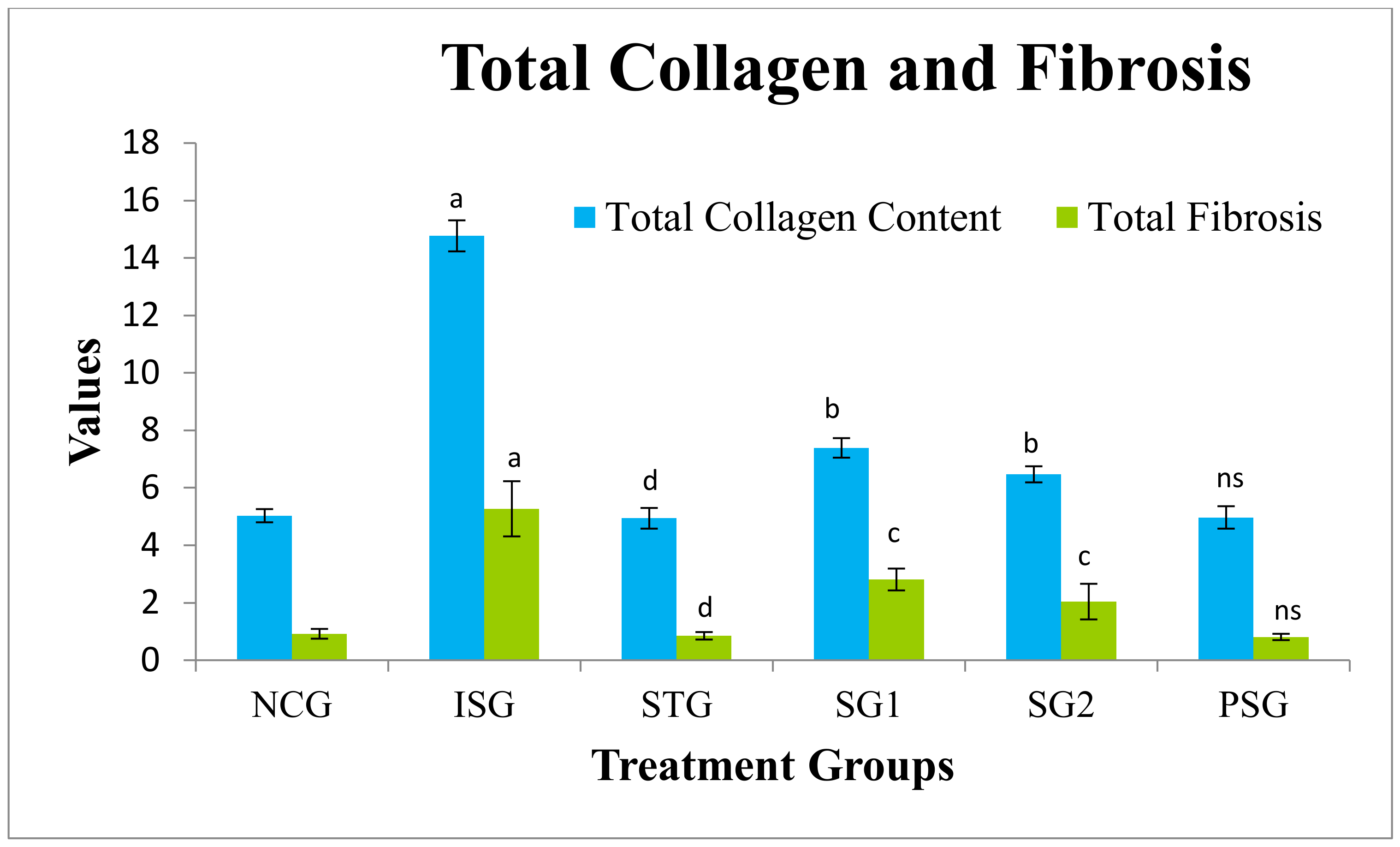
2.5.15. Total Myocardial Collagen Content (Heart)
2.5.16. Fibrosis
2.5.17. Cell (Myocyte) Size Determination
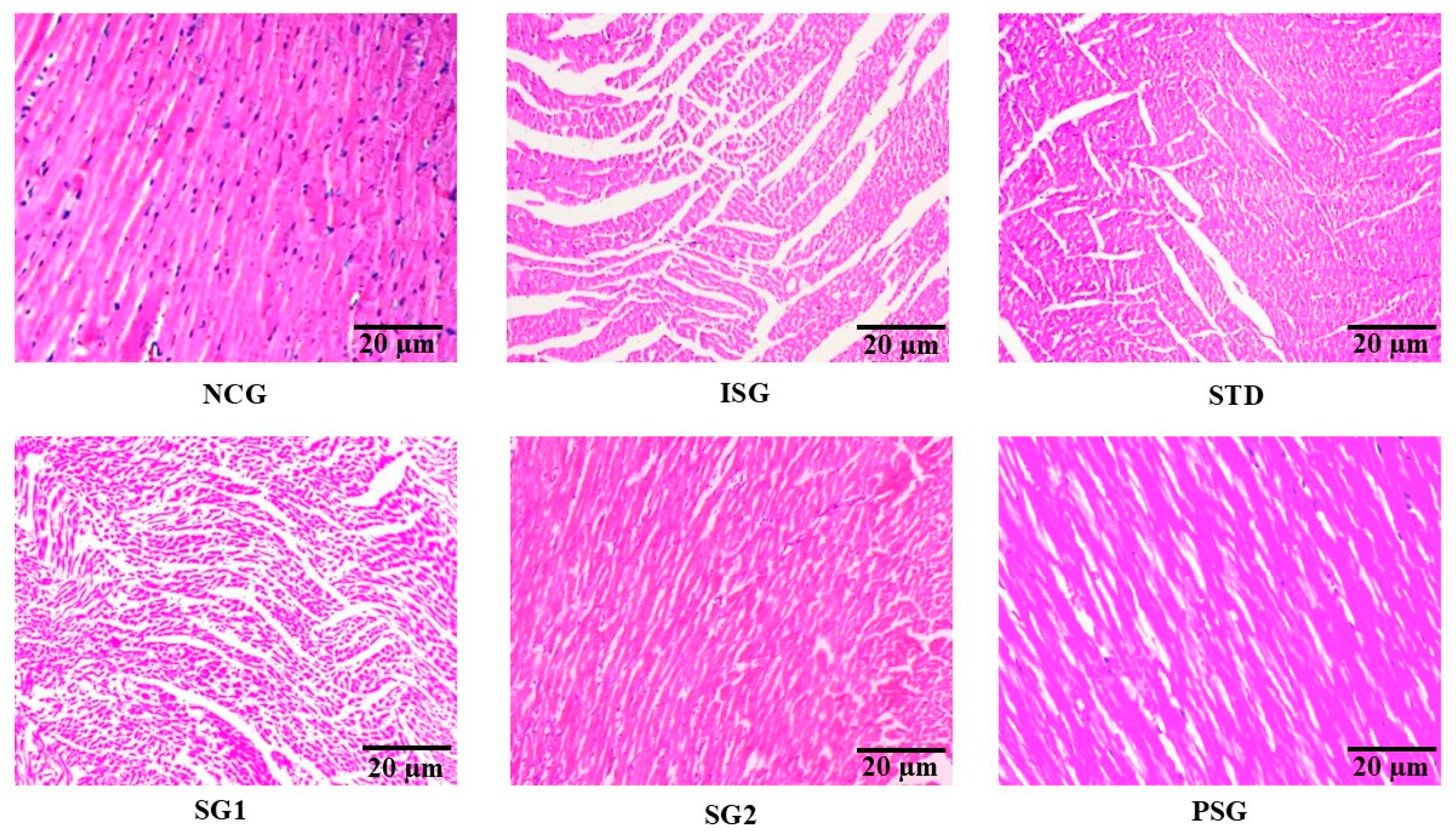
2.5.18. Histopathology
3. Results
3.1. Grading of Heart
3.2. Heart:Body Weight Ratio
3.3. Gross Morphology
3.4. Serum Biochemical Markers
3.5. Troponin
3.6. Oxidative Stress Parameters
3.7. Antioxidant Parameters
3.7.1. Non-Enzymatic Antioxidants Marker in Serum/Plasma/Tissue
3.7.2. Antioxidant Marker (Enzymatic) in Heart
3.8. Protein Estimation
3.9. Lipid Profile
3.10. Lysosomal Hydrolases
3.11. Heart Mitochondrial Enzymes
3.12. Apoptotic Marker
3.12.1. Expression of α-and β-Myosin Heavy Chain (α, β-MHC)
3.12.2. Flow Cytometry
3.13. Total Myocardial Collagen Content and Fibrosis Estimation
3.14. Cell (Myocyte) Size Determination
3.15. Histopathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPX | Glutathione peroxidase |
| GR | Glutathione reductase |
| GST | Glutathione S-Transferase |
| IDH | Isocitrate dehydrogenase |
| MDH | Malate Dehydrogenase |
| SDH | Succinate dehydrogenase |
| α-KGDH | α-ketoglutarate dehydrogenase |
| TP | Total Protein |
| AL | Albumin |
| GL | Globulin |
| A/G ratio | Albumin/globulin ratio |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| LDH | Lactate dehydrogenase |
| CK | Creatine kinase |
| CK-MB | Creatine kinase myocardial band |
| ALP | Alkaline phosphatase |
| TBARS | Thiobarbituric acid reactive substances |
| GC | Glucose |
| TC | Total cholesterol |
| TG | Triglyceride |
| HDL | High density lipoprotein |
| LDL | Low density lipoprotein |
| VLDL | Very low density lipoprotein |
| CVD | cardiovascular diseases |
| CHD | Coronary heart disease |
| IHD | Ischemic heart disease |
| SPF | Specific pathogen-free |
| CDRI | Central drug research institute |
| CPCSEA | Committee for the purpose of control and supervision of experiments on animals |
| IAEC | Institutional animal ethical committee |
| p.o. | Per oral |
| b.w. | Body weight |
| w/v | weight/volume |
| LV | Left ventricle |
| RV | Right ventricle |
| IVS | Intra ventricular septum |
| RIPA | Radioimmunoprecipitation assay |
| Tris-HCl | Tris(hydroxymethyl)aminomethane hydrochloride |
| NaCl | Sodium chloride |
| EGTA | Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid |
| Na2EDTA | Ethylenediaminetetraacetic acid disodium salt dihydrate |
| Na3VO4 | Sodium orthovanadate |
| PMSF | Phenylmethylsulfonyl fluoride |
| SDS–PAGE | Sodium dodecyl sulphate–polyacrylamide |
| PVDF | Polyvinylidene fluoride |
| TBST | Tris-buffered saline and Polysorbate 20 |
| ECL | Enhanced chemiluminescence |
| FITC | Fluorescein isothiocyanate |
| PBS | Phosphate buffered saline |
| PI | Propidium iodide |
| FACScan | Fluorescence activated cell analyzer |
| THP | Total hydroxyproline content |
| CF | Conversion factor |
| H&E | Hematoxylin and eosin |
| NIH | National institutes of health |
| NCG | Normal control group |
| PSG | Per se group |
| ISG | Isoprenaline control group |
| STG | Standard treated group |
| SG2 | Sericin group 2 |
| SG1 | Sericin group 1 |
| MHC | Myosin heavy chain |
| ECF | Extra cellular fluid |
| cTn | cardiovascular troponins |
| cTnI | Cardiovascular troponin I |
| HP | Hydroperoxides |
| CD | Conjugated dienes |
| ROS | Reactive oxygen species |
| ISO | Isoprenaline (Isoproterenol) |
| MI | Myocardial Infarction |
| TCA | Tricarboxylic acid |
| PS | Phosphatidylserine |
| ECM | Extra cellular matrix |
References
- American Heart Association Report—Annual Statistical Update. Available online: https://newsroom.heart.org/news/heart-disease?preview=f75e (accessed on 26 May 2022).
- Arafath, M.M.; Viswanathan, R. Study of Clinical Profile of Acute Myocardial Infarction in Tertiary Care Center: A Retrospective Study. Int. J. Sci. Study 2021, 186, 189. [Google Scholar]
- Huffman, M.D.; Prabhakaran, D.; Osmond, C.; Fall, C.H.D.; Tandon, N.; Lakshmy, R.; Ramji, S.; Khalil, A.; Gera, T.; Prabhakaran, P.; et al. Incidence of cardiovascular risk factors in an Indian urban cohort: Results from the New Delhi Birth Cohort. J. Am. Coll. Cardiol. 2011, 57, 1765–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Liu, M.; Sun, R.R.; Zheng, Y.; Zhang, P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid. Med. Cell. Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef]
- Ahsan, F.; Mahmood, T.; Usmani, S.; Bagga, P.; Shamim, A.; Tiwari, R.; Verma, N.; Siddiqui, M.H. A conglomeration of preclinical models related to myocardial infarction. Braz. J. Pharm. Sci. 2020, 56, 1–23. [Google Scholar] [CrossRef]
- Ahsan, F.; Ansari, T.M.; Usmani, S.; Bagga, P. An Insight on Silk Protein Sericin: From Processing to Biomedical Application. Drug Res. 2018, 68, 317–327. [Google Scholar] [CrossRef]
- Srivastav, R.K.; Siddiqui, H.H.; Mahmood, T.; Ahsan, F. Evaluation of cardioprotective effect of silk cocoon (Abresham) on isoprenaline-induced myocardial infarction in rats. Avicenna J. Phytomed. 2013, 3, 216–223. [Google Scholar] [CrossRef]
- Rona, G.; Kahn, D.S.; Chappel, C.I. Study on the healing of cardiac necrosis in the rat. Am. J. Pathol. 1961, 39, 473–489. [Google Scholar]
- Tiwari, R.; Siddiqui, M.H.; Mahmood, T.; Farooqui, A.; Bagga, P.; Ahsan, F.; Shamim, A. An exploratory analysis on the toxicity & safety profile of Polyherbal combination of curcumin, quercetin and rutin. Clin. Phytosci. 2020, 6, 82. [Google Scholar] [CrossRef]
- Rona, G.; Chappel, C.I.; Kahn, D.S. The significance of factors modifying the development of isoproterenol-induced myocardial necrosis. Am. Heart J. 1963, 66, 389–395. [Google Scholar] [CrossRef]
- Chauhan, K.; Parmar, L.; Solanki, R.; Kagathara, V.; Madat, D.; Patel, T. Effect of Piper longum linn on histopathological and biochemical changes in isoproterenol induced myocardial infarction in rats. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 759–766. [Google Scholar]
- Ahsan, F.; Siddiqui, H.H.; Mahmood, T.; Srivastav, R.K.; Nayeem, A. Evaluation of Cardioprotective effect of Coleus forskohlii against Isoprenaline induced myocardial infarction in rats. Indian J. Pharm. Biol. Res. 2014, 2, 17–25. [Google Scholar] [CrossRef]
- Iaccarino, G.; Dolber, P.C.; Lefkowitz, R.J.; Koch, W.J. β-Adrenergic receptor kinase-1 levels in catecholamine-induced myocardial hypertrophy: Regulation by β- but not α1-adrenergic stimulation. Hypertension 1999, 33, 396–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Suryanarayana Rao, K.; Recknagel, R.O. Early onset of lipoperoxidation in rat liver after carbon tetrachloride administration. Exp. Mol. Pathol. 1968, 9, 271–278. [Google Scholar] [CrossRef]
- Kyaw, A. A simple colorimetric method for ascorbic acid determination in blood plasma. Clin. Chim. Acta 1978, 86, 153–157. [Google Scholar] [CrossRef]
- Baker, H.; Frank, O. Tocopherol. In Clinical Vitaminology. Methods and Interpretation; New York Inter Science: New York, NY, USA, 1968; pp. 172–173. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manickam, A. Biochemical Methods, 2nd ed.; New Age International (P) Ltd.: New Delhi, India, 1997; pp. 185–186. [Google Scholar]
- Varley, H. Practical Clinical Biochemistry, 3rd ed.; Interscience Publishers, Inc.: Hoboken, NJ, USA, 1976. [Google Scholar]
- Boyne, A.F.; Ellman, G.L. A methodology for analysis of tissue sulfhydryl components. Anal. Biochem. 1972, 46, 639–653. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E. Selenium: Biochemical role as a component of glutathione peroxidase. Nutr. Rev. 1980, 38, 280–283. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione-S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.G.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Foster, L.B.; Dunn, R.T. Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clin. Chem. 1973, 19, 338–340. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Burstein, M.; Scholnick, H.R.; Morfin, R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 1970, 11, 583–595. [Google Scholar] [CrossRef]
- Falholt, K.; Lund, B.; Falholt, W. An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clin. Chim. Acta 1973, 46, 105–111. [Google Scholar] [CrossRef]
- Zilversmit, D.B.; Davis, A.K. Microdetermination of plasma phospholipids by trichloroacetic acid precipitation. J. Lab. Clin. Med. 1950, 35, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Conchie, J.; Gelman, A.L.; Levvy, G.A. Inhibition of glycosidases by aldonolactones of corresponding configuration. The C-4- and C-6-specificity of beta-glucosidase and beta-galactosidase. Biochem. J. 1967, 103, 609–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hoof, F.; Hers, H.G. The Abnormalities of Lysosomal Enzymes in Mucopolysaccharidoses. Eur. J. Biochem. 1968, 7, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Barrett, A.J. A new assay for cathepsin B1 and other thiol proteinases. Anal. Biochem. 1972, 47, 280–293. [Google Scholar] [CrossRef]
- Sapolsky, A.I.; Altman, R.D.; Howell, D.S. Cathepsin D activity in normal and osteoarthritic human cartilage. Fed. Proc. 1973, 32, 1489–1493. [Google Scholar]
- Fontana-Ayoub, M.; Krumschnabel, G. Laboratory protocol: Isolation of mouse heart mitochondria. Mitochondrial Physiol. Netw. 2015, 6, 1–2. [Google Scholar]
- Bell, J.L.; Baron, D.N. A colorimetric method for determination of isocitric dehydrogenase. Clin. Chim. Acta 1960, 5, 740–747. [Google Scholar] [CrossRef]
- Mehler, A.H.; Kobnberg, A.; Gbisolia, S.; Ochoa, S. The enzymatic mechanism of oxidation-redactions between malate or isocitrate and pyravate. J. Biol. Chem. 1948, 174, 961–977. [Google Scholar] [CrossRef]
- Reed, L.J.; Mukherjee, B.B. α-ketoglutarate dehydrogenase complex from Escherichia coli. Methods Enzymol. 1969, 13, 55–61. [Google Scholar] [CrossRef]
- Slater, E.C.; Bonner, W.D. The effect of fluoride on the succinic oxidase system. Biochem. J. 1952, 52, 185–196. [Google Scholar] [CrossRef]
- Kumar, S.; Sood, S.; Enjamoori, R.; Jaiswal, A.; Dinda, A.K.; Roy, T.S.; Maulik, S.K. Method for Enhanced Separation of Cardiac Myosin Heavy Chain Isoforms by Nongradient SDS-PAGE Muscle autografts in nerve gaps View project Whole exome and Targeted Sequencing in HCM patients from India View project. Anal. Lett. 2009, 42, 2403–2410. [Google Scholar] [CrossRef]
- Jaiswal, A.; Kumar, S.; Seth, S.; Dinda, A.K.; Maulik, S.K. Effect of U50,488H, a κ-opioid receptor agonist on myocardial α-and β-myosin heavy chain expression and oxidative stress associated with isoproterenol-induced cardiac hypertrophy in rat. Mol. Cell. Biochem. 2010, 345, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Y.; Wang, Z.; Zhong, S.; Hu, G.; Zuo, W. The Effects of Mobile Phone Radiofrequency Radiation on Cochlear Stria Marginal Cells in Sprague–Dawley Rats. Bioelectromagnetics 2020, 41, 219–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Luo, S.; Pan, C.; Cheng, X. Modulation of fatty acid metabolism is involved in the alleviation of isoproterenol-induced rat heart failure by fenofibrate. Mol. Med. Rep. 2015, 12, 7899–7906. [Google Scholar] [CrossRef] [Green Version]
- Woessner, J.J. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef]
- Benjamin, I.J.; Jalil, J.E.; Tan, L.B.; Cho, K.; Weber, K.T.; Clark, W.A. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ. Res. 1989, 65, 657–670. [Google Scholar] [CrossRef] [Green Version]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Seabrooks, L.; Hu, L. Insects: An underrepresented resource for the discovery of biologically active natural products. Acta Pharm. Sin. B 2017, 7, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, D.; Jeemon, P.; Roy, A. Cardiovascular Diseases in India: Current Epidemiology and Future Directions. Circulation 2016, 133, 1605–1620. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Goyal, V.K.; Jana, S.; Pandey, S.K.; Gothi, A. What suits best for organ weight analysis: Review of relationship between organ weight and body/brain weight for rodent toxicity studies. Int. J. Pharm. Sci. Res. 2014, 5, 1525–1532. [Google Scholar] [CrossRef]
- Henry, J.P.; Stephens-Larson, P. Reduction of chronic psychosocial hypertension in mice by decaffeinated tea. Hypertension 1984, 6, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litwin, S.E.; Katz, S.E.; Weinberg, E.O.; Lorell, B.H.; Aurigemma, G.P.; Douglas, P.S. Serial echocardiographic-doppler assessment of left ventricular geometry and function in rats with pressure-overload hypertrophy chronic angiotensin-converting enzyme inhibition attenuates the transition to heart failure. Circulation 1995, 91, 2642–2654. [Google Scholar] [CrossRef]
- Suchalatha, S.; Devi, S. Protective effect of Terminalia chebula against experimental myocardial injury induced by isoproterenol. Indian J. Exp. Biol. 2004, 42, 174–178. [Google Scholar]
- Sushamakumari, S.; Jayadeep, A.; Kumar, J.S.S.; Menon, V.P. Effect of carnitine on malondialdehyde, taurine and glutathione levels in heart of rats subjected to myocardial stress by isoproterenol. Indian J. Exp. Biol. 1989, 27, 134–137. [Google Scholar]
- Li, R.; Yan, G.; Zhang, Q.; Jiang, Y.; Sun, H.; Hu, Y.; Sun, J.; Xu, B. miR-145 inhibits isoproterenol-induced cardiomyocyte hypertrophy by targeting the expression and localization of GATA6. FEBS Lett. 2013, 587, 1754–1761. [Google Scholar] [CrossRef] [Green Version]
- Mair, J. Progress in myocardial damage detection: New biochemical markers for clinicians. Crit. Rev. Clin. Lab. Sci. 1997, 34, 1–66. [Google Scholar] [CrossRef]
- Bertinchant, J.P.; Robert, E.; Polge, A.; Marty-Double, C.; Fabbro-Peray, P.; Poirey, S.; Aya, G.; Juan, J.M.; Ledermann, B.; De la Coussaye, J.E.; et al. Comparison of the diagnostic value of cardiac troponin I and T determinations for detecting early myocardial damage and the relationship with histological findings after isoprenaline-induced cardiac injury in rats. Clin. Chim. Acta 2000, 298, 13–28. [Google Scholar] [CrossRef]
- Brady, S.; York, M.; Scudamore, C.; Williams, T.; Griffiths, W.; Turton, J. Cardiac troponin i in isoproterenol-induced cardiac injury in the Hanover Wistar rat: Studies on low dose levels and routes of administration. Toxicol. Pathol. 2010, 38, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Zaltzberg, H.; Ben-Amotz, A.; Kanter, Y.; Aviram, M. β-Carotene affects antioxidant status in non-insulin-dependent diabetes mellitus. Pathophysiology 1999, 6, 157–161. [Google Scholar] [CrossRef]
- Senthil, S.; Chandramohan, G.; Pugalendi, K.V. Isomers (oleanolic and ursolic acids) differ in their protective effect against isoproterenol-induced myocardial ischemia in rats. Int. J. Cardiol. 2007, 119, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Dutta-Roy, A.K.; Gordon, M.J.; Campbell, F.M.; Duthie, G.G.; James, W.P.T. Vitamin E requirements, transport, and metabolism: Role of α-tocopherol-binding proteins. J. Nutr. Biochem. 1994, 5, 562–570. [Google Scholar] [CrossRef]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D.M. The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Tappel, A.L. Vitamin E and selenium protection from in vivo lipid peroxidation. Ann. N. Y. Acad. Sci. 1980, 355, 18–31. [Google Scholar] [CrossRef]
- Vennila, L.; Pugalendi, K.V. Protective effect of sesamol against myocardial infarction caused by isoproterenol in Wistar rats. Redox Rep. 2010, 15, 36–42. [Google Scholar] [CrossRef]
- Devi, R.; Deori, M. Evaluation of antioxidant activities of silk protein sericin secreted by silkworm Antheraea assamensis (Lepidoptera: Saturniidae). J. Pharm. Res. 2011, 4, 4688–4691. [Google Scholar]
- Eram, S.; Mujahid, M.; Bagga, P.; Ahsan, F.; Rahman, M.A. Hepatoprotective Evaluation of Galanga (Alpinia officinarum) Rhizome Extract against Antitubercular Drugs Induced Hepatotoxicity in Rats. J. Herbs Spices Med. Plants 2020, 26, 113–125. [Google Scholar] [CrossRef]
- Karthikeyan, J. Enzymatic and non-enzymatic antioxidants in selected Piper species. Indian J. Exp. Biol. 2003, 41, 135–140. [Google Scholar] [PubMed]
- Polidoro, G.; Di Ilio, C.; Arduini, A.; La Rovere, G.; Federici, G. Superoxide dismutase, reduced glutathione and TBA-reactive products in erythrocytes of patients with multiple sclerosis. Int. J. Biochem. 1984, 16, 505–509. [Google Scholar] [CrossRef]
- Guarnieri, C.; Flamigni, F.; Caldarera, C.M. Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J. Mol. Cell. Cardiol. 1980, 12, 797–808. [Google Scholar] [CrossRef]
- Saravanan, G.; Prakash, J. Effect of garlic (Allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J. Ethnopharmacol. 2004, 94, 155–158. [Google Scholar] [CrossRef]
- Sheela, S.C.; Shyamala, D.C. Protective effect of Abana®, a poly-herbal formulation, on isoproterenol-induced myocardial infarction in rats. Indian J. Pharmacol. 2021, 32, 201. [Google Scholar]
- Ahmed, K.K.M.; Rana, A.C.; Dixit, V.K. Effect of Calotropis procera latex on isoproterenol induced myocardial infarction in albino rats. Phytomedicine 2004, 11, 327–330. [Google Scholar] [CrossRef]
- Mohanty, I.; Arya, D.S.; Dinda, A.; Talwar, K.K.; Joshi, S.; Gupta, S.K. Mechanisms of cardioprotective effect of Withania somnifera in experimentally induced myocardial infarction. Basic Clin. Pharmacol. Toxicol. 2004, 94, 184–190. [Google Scholar] [CrossRef]
- Farvin, S.K.; Anandan, R.; Kumar, S.H.S.; Shiny, K.S.; Sankar, T.V.; Thankappan, T.K. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol. Res. 2004, 50, 231–236. [Google Scholar] [CrossRef]
- Deori, M.; Devi, D.; Kumari, S.; Hazarika, A.; Kalita, H.; Sarma, R.; Devi, R. Antioxidant effect of sericin in brain and peripheral tissues of oxidative stress induced hypercholesterolemic rats. Front. Pharmacol. 2016, 7, 326. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.S.; Varghese, A.; Muraleedharan, D.; Menon, V. Protective action of aspirin in experimental myocardial infarction induced by isoproterenol in rats and its effect on lipid peroxidation. Indian J. Exp. Biol. 1990, 28, 480–485. [Google Scholar]
- Padmanabhan, M.; Rajadurai, M.; Prince, P.S.M. Preventive effect of S-allylcysteine on membrane-bound enzymes and glycoproteins in normal and isoproterenol-induced cardiac toxicity in male Wistar rats. Basic Clin. Pharmacol. Toxicol. 2008, 103, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.B.; Goldfine, A.B.; Timimi, F.K.; Ting, H.H.; Roddy, M.A.; Simonson, D.C.; Creager, M.A. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation 1998, 97, 1695–1701. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.J.; Eberle, H.C.; Nassenstein, K.; Schlosser, T.; Farazandeh, M.; Naber, C.K.; Sabin, G.V.; Bruder, O. Impact of hyperglycemia at admission in patients with acute ST-segment elevation myocardial infarction as assessed by contrast-enhanced MRI. Clin. Res. Cardiol. 2011, 100, 649–659. [Google Scholar] [CrossRef]
- Khan, A.A.; Siddiqui, H.H.; Ansari, T.M.; Ahsan, F. A Comparative Evaluation Study of Citrus limetta and Metformin against Hyperlipidemia in Diabetic and Non-diabetic rats. Res. J. Pharm. Technol. 2019, 12, 1244–1250. [Google Scholar] [CrossRef]
- Shiomi, T.; Tsutsui, H.; Ikeuchi, M.; Matsusaka, H.; Hayashidani, S.; Suematsu, N.; Wen, J.; Kubota, T.; Takeshita, A. Streptozotocin-induced hyperglycemia exacerbates left ventricular remodeling and failure after experimental myocardial infarction. J. Am. Coll. Cardiol. 2003, 42, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Yeagle, P.L. Cholesterol and the cell membrane. Rev. Biomembr. 1985, 822, 267–287. [Google Scholar] [CrossRef]
- Rajadurai, M.; Mainzen Prince, P.S. Preventive effect of naringin on lipids, lipoproteins and lipid metabolic enzymes in isoproterenol-induced myocardial infarction in Wistar rats. J. Biochem. Mol. Toxicol. 2006, 20, 191–197. [Google Scholar] [CrossRef]
- Ravichandran, L.V.; Puvanakrishnan, R.; Joseph, K.T. Influence of isoproterenol-induced myocardial infarction on certain glycohydrolases and cathepsins in rats. Biochem. Med. Metab. Biol. 1991, 45, 6–15. [Google Scholar] [CrossRef]
- Akila, P.; Asaikumar, L.; Vennila, L. Chlorogenic acid ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes. Biomed. Pharmacother. 2017, 85, 582–591. [Google Scholar] [CrossRef]
- Chambers, D.E.; Parks, D.A.; Patterson, G.; Roy, R.; McCord, J.M.; Yoshida, S.; Parmley, L.F.; Downey, J.M. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J. Mol. Cell. Cardiol. 1985, 17, 145–152. [Google Scholar] [CrossRef]
- Ricciutti, M.A. Myocardial lysosome stability in the early stages of acute ischemic injury. Am. J. Cardiol. 1972, 30, 492–497. [Google Scholar] [CrossRef]
- Asghar, A.; Samejima, K.; Yasui, T. Functionality of muscle proteins in gelation mechanisms of structured meat products. Crit. Rev. Food Sci. Nutr. 1985, 22, 27–106. [Google Scholar] [CrossRef] [PubMed]
- Porzio, M.A.; Pearson, A.M. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim. Biophys. Acta 1977, 490, 27–34. [Google Scholar] [CrossRef]
- Yates, L.D.; Greaser, M.L.; Huxley, H.E. Quantitative determination of myosin and actin in rabbit skeletal muscle. J. Mol. Biol. 1983, 168, 123–141. [Google Scholar] [CrossRef]
- Hong, H.Q.; Lu, J.; Fang, X.L.; Zhang, Y.H.; Cai, Y.; Yuan, J.; Liu, P.Q.; Ye, J.T. G3BP2 is involved in isoproterenol-induced cardiac hypertrophy through activating the NF-κB signaling pathway. Acta Pharmacol. Sin. 2018, 39, 184–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, B.A.; Drujan, D.; Willis, M.S.; Murphy, L.O.; Corpina, R.A.; Burova, E.; Rakhilin, S.V.; Stitt, T.N.; Patterson, C.; Latres, E.; et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007, 6, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Brault, J.J.; Gygi, S.P.; Glass, D.J.; Valenzuela, D.M.; Gartner, C.; Latres, E.; Goldberg, A.L. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J. Cell Biol. 2009, 185, 1083–1095. [Google Scholar] [CrossRef] [Green Version]
- El-Borm, H.T.; Gobara, M.S.; Badawy, G.M. Ginger extract attenuates labetalol induced apoptosis, DNA damage, histological and ultrastructural changes in the heart of rat fetuses. Saudi J. Biol. Sci. 2021, 28, 440–447. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Zhang, J.; Sun, N.; Huang, K.; Li, H.; Wang, Z.; Huang, K.; Wang, L. An injectable silk sericin hydrogel promotes cardiac functional recovery after ischemic myocardial infarction. Acta Biomater. 2016, 41, 210–223. [Google Scholar] [CrossRef]
- Reddy, G.K.; Enwemeka, C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem. 1996, 29, 225–229. [Google Scholar] [CrossRef]
- Kitchener, R.L.; Grunden, A.M. Prolidase function in proline metabolism and its medical and biotechnological applications. J. Appl. Microbiol. 2012, 113, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Murtha, L.A.; Schuliga, M.J.; Mabotuwana, N.S.; Hardy, S.A.; Waters, D.W.; Burgess, J.K.; Knight, D.A.; Boyle, A.J. The processes and mechanisms of cardiac and pulmonary fibrosis. Front. Physiol. 2017, 8, 782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jianzhe, L.; Liu, B.; Wang, C.; Li, Y.; Peng, J. Effect of calcitonin gene-related peptide on isoprenaline-induced cardiac fibroblast proliferation and collagen expression. J. Cent. South Univ. Med. Sci. 2013, 38, 545–552. [Google Scholar] [CrossRef]
- Nirmala, C.; Anand, S.; Puvanakrishnan, R. Influence of curcumin treatment on collagen metabolism in experimental myocardial necrosis in rats. Proc. Indian Acad. Sci. 1999, 111, 231–237. [Google Scholar] [CrossRef]







| Groups | Treatment Provided | Dose, Route and Duration |
|---|---|---|
| Normal Control Group (NCG) | Rats were administered with distilled water | 2 mL/kg/day/p.o. for continuous 28 days |
| Isoproterenol Control Group (ISG) | Rats were administered with Isoproterenol | 85 mg/kg/s.c./once a day administered on the 29th and 30th days. |
| Standard Group (STG) | Rats were administered with Metoprolol + Isoproterenol | 10 mg/kg/day/p.o. for continuous 28 days + 85 mg/kg/s.c. once a day administered on the 29th and 30th days |
| Sericin Group 1 (SG1) | Rats were administered with Sericin + Isoproterenol | 500 mg/kg/day/p.o. for continuous 28 days + 85 mg/kg/s.c. once a day administered on the 29th and 30th days |
| Sericin Group 2 (SG2) | Rats were administered with Sericin + Isoproterenol | 1000 mg/kg/day/p.o. for continuous 28 days + 85 mg/kg/s.c. once a day administered on 29th and 30th day |
| Per se Group (PSG) | Rats were administered with Sericin alone | 1000 mg/kg/day/p.o. administered continuously for 28 days |
| Grade No. | Characteristics |
|---|---|
| Grade-0 | No lesion |
| Grade-1 | Inflammation and redness, capillary dilatation |
| Grade-2 | Edema, capillary dilatation, ventricle portion yellowish |
| Grade-3 | Scar formation, yellowish color of atrium and ventricle part of heart |
| Grade-4 | Diffuse heart, scar formation, and yellowish color of atrium and ventricle |
| Treatment Groups | Grade |
|---|---|
| Normal Control (NCG) | Grade 0 |
| Isoproterenol Control Group (ISG) | Grade 4 |
| Standard Group (STG) | Grade 1 |
| Sericin Group 1(SG1) | Grade 2 |
| Sericin Group 2(SG2) | Grade 1 |
| Per se Group (PSG) | Grade 0 |
| Treatment Group | Number of Animals | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| NCG | −ve | −ve | −ve | −ve | −ve | −ve |
| ISG | +ve | +ve | +ve | +ve | +ve | +ve |
| STG | −ve | −ve | −ve | −ve | −ve | −ve |
| SG1 | −ve | +ve | −ve | +ve | +ve | −ve |
| SG2 | +ve | +ve | −ve | −ve | −ve | −ve |
| PSG | −ve | −ve | −ve | −ve | −ve | −ve |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahsan, F.; Mahmood, T.; Wani, T.A.; Zargar, S.; Siddiqui, M.H.; Usmani, S.; Shamim, A.; Wahajuddin, M. Effectual Endeavors of Silk Protein Sericin against Isoproterenol Induced Cardiac Toxicity and Hypertrophy in Wistar Rats. Life 2022, 12, 1063. https://doi.org/10.3390/life12071063
Ahsan F, Mahmood T, Wani TA, Zargar S, Siddiqui MH, Usmani S, Shamim A, Wahajuddin M. Effectual Endeavors of Silk Protein Sericin against Isoproterenol Induced Cardiac Toxicity and Hypertrophy in Wistar Rats. Life. 2022; 12(7):1063. https://doi.org/10.3390/life12071063
Chicago/Turabian StyleAhsan, Farogh, Tarique Mahmood, Tanveer A. Wani, Seema Zargar, Mohammed Haris Siddiqui, Shazia Usmani, Arshiya Shamim, and Muhammad Wahajuddin. 2022. "Effectual Endeavors of Silk Protein Sericin against Isoproterenol Induced Cardiac Toxicity and Hypertrophy in Wistar Rats" Life 12, no. 7: 1063. https://doi.org/10.3390/life12071063
APA StyleAhsan, F., Mahmood, T., Wani, T. A., Zargar, S., Siddiqui, M. H., Usmani, S., Shamim, A., & Wahajuddin, M. (2022). Effectual Endeavors of Silk Protein Sericin against Isoproterenol Induced Cardiac Toxicity and Hypertrophy in Wistar Rats. Life, 12(7), 1063. https://doi.org/10.3390/life12071063






